Abstract
Background
CO2 emissions from cleared mangrove areas may be substantial, increasing the costs of continued losses of these ecosystems, particularly in mangroves that have highly organic soils.
Methodology/Principal Findings
We measured CO2 efflux from mangrove soils that had been cleared for up to 20 years on the islands of Twin Cays, Belize. We also disturbed these cleared peat soils to assess what disturbance of soils after clearing may have on CO2 efflux. CO2 efflux from soils declines from time of clearing from ∼10 600 tonnes km−2 year−1 in the first year to 3000 tonnes km2 year−1 after 20 years since clearing. Disturbing peat leads to short term increases in CO2 efflux (27 umol m−2 s−1), but this had returned to baseline levels within 2 days.
Conclusions/Significance
Deforesting mangroves that grow on peat soils results in CO2 emissions that are comparable to rates estimated for peat collapse in other tropical ecosystems. Preventing deforestation presents an opportunity for countries to benefit from carbon payments for preservation of threatened carbon stocks.
Introduction
Mangroves are being cleared at a rapid rate, exceeding that of tropical forests [1], [2]. Clearing of above-ground biomass in mangrove forests results in changes in ecosystem processes [3] and losses of ecosystem services, including fisheries and storm protection [4], [5]. Additionally, clearing of forests reduces carbon sequestration and may lead to CO2 emissions due to loss of aboveground carbon stocks and increased rates of soil decomposition [6]. In terrestrial ecosystems land-use change is one of the major sources of CO2 emissions above the burning of fossil fuels [7]. In the tropics clearing of rainforests has led to high levels of CO2 emissions [8] which have made these forests particularly valuable for conservation schemes developed to reduce emissions from deforestation and forest degradation, and to enhance carbon storage (REDD and REDD+) [9], [10], [11]. Similar schemes are proposed for carbon rich marine ecosystems, including mangroves, but there are many uncertainties around factors influencing carbon sequestration and carbon stocks in these coastal systems [12], [13].
There are few estimates of ecosystem carbon stocks in mangroves [14], [15]. The few that are available indicate a large proportion of carbon is in soils [14], [15], [16]. Carbon stocks in mangrove soils can be extremely high at some sites, as they contain accumulated peat (>20% carbon) derived mainly from roots as sea level has risen in the last interglacial period and anoxic conditions have slowed decomposition [17], [18]. The high levels of carbon in mangrove soils, the potential oxidation of peat deposits with land use change [6] indicate that once cleared mangrove forests on peat soils may become significant sources of CO2.
In terrestrial tropical forest settings, clearing and draining of peat soils results in oxidation of carbon leading to peat collapse and the emission of CO2 and other greenhouse gases [11]. Peat collapse and CO2 emissions from cleared peat lands correlate with the level of the water table, increasing with the lowering of the water table and thus the exposure of peat to aerobic conditions [11], [19], [20]. Similarly, clearing of mangrove forests could result in significant CO2 emissions due to oxidation of C in mangrove peat. In mangrove ecosystems that have been damaged by hurricanes peat collapse has been observed [21]. Additional oxidation may occur if peat is disturbed and contact with air is increased, as would be the case when shrimp ponds are constructed in peat soils and peat is pushed up on to banks or levees. The increase in CO2 emissions with clearing of mangroves may be a major cost of disturbance of mangrove world-wide, and thus may contribute to the case for strengthening protection of these ecosystems through abating CO2 emissions [12], [22].
In this study we measured CO2 emissions from mangrove peat in Belize that had been cleared of vegetation over the last 2 decades in anticipation of tourism development. This mangrove peat at the site has a carbon (C) concentration of approximately 300 mg C g−1 [23]. We used a chronosequence of clearing to assess the potential change in CO2 efflux over time since disturbance. Additionally we experimentally disturbed cleared peat to assess the potential enhancements in CO2 efflux through increasing contact with air.
Results
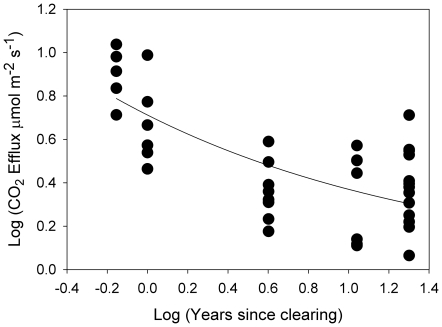
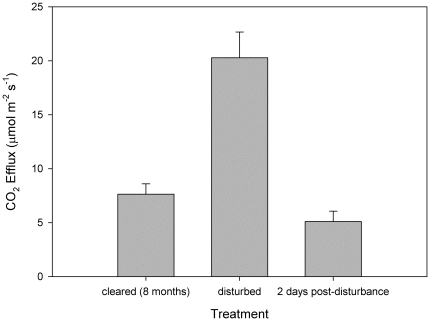
Over our chronosequence of sites representing time since clearing of mangroves, CO2 efflux declined logarithmically with time, from 7.6 to 2.1 µmol m−2 s−1 over 20 years (Figure 1, F1, 30 = 40.50, P<0.0001). Soil temperature varied during the measurements, but there was no significant correlation between CO2 efflux and soil temperature. At 4 years after clearing, CO2 efflux had reached a relatively constant level of approximately 2 µmol m−2 s−1. Extrapolation of CO2 efflux rates to annual CO2 loss indicates that CO2 emissions from cleared peat would be ∼10 600 tonnes km−2 year−1 in the first year after clearing, falling to ∼2900 tonnes CO2 km−2 year−1 (Table 1). Higher rates of CO2 efflux were observed with acute disturbance of the peat, reaching a mean of 27 µmol m−2 s−1 when blocks of peat were cut from the soil (Figure 2, F2,15 = 25.37, P<0.0001). However this increase was transitory, as CO2 efflux had returned to ambient levels within 2 days of disturbance.
Figure 1. Variation in CO2 efflux from peat soils over the time since the mangrove forest was cleared from Twin Cays Belize.
The fitted line is of the form: Log CO2 Efflux = a x exp (-b x time) where a = 0.712 and b = 0.656; R2 = 0.51. The model is significant: F1,30 = 40.4988, P<0.0001.
Table 1. Estimates of CO2 efflux from modified mangrove and other habitats with peat soils.
| Habitat | Modification | CO2 efflux tonnes km−2 year−1 | Method | Reference |
| Mangrove, Belize | Cleared | 2900 | CO2 efflux | THIS STUDY |
| Mangrove, Honduras | Forest damaged by hurricane | 1500 | Inferred from peat collapse | Cahoon et al. 2003 |
| Mangrove, Australia | Shrimp pond | 1750 (220–5000) | CO2 efflux | Burford and Longmore 2001 |
| Rainforest, Indonesia | Drained for agriculture | 3200 | Inferred from peat collapse and measured as CO2 efflux | Couwenburg et al. 2010 and references therein |
| Tundra, Alaska | Thawed (vegetation intact) | 150–430 | Net CO2 exchange | Schuur et al. 2009 |
Figure 2. CO2 efflux from peat soils that were cleared of forest (cleared 8 months) where peat was disturbed by cutting blocks from the soils (disturbed) and two days after the blocks of peat were cut (2 days post-disturbance).
There was a significant effect of the disturbance treatment (F2,15 = 25.37, P<0.0001) but after two days there was no significant difference in soil CO2 efflux between disturbed and undisturbed samples.
Discussion
Based on short term measurements of CO2 efflux from the soil surface of cleared mangrove forests, we found that CO2 efflux is substantial, estimated to be approximately 2900 tonnes km−2 year−1 (Table 1). This value is similar to CO2 losses estimated for collapsing terrestrial peat soils in Indonesia [11], similar to that which can be estimated from peat collapse (losses in elevation) after hurricane damage in mangroves in Honduras [21], and greater than estimates of CO2 emissions with permafrost thaw and decomposition of tundra peat [24]. In contrast, intact mangrove forests absorb approximately 5000 tonnes CO2 km−2 year−1 of which only ∼20% is respired as CO2 [14], [15]. Carbon export from mangroves to adjacent systems (which could be up to 70% of total production) may potentially contribute to CO2 emissions, but also support secondary production [14], [15]. Clearing mangroves from peat soils will clearly be unfavourable for regional and global carbon budgets [6] as well as reducing other ecosystem services offered by mangroves [5].
While CO2 efflux from intact forest soils is strongly associated with root respiration [25], CO2 efflux from cleared and disturbed mangrove soils reflects microbial degradation of organic matter within soils [15]. The large, but transient increase in CO2 efflux with disturbance of the peat (Figure 2) probably reflects oxidation of relative labile fractions (e.g. sugars and phenols) as they are exposed to enhanced oxygen concentrations [26]. However, this fraction is rapidly depleted before relatively slower decomposition of refractory pools (e.g. lignin) dominates CO2 efflux. Short term high levels of CO2 efflux from soil directly after clearing (Figure 1, 8 months) or from disturbing the peat are not included in our annual estimate of CO2 emissions but may contribute a significant proportion to total emissions.
Once cleared, mangroves are often converted to shrimp ponds [1], [2]. Rates of CO2 emissions from cleared mangroves are within the same range as those measured from shrimp ponds [27]. Thus, once established this alternative land-use, unlike conversion to agriculture [28] does not mitigate CO2 emissions from clearing mangroves. Additionally, aquaculture and agriculture often increase nutrient availability of coastal waters [29]. Mangrove peat collapse has been observed to be enhanced by addition of nitrogen due to increases in decomposition and compaction [18]. Thus, increasing levels of nutrients in cleared mangrove areas may contribute to loss of habitat and possibly to increased CO2 emissions associated with decomposition of peat.
Approximately half of Caribbean mangrove forests are anticipated to be growing on carbon rich peat soils [30]. The proportion of mangrove forests on peat soils is not known for the Indo-Pacific region and Africa, but could be substantial, particularly if mangrove peat is associated with upland peat forest soils which are common in the Indo-Pacific region [31]. The documentation of acid sulphate soils in shrimp farm developments from South East Asia and elsewhere [32] also indicates the presence of high concentrations of organic matter in many mangrove soils that have already been cleared for aquaculture. CO2 emissions from cleared mangroves growing on mineral soils have not been assessed, but are needed in conjunction with improved soil mapping of soil carbon stocks within mangrove forest soils in order to estimate the global effects of clearing mangroves on CO2 emissions.
Our annual estimate of CO2 emissions of 2900 tonnes km−2 year−1 may be improved through measurement of CO2 efflux over seasons which vary in tidal height, temperature and rainfall, however the timing of our measurements have probably lead to a underestimate of CO2 emissions. Our measurements were made in the winter months in Belize when temperatures are relatively low and may limit bacterial activity. Although tidal variation is low in Belize (∼0.5 m) in the winter months tides are higher than in summer [33] and thus we may have underestimated CO2 flux compared to periods when tides are lower and peat maybe exposed to air at greater depth in the soil. Increases in sea level may also influence CO2 emissions from cleared forest soils, changing oxidation status and potentially altering decomposition processes [26].
We conclude that the clearing of mangroves and the use of mangrove peat soils for alternative uses (e.g. cleared, shrimp ponds) results in increases in CO2 emissions, in addition to resulting in losses in other ecosystem functions including fisheries and coastal protection. Incentive payments for maintaining intact forests, thus avoiding carbon emissions, as proposed by REDD and REDD+ [9], would be beneficial for conservation of mangroves in the tropics. There are significant gaps in our knowledge in: 1) the global extent of carbon currently stored in peat and mineral soils in mangrove forests, 2) the rate of CO2 emissions from clearing mangroves growing on mineral soils, 3) the spatial and temporal variation in CO2 emissions from cleared mangrove forests and alternative land-uses, and 4) the loss of carbon as dissolved organic and inorganic forms of carbon from intact and disturbed forest systems [12], [14], [15]. Filling these knowledge gaps will improve arguments for conservation of mangroves based on carbon stocks and sequestration.
Materials and Methods
Study site
This study was conducted at Twin Cays, a peat-based, 92-ha archipelago of intertidal mangrove islands in a carbonate setting, just inside the crest of the Mesoamerican Barrier Reef System of central Belize, 12 km off shore (16°50′N, 88°06′W). These islands receive no terrigenous inputs of freshwater or sediments. Mangrove islands in this part of the reef, which originated approximately 8000 yr B.P. on a limestone base formed by a Pleistocene patch reef, have an underlying peat deposit ∼7–10 m thick and have been mangrove communities throughout the Holocene [18]. Mangrove forests are dominated by Rhizophora mangle the roots of which the peat is derived [18]. Since 1980, this group of islands has been the primary study site for the Smithsonian Institution's National Museum of Natural History Field Station on nearby Carrie Bow Cay [34].
Illegal clearing of mangroves has occurred on Twin Cays over the last 20 years, primarily for housing and prospective tourism developments. Multiple clearing events allowed us to measure CO2 efflux over soils that have been cleared of vegetation over 20 years. We measured CO2 efflux from 3–5 ha patches that had been cleared for durations of 8 months, 12 months, 4 years, 11 years and 20 years. We measured efflux at 6–12 locations within each aged clearing.
In order to test whether disturbance of the peat increased soil CO2 efflux we cut blocks of peat from the area that had been cleared 8 months previously. Six replicate blocks approximately 30×30×30 cm were cut with a shovel and placed on the soil surface. We measured CO2 efflux from the soil, from the peat blocks directly after cutting them from the peat and then again after 2 days.
CO2 efflux from soils was measured using a LiCor 6400 portable photosynthesis system configured with the LiCor Soil Respiration chamber (LiCor Corp, Lincoln, NE, USA). The chamber was set to penetrate 5 mm into the soil. Settings for measurement were determined at each site following the procedure described by the manufacturer. Soil temperature was measured at 2 cm depth simultaneously with CO2 efflux. Soil temperatures varied from 28 to 34 C during the measurements. Measurements were made in February of 2004 and January of 2007.
Data analysis
Differences in soil CO2 flux over time among areas of differing time since clearing were assessed using linear models where time was considered a random, continuous variable in the model. Changes in CO2 efflux with disturbance of peat was assessed using repeated measures ANOVA. Scaling instantaneous CO2 efflux data was done by simply multiplying CO2 efflux (µmol m−2 s−1) by time to give tonnes CO2 km−2 year−1.
Acknowledgments
We thank the Smithsonian Marine Science Network and the staff of the Carrie Bow Cay Research Station. We thank Dr Stephen Crooks, Dr Emily Pidgeon and other members of the Blue Carbon Working Group and acknowledge the support of the South East Queensland Climate Adaptation Research Initiative.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Smithsonian Marine Science Network and a National Science Foundation Biocomplexity award (DEB-9981535). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Valiela I, Bowen JL, York JK. Mangrove forests: one of the world's threatened major tropical environments. Bioscience. 2001;51:807–815. [Google Scholar]
- 2.Alongi DM. Present state and future of the world's mangrove forests. Environ Conserv. 2002;29:331–349. [Google Scholar]
- 3.Granek EF, Ruttenberg BI. Changes in biotic and abiotic processes following mangrove clearing. Estuar Coastal Shelf Sci. 2008;80:555–562. [Google Scholar]
- 4.Aburto-Oropeza O, Ezcurra E, Danemann G, Valdez V, Murray J, et al. Mangroves in the Gulf of California increase fishery yields. Proc Nat Acad Sci USA. 2008;105:10456–10459. doi: 10.1073/pnas.0804601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbier EB, Koch EW, Silliman B. Coastal ecosystem-based management with nonlinear ecological functions and values. Science. 2008;319:321–323. doi: 10.1126/science.1150349. [DOI] [PubMed] [Google Scholar]
- 6.Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, et al. Mangroves among the most carbon-rich tropical forests and key in land-use carbon emissions. Nature Geosci. 2011;4:293–297. [Google Scholar]
- 7.Houghton RA. Land-use change and the carbon cycle. Glob Chan Biol. 1995;1:275–287. [Google Scholar]
- 8.Kauffman JB, Hughes RF, Heider C. Dynamics of carbon and nutrient pools associated with land conversion and abandonment in Neotropical landscapes. Ecol Appl. 2009;19:1211–1222. doi: 10.1890/08-1696.1. [DOI] [PubMed] [Google Scholar]
- 9.Anglesen A. Bogor, Indonesia: Center for International Forestry Research; 2009. Realising REDD+: National Strategy and Policy Options.362 [Google Scholar]
- 10.Muradian R, Kumar P. Payment for ecosystem services and valuation: challenges and research gaps. In: Kumar P, Muradian R, editors. Payment for Ecosystem Services. New Delhi: Oxford University Press; 2009. pp. 1–16. [Google Scholar]
- 11.Couwenberg J, Dommain R, Joosten H. Greenhouse gas fluxes from tropical peatlands in south-east Asia. Glob Chan Biol. 2010;16:1715–1732. [Google Scholar]
- 12.Alongi DM. Carbon payments for mangrove conservation: ecosystem constraints and uncertainties in sequestration potential. Environ Sci Pol. 2011;14:462–470. [Google Scholar]
- 13.McCleod E, Chmura GL, Bouillon S, Salm R, Bjork M, et al. Front Ecol Environ; 2011. A Blueprint for Blue Carbon: Towards an improved understanding of the role of vegetated coastal habitats in sequestering CO2. in press. [Google Scholar]
- 14.Alongi DM. Dordrecht, The Netherlands: Springer; 2009. The Energetics of Mangrove Forests.216 [Google Scholar]
- 15.Bouillon S, Borges AV, Castañeda-Moya E, Diele K, Dittmar T, et al. Mangrove production and carbon sinks: a revision of global budget estimates. Glob Biogeochem Cycl. 2008;22 doi: 10.1029/2007GB003052. [Google Scholar]
- 16.Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC. Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands. 2011;31:343–352. [Google Scholar]
- 17.Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC. Global carbon sequestration in tidal, saline wetland soils. Glob Biogeochem Cycl. 2003;17:1111–1120. [Google Scholar]
- 18.McKee KL, Feller IC, Cahoon DR. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob Ecol Biogeogr. 2007;16:545–556. [Google Scholar]
- 19.DeLaune RD, Nyman JA, Patrick WH. Peat collapse, ponding and wetland loss in a rapidly submerging coastal marsh. J Coast Res. 1994;10:1021–1030. [Google Scholar]
- 20.Crow SE, Wieder K. Sources of CO2 emission from a northern peatland: root respiration, exudation, and decomposition. Ecology. 2005;86:1825–1834. [Google Scholar]
- 21.Cahoon DR, Hensel P, Rybczyk J, McKee KL, Proffitt CE, et al. Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after Hurricane Mitch. J Ecol. 2003;91:1093–1105. [Google Scholar]
- 22.Emmett-Mattox S, Crooks S, Findsen J. Wetland grasses and gases: Are tidal wetlands ready for the carbon markets? Nat Wetl Newslet. 2010;32:6–10. [Google Scholar]
- 23.McKee KL, Feller IC, Popp M, Wanek W. Mangrove isotopic fractionation (d15N and d13C) across a nitrogen versus phosphorus limitation gradient. Ecology. 2002;83:1065–1075. [Google Scholar]
- 24.Schuur EAG, Vogel JG, Crummer KG, Lee H, Sickman JO, et al. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature. 2009;459:556–559. doi: 10.1038/nature08031. [DOI] [PubMed] [Google Scholar]
- 25.Lovelock CE. Soil respiration in tropical and subtropical mangrove forests. Ecosystems. 2008;11:342–354. [Google Scholar]
- 26.Lallier-Vergès E, Marchand C, Disnar J-R, Lottier N. Origin and diagenesis of lignin and carbohydrates in mangrove sediments of Guadeloupe (French West Indies): Evidence for a two-step evolution of organic deposits. Chem Geol. 2008;255:388–398. [Google Scholar]
- 27.Burford M, Longmore High ammonium production from sediments in hypereutrophic shrimp ponds. Mar Ecol Prog Ser. 2001;224:187–195. [Google Scholar]
- 28.Chimner RA, Ewel KC. Differences in carbon fluxes between forested and cultivated micronesian tropical peatlands. Wetl Ecol Manag. 2004;12:419–427. [Google Scholar]
- 29.Burford M, Costanzo SD, Dennison WC, Jackson CJ, Jones AB, et al. A synthesis of dominant ecological processes in intensive shrimp ponds and adjacent coastal environments in NE Australia. Mar Poll Bull. 2003;46:1456–1469. doi: 10.1016/S0025-326X(03)00282-0. [DOI] [PubMed] [Google Scholar]
- 30.Ellison AM, Farnsworth EJ. Anthropogenic disturbance of Caribbean mangrove ecosystems: past impacts, present trends, and future predictions. Biotropica. 1996;28:549–565. [Google Scholar]
- 31.Ewel KC. Appreciating tropical coastal wetlands from a landscape perspective. Front Ecol Environ. 2010;8:20–26. [Google Scholar]
- 32.Boyd CE. Shrimp pond bottom soil and sediment management. In: Wyban J, editor. Proceedings of the Special Session on Shrimp Farming. Baton Rouge, LA: World Aquaculture Society; 1992. pp. 166–181. [Google Scholar]
- 33.Lee RY, Porubsky WP, Feller IC, McKee KL, Joye SB. Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (Twin Cays, Belize). Biogeochemistry. 2008;87:181–198. [Google Scholar]
- 34.Rützler K, Feller IC. Caribbean mangrove swamps. Sci Am. 1996;274:94–99. [Google Scholar]