Abstract
CodY, a highly conserved protein in the low G + C, gram-positive bacteria, regulates the expression of many Bacillus subtilis genes that are induced as cells make the transition from rapid exponential growth to stationary phase and sporulation. This transition has been associated with a transient drop in the intracellular pool of GTP. Many stationary-phase genes are also induced during exponential-growth phase by treatment of cells with decoyinine, a GMP synthetase inhibitor. The effect of decoyinine on an early-stationary-phase gene is shown here to be mediated through CodY and to reflect a reduction in guanine nucleotide accumulation. CodY proved to bind GTP in vitro. Moreover, CodY-mediated repression of target promoters was dependent on a high concentration of GTP, comparable to that found in rapidly growing exponential-phase cells. Because a codY-null mutant was able to sporulate under conditions of nutrient excess, CodY also appears to be a critical factor that normally prevents sporulation under such conditions. Thus, B. subtilis CodY is a novel GTP-binding protein that senses the intracellular GTP concentration as an indicator of nutritional conditions and regulates the transcription of early-stationary-phase and sporulation genes, allowing the cell to adapt to nutrient limitation.
Keywords: CodY, GTP, decoyinine, sporulation, amino acid repression
Our understanding of the relationship between environmental signals and global changes in gene expression is limited by the difficulty in identifying intracellular signaling molecules that interact with key regulatory proteins. This gap is particularly apparent for cases of general nutrient limitation. When Bacillus subtilis cells encounter nutrient limitation and enter stationary phase, a variety of adaptive processes—such as genetic competence, secretion of macromolecule-degrading enzymes, import of secondary nutrients, activation of metabolic pathways, chemotaxis and motility, production of antibiotics, and sporulation—are initiated (Sonenshein 1989). A network of global regulatory proteins modulates the cell's response and regulates the choice between adaptation to poor growth conditions and sporulation (Sonenshein 1989, 2000; Burkholder and Grossman 2000), but the specific signals to which these regulators respond have remained a mystery.
Many B. subtilis genes that are expressed early in stationary phase are repressed by CodY (Table 1). Preliminary results indicate that CodY also contributes to regulation of at least two genes (citB, spo0A) whose products are necessary for sporulation (M. Ratnayake-Lecamwasam and A.L. Sonenshein, unpubl.). Genome sequencing has revealed CodY homologs in Bacillus anthracis, Bacillus halodurans, Bacillus stearothermophilus, Clostridium acetobutylicum, Clostridium difficile, Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, Streptococcus pneumoniae, Streptococcus pyogenes, and Lactococcus lactis (www.ncbi.nlm.nih.gov/Microb_blast/unfinishedgenome.html; Bolotin et al. 1999).
Table 1.
Promoters regulated by CodY
| Target promoters
|
Function of gene product
|
Induced by decoyinine
|
References
|
|---|---|---|---|
| Transport | |||
| dpp | dipeptide transport | + | Mathiopoulos et al. 1991; Slack et al. 1995 |
| gabP | γ-aminobutyrate transport | ND | Ferson et al. 1996 |
| Metabolism | |||
| hut | histidine degradation | ND | Slack et al. 1995; Fisher et al. 1996 |
| bkd | branched chain aa degradation | ND | Debarbouille et al. 1999 |
| citB | aconitase | + | See below |
| ure | urea degradation | ND | Wray et al. 1997 |
| Chemotaxis/motility | |||
| hag | flagellin | ND | Mirel et al. 2000 |
| Competence/antibiotics | |||
| srfA | surfactin synthesis | + | Serror and Sonenshein 1996b; Lazazzera et al. 1999 |
| comK | genetic competition | ND | Serror and Sonenshein 1996b |
| Regulation | |||
| codVWXY | ND | Ratnayake-Lecamwasam and Sonenshein, unpubl. | |
| rapA | Spo0F∼P phosphatase | + | Mueller et al. 1992; Lazazzera et al. 1999 |
| rapC | ComA∼P phosphatase | ND | Lazazzera et al. 1999 |
| Sporulation | |||
| citB | aconitase | + | Dingman et al. 1987; Ratnayake-Lecamwasam and Sonenshein, unpubl. |
| spo0A (sporulation promoter) | transcription factor | ND | Ratnayake-Lecamwasam and Sonenshein, unpubl. |
CodY was first identified as a repressor of the B. subtilis dipeptide transport (dpp) operon and was found to be active when cells are grown with an excess of glucose or Casamino acids (CAA) as reported by Slack et al. (1995). During vegetative growth, the dpp operon is also directly repressed by AbrB, a second global regulator of early-stationary-phase genes (Slack et al. 1991; Strauch 1993; Serror and Sonenshein 1996b), but the repressive effects of nutrient excess are mediated through CodY, not AbrB (Slack et al. 1993, 1995). CodY also mediates amino acid repression of the B. subtilis hut, srfA, ureABC, and hag operons and the comK and gabP genes (Ferson et al. 1996; Fisher et al. 1996; Serror and Sonenshein 1996b; Wray et al. 1997; Mirel et al. 2000) and the L. lactis opp-pepO1, pepN, and pepC genes (Guédon et al. 2001) In this work, we sought to identify the signal that indicates nutrient excess or limitation and thereby regulates CodY activity.
Although CodY might sense the intracellular concentration of one or more amino acids, its activity might also be regulated, as Wray et al. (1997) have suggested, by the decrease in growth rate that is observed when B. subtilis cells encounter nutrient-limiting conditions. When bacterial growth rate drops, due to carbon source or amino acid limitation, the stringent response is activated, leading to conversion of GTP to pppGpp and ppGpp (Cashel et al. 1996), and biosynthesis is attenuated. Thus, accumulation of (p)ppGpp or a decrease in GTP might be the signal that inactivates CodY and derepresses CodY-regulated genes.
It is, in fact, well-established that B. subtilis cells, as they enter stationary phase, undergo a transient decrease in their GDP and GTP pools (Mitani et al. 1977; Freese et al. 1979a; Lopez et al. 1979), which may be due either to conversion of GTP to (p)ppGpp or to the depletion of precursors necessary for guanine nucleotide synthesis (Mitani et al. 1977; Freese et al. 1979a; Lopez et al. 1979; Ochi et al. 1981; Mathiopoulos et al. 1991; Sonenshein 2000). A forced reduction in guanine nucleotide pools by treatment of cells with decoyinine, an inhibitor of GMP synthetase (Lopez et al. 1979), also induces stationary phase genes (Table 1).
In this study, we show that the decoyinine-dependent derepression of dpp is mediated through CodY. We further show that CodY is a GTP-binding protein and that CodY-mediated repression of the dpp promoter is dependent in vitro on a high concentration of GTP, comparable to that found in rapidly growing exponential-phase cells. Our results also indicate that CodY is a critical factor that prevents the initiation of sporulation under conditions of nutrient excess.
Results
CodY concentration during exponential-growth and stationary phase
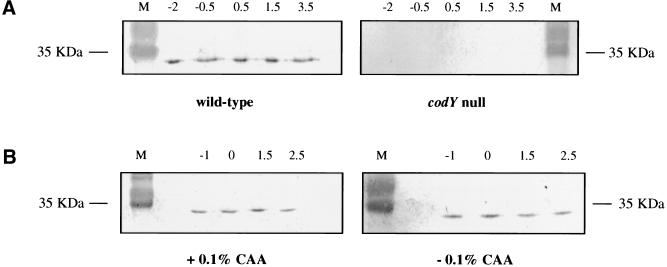
In B. subtilis cells grown in nutrient broth sporulation (DS) medium, CodY-regulated genes are repressed during exponential-growth phase and induced at the entry into stationary phase. To determine whether expression of these genes correlates with the presence or absence of CodY protein, cultures of wild-type (PS29) and ΔcodY (PS37) mutant strains in DS medium were sampled at various times during growth. Proteins released by sonication were analyzed by Western blotting, using a serum polyclonal antibody to CodY. Figure 1A indicates that during both exponential-growth and stationary phase, the CodY concentration of the wild-type B. subtilis strain remained about the same. Even 8 h after the onset of stationary phase, the CodY concentration was similar to that in exponential-phase cells (results not shown). The codY-null strain, as expected, did not contain any protein that reacted with the antibody. When the wild-type strain was grown in S7, a defined medium containing 0.5% glucose, the concentration of CodY again did not change significantly, irrespective of the presence or absence of 0.1% CAA (Fig. 1B). In S7 medium, the presence of glucose and CAA strongly inhibits expression of CodY-regulated genes (Serror and Sonenshein 1996b). These results indicate that the presence of CodY is not affected by the growth phase or by the composition of the medium, that is, by conditions that affect expression of CodY-regulated genes.
Figure 1.
CodY concentration during exponential growth and stationary phase. Samples were taken at the indicated time points (in h) before and after entry into stationary phase (T0) and proteins of each crude lysate were separated by SDS-PAGE. The proteins were electrotransferred and immunoblotted with a polyclonal CodY antibody. Lane M contains prestained molecular weight markers (GIBCO-BRL). (A) Immunoblots of lysates from wild-type and ΔcodY strains grown in DS medium. (B) Immunoblots of lysates from a wild-type strain grown in S6 medium in the presence (S6C) or absence (S6) of 0.1% CAA.
The target of decoyinine
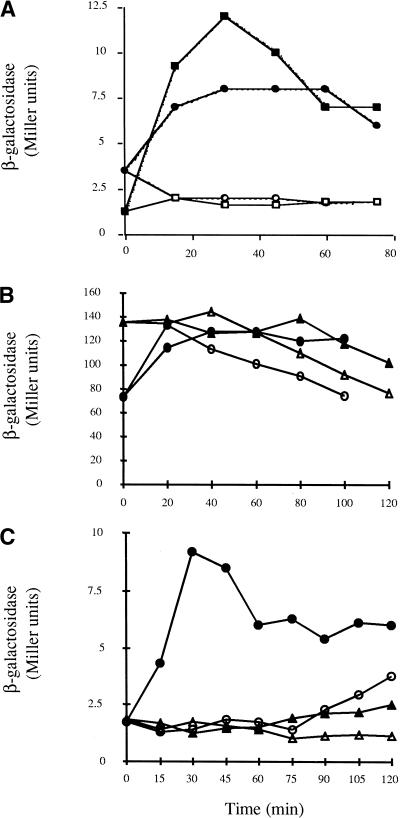
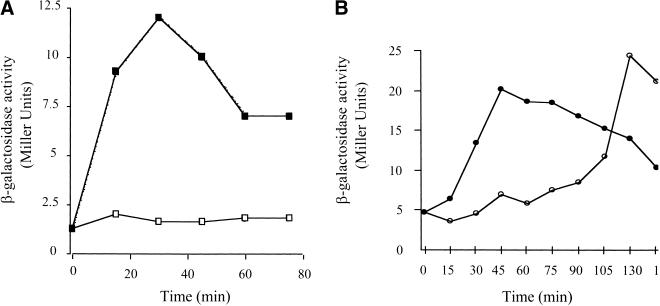
Mathiopoulos et al. (1991) found that dpp expression in B. subtilis cells growing rapidly under conditions of nutrient excess is derepressed ∼8 min after the addition of decoyinine, a GMP synthetase inhibitor. Two proteins, AbrB and CodY, are known to regulate dpp expression (Slack et al. 1995). To see which, if either, of these proteins is the target of decoyinine, dpp expression was studied in decoyinine-treated wild-type, abrB, ΔcodY, and abrB ΔcodY strains. The addition of decoyinine to strains PS59 (WT) and PS56 (abrB) had the same effect; dpp expression was derepressed rapidly (Fig. 2A). However, in PS164 (ΔcodY) cells, in which dpp expression is partially derepressed during exponential phase, no additional derepression of dpp expression was observed after the addition of decoyinine (Fig. 2B). Decoyinine also had no added effect on dpp expression in PS83 (abrB ΔcodY) cells in which dpp expression is derepressed to the highest level seen under any condition. These results show that the decoyinine effect on dpp expression is not mediated through AbrB or another unidentified regulator of dpp and imply strongly that CodY is the direct or indirect target of decoyinine.
Figure 2.
The effect of decoyinine on dpp-lacZ directed β-galactosidase expression in cells grown in S6C medium. When the cultures reached a turbidity at 600 nm of 0.3 to 0.4 (T0 for this experiment), the cultures were split into two 25-ml cultures and 0.125 ml of either 1N KOH (open symbols) or decoyinine (100 mg/mL in 1N KOH) was added (closed symbols). (A) Strains PS59 (abrB+ codY+), squares; PS56 (abrB codY+), circles. (B) Strains PS164 (abrB+ codY), circles; PS83 (abrB codY), triangles. (C) Strain PS59 was grown in the presence or absence of 1 mM guanosine. Triangles, β-galactosidase activity in the presence of guanosine; circles, in the absence of guanosine. The experiments were performed in duplicate and any variations in data points were <10% of the represented values.
B. subtilis cells can take up exogenous guanosine from the culture medium and convert it via the salvage pathway to GMP, GDP, and GTP (Nygaard 1993), bypassing the metabolic block caused by decoyinine. When B. subtilis wild-type strain PS59 was grown in S6C medium supplemented with 1 mM guanosine, the addition of decoyinine failed to induce dpp expression (Fig. 2C). Thus, dpp expression and, by extension, CodY respond directly or indirectly to guanine nucleotide levels.
Effect of decoyinine on dpp expression in a B. subtilis relA strain
The relA gene encodes the only (p)ppGpp synthetase in B. subtilis (Lopez et al. 1981; Wendrich and Marahiel 1997). Partial amino acid limitation, leading to an increase in (p)ppGpp and a reduction in GTP levels, induces spore formation in relA+ cells but not in relA-mutant cells (Lopez et al. 1981; Ochi et al. 1981). Although a relA-mutant strain sporulates poorly (Lopez et al. 1981; Eymann et al. 2001), such a strain can be made to sporulate by adding decoyinine (Ochi et al. 1981). We have found that activation of the stringent response also induces the dpp operon (Ratnayake-Lecamwasam 2001).
To see if derepression of CodY-regulated genes is dependent on synthesis of (p)ppGpp under all conditions, decoyinine was added to rapidly growing cells of a B. subtilis strain carrying a relA mutation. In wild-type cells, dpp expression reached its maximal level 30 min after the addition of decoyinine (Fig. 3A). In relA cells, dpp expression was derepressed to the same extent within 45 min after decoyinine addition (Fig. 3B). In control cells that had not been treated with decoyinine, dpp expression increased at the point of transition from exponential-growth to stationary phase even in relA mutant cells (Fig. 3B). Thus, the stringent response is not essential for decoyinine-mediated and stationary-phase-dependent derepression of dpp expression.
Figure 3.
Effect of a relA mutation on induction of dpp-lacZ by decoyinine. When cultures in S6C medium reached a turbidity at 600 nm of 0.3 to 0.4 (T0), the cultures were split into two 25-ml cultures and 0.125 ml of either 1N KOH (open symbols) or decoyinine (100 mg/mL in 1N KOH; closed symbols) was added. (A) Strain PS59 (relA +); (B) Strain MRLB10 (relA). The experiments were performed in duplicate and any variations in data points were <15% of the represented values.
Effect of decoyinine on sporulation of wild-type and ΔcodY strains
Wild-type B. subtilis cells sporulate very poorly in S6 medium supplemented with 0.1% CAA and 0.5% glucose (S6C) but sporulate quite well in this medium when decoyinine is added (Freese et al. 1979b). As Table 2 indicates, treatment with decoyinine increased spore production of PS59 (wild-type) cells by nearly 200-fold. Interestingly, the PS164 (ΔcodY) strain formed 400 times more spores than did PS59 in S6C medium. The addition of decoyinine to PS164 cells led to a slight decrease in sporulation. Because the sporulation defect of B. subtilis cells grown in S6C medium is overcome by a mutation in codY or by a decrease in guanine nucleotide synthesis, CodY appears to be an important guanine nucleotide sensor or a component of a sensory pathway for regulation of sporulation in this medium.
Table 2.
Effect of a ΔcodY mutation on sporulation under nutrient excess conditions
| Strain
|
Decoyinine addition
|
Viable cells* (cfu/mL)
|
Spores* (cfu/mL)
|
(Spores/viable cells) ×100 (%)
|
|---|---|---|---|---|
| Wild-type | − | 8.75 × 107 | 1.5 × 105 | 0.15 |
| Wild-type | + | 8.25 × 107 | 2.6 × 107 | 31 |
| ΔcodY | − | 7.40 × 107 | 6.7 × 107 | 91 |
| ΔcodY | + | 6.50 × 107 | 3.7 × 107 | 58 |
Bacillus subtilis wild-type (PS59) and ΔcodY (PS164) strains were grown in S6C medium. When the cultures reached a turbidity at 600 nm of 0.3 to 0.4, they were split. One subculture received decoyinine dissolved in KOH, and the other KOH only (see Materials and Methods). After further incubation for ∼20 h, a sample of each culture was serially diluted; each dilution was divided in half, and one half was incubated at 80°C for 10 min. The heat-treated and unheated cells were plated on DS medium and the mean values for viable cells and spores were calculated.
The values in each column are the average of two independent experiments.
GTP-binding motifs in CodY
Small GTPases have conserved G1 (A/GX4GKT/S), G3 (DX2G), and G4 (T/NKXD) GTP-binding motifs. A putative G1 motif (GGERLGTL) was found in CodY between amino acids 119 and 126, a putative G3 motif (DRVG) between amino acids 208 and 211, and a putative G4 motif (NKFL) between amino acids 247 and 250. Unlike the G1 consensus sequence, the CodY G1-like motif does not contain a lysine residue at the seventh position. The CodY G3 overlaps the HTH domain of CodY, spanning the last three amino acids of helix I (Asp, Arg, and Val) and the first amino acid of the turn (Gly). The CodY G4 motif varies from the consensus at the fourth position, where in CodY, leucine is substituted for aspartate. These putative GTP-binding motifs are also present in CodY homologs identified by sequence analysis in other gram-positive bacteria (Table 3).
Table 3.
Comparison of putative GTP binding motifs in CodY homologs
| Small GTPases Consensus sequences | G1 GXXXXGXT | G3 DXXG | G4 NKXD |
|---|---|---|---|
| A S | TQ | ||
| FtsZ | |||
| E. coli | LGGGTGTG | DAFG | TSLD |
| CodY | |||
| B. subtilis | GGERLGTL | DRVG | NKFL |
| B. stearothermophilus | GGERLGTL | DRVG | DKFL |
| B. halodurans | GGQRLGTL | DRVG | DKFL |
| B. anthracis | GGERLGTL | NAa | NAa |
| C. difficile | GGMRLGSL | DRIG | NEGI |
| C. acetobutylicum | NRERLGTL | DRVG | ILND |
| S. pneumoniae | SGIRLGSL | DRIG | LISD |
| E. faecalis | AGKRLGTI | DRVG | NQQF |
| S. mutans | GGMRLGSL | DRIG | NEGI |
| S. aureus | GGERLGTL | DRIG | EKGI |
| S. pyogenes | GGMRLGSL | DRIG | NEGI |
| L. lactis | SGMRLGTF | DKIG | TGLF |
CodY sequences of B. stearothermophilus, B. halodurans, B. anthracis, C. difficile, C. acetobutylicum, S. pneumoniae, E. faecalis, S. mutans, S. aureus, and S. pyogenes were obtained from http://www.ncbi.nlm.nih.gov/Microb_blast/unfinishedgenome.html, and compared using the BLAST program (Altschuhl et al. 1997). The E. coli FtsZ motifs were from RayChaudhuri and Park (1992), the B. subtilis CodY sequence was from Slack et al. (1995), and L. lactis CodY was from Bolotin et al. (1999). The consensus sequences for G1, G3, and G4 motifs were from Bourne et al. (1991).
Not available.
Binding of purine nucleotides to CodY
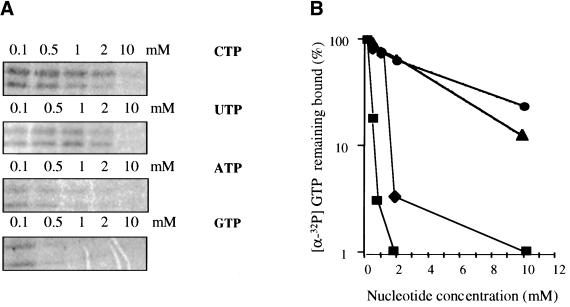
To determine if CodY interacts directly with GTP, a mixture of CodY (overexpressed in and purified from E. coli; Serror and Sonenshein 1996a) and [α-32P] GTP was tested for UV-induced cross-linking (Fig. 4A). Two radioactive bands were seen; the larger was a full-length CodY protein (∼30 kD) and the smaller (20–25 kD) was a truncated form of CodY (as identified by immunoblotting with the CodY antibody) that most likely resulted from degradation during purification. CodY-His6 (see Materials and Methods) purified from B. subtilis could also be cross-linked to [α-32P] GTP (data not shown). The formation of the cross-linked, radioactive GTP-CodY complexes was inhibited when excess unlabelled GTP was present (Fig. 4). We found that CodY-His6 could also be cross-linked to [α-32P] ATP (data not shown). These results show that CodY interacts directly with purine nucleotides.
Figure 4.
Ultraviolet-induced crosslinking of [α-32P] GTP to CodY. (A) CodY (3 μM), overexpressed and purified from Escherichia coli BL21/λDE3, was incubated with 0.1 mM [α-32P] GTP and 0.1, 0.5, 1, 2, and 10 mM unlabelled GTP, ATP, CTP, or UTP and irradiated with UV light. Irradiated samples were analyzed by SDS-PAGE (12% polyacrylamide) and exposed to a phosphorimager screen. (B) Quantitation of results in part (A). Unlabelled GTP, closed squares; unlabelled ATP, closed diamonds; unlabelled CTP, closed circles; unlabelled UTP, closed triangles.
To test the specificity of GTP binding to CodY, we performed UV-mediated cross-linking experiments with 0.1 mM [α-32P] GTP in the presence of unlabelled GTP, ATP, CTP, or UTP. As expected, 0.5 mM unlabelled GTP reduced the [α-32P] GTP bound to CodY by 85% (Fig. 4B). Competition experiments with other unlabelled nucleoside triphosphates showed that CodY binds more tightly to GTP than to the other NTPs but that the affinity for ATP is higher than that observed for pyrimidine nucleotides.
Effect of GTP on repression by CodY
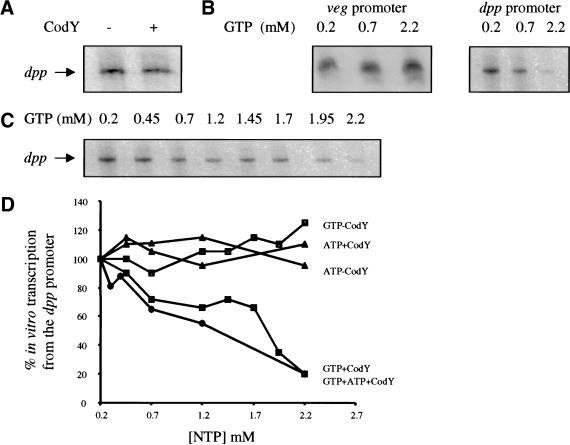
Gel mobility-shift and DNase I footprinting assays showed that CodY binds to the dpp, srfA, and comK promoters in the absence of added GTP (Serror and Sonenshein 1996a,b). When GTP was added to 2 mM, the apparent affinity of CodY for the dpp and srfA promoter regions increased only three- to fourfold (data not shown). To test whether CodY represses transcription directly and whether GTP has a greater effect on repression than on DNA binding, we performed in vitro transcription experiments, initiating at the dpp promoter as described in Materials and Methods. Figure 5 shows that a transcript of the expected size (155 nt) was obtained in the absence of CodY (panel A, lane 1) and that the addition of CodY-His6 had little or no effect on the production of this transcript (panel A, lane 2). Unmodified CodY purified after overexpression in E. coli also failed to repress dpp transcription in vitro (data not shown). Thus, CodY is either not a direct repressor of dpp or requires an effector in order to repress transcription.
Figure 5.
The effects of GTP and ATP on CodY-mediated repression. In vitro transcription reactions were performed with dpp promoter- or veg promoter-containing fragments as the template. The reactions were initiated by adding Bacillus subtilis EςA RNA polymerase (see Materials and Methods). The dpp transcript is 155 n; the veg transcript is 104 n. (A) The dpp promoter fragment (80 nM) was incubated with RNA polymerase (37 nM); CodY-His6 (300 nM) was added as indicated. (B) The veg or dpp promoter fragments (40 nM) were incubated with RNA polymerase (19 nM) and CodY-His6 (180 nM) in the presence of varying concentrations of GTP. (C) Transcription from the dpp promoter (80 nM) by RNA polymerase (37 nM) in the presence of CodY-His6 (300 nM) and varying concentrations of GTP. (D) Quantitation of in vitro transcription results. The data from part (C) and from parallel experiments in which the concentrations of ATP or ATP and GTP were varied were analyzed using the ImageQuant program.
To test whether GTP is the co-repressor for CodY, we performed in vitro transcription reactions with the dpp promoter in the presence of purified CodY-His6 and GTP at concentrations ranging from 0.2 mM to 2.2 mM. As shown in Figure 5 (panels C,D) dpp transcription was inhibited in reactions that contained CodY and a high concentration of GTP. At 2.2 mM GTP, residual dpp transcription was 20% (Fig. 5D). In reactions that did not contain CodY, dpp transcription was not inhibited at any concentration of GTP tested (Fig. 5D). Thus, CodY represses transcription from the dpp promoter but only when GTP is present at high concentration. The intracellular concentration of GTP in rapidly growing B. subtilis cells has been estimated at 1–3 mM (Lopez et al. 1979; Neidhardt et al. 1990).
Because we found that CodY binds ATP with an affinity similar to that for GTP, we tested whether CodY could repress dpp transcription in the presence of ATP. Even at 2.2 mM ATP, no inhibition of dpp transcription was seen (Fig. 5D). CTP (3.2 mM) was also ineffective as a co-repressor for CodY (data not shown). Additionally, we have shown that ATP (2.2 mM) does not interfere with the effect of GTP (2.2 mM) on CodY-mediated repression of dpp transcription (Fig. 5D). To investigate the specificity of GTP-bound CodY, we studied the in vitro transcription of the veg promoter in the presence of GTP and CodY-His6 (Fig. 5B). The veg promoter is strongly transcribed during vegetative growth (Le Grice et al. 1986) and is not a known target of CodY. Transcription of the veg promoter was not inhibited by CodY, even in the presence of 2.2 mM GTP.
Discussion
Our results suggest a mechanism for the effect of nutrient limitation on the expression of stationary-phase and early-sporulation genes in B. subtilis. It appears that CodY bound to GTP represses many stationary-phase and some early-sporulation genes during growth in excess nutrients. The intracellular concentration of GTP is 1–3 mM in exponential-phase cells grown in rich medium (Lopez et al. 1979; Neidhardt et al. 1990). When the GTP concentration is within this range during in vitro transcription, CodY represses the dpp promoter. Under slow-growth conditions or as stationary phase approaches, the GTP level decreases, due to (p)ppGpp production or a reduction in GTP synthesis or both. As a result, CodY is no longer bound to GTP and can no longer repress transcription. Lopez et al. (1979) found that the pool of GTP drops by 70%–80% at the entry into stationary phase. If our in vitro experiments are a faithful reflection of in vivo conditions, such a drop would lead to substantial derepression of CodY-regulated genes. Although GTP activates CodY as a repressor, at least 30 other metabolites tested for stimulation or inhibition of CodY binding and repression in vitro were ineffective (P. Serror and A.L. Sonenshein, unpubl.). The strong conservation of CodY in other gram-positive bacteria suggests that it may have similar functions in these other organisms as well.
Expression of dpp is derepressed in a relA-dependent manner when the stringent response is activated (Ratnayake-Lecamwasam 2001) but can also be derepressed in a relA-independent manner by the addition of decoyinine, a GMP synthetase inhibitor. The stringent response appears to participate in inactivation of CodY in two indirect ways. First, synthesis of (p)ppGpp is at the expense of GTP. Second, Freese et al. (1979a) showed that (p)ppGpp inhibits IMP dehydrogenase, the first enzyme of the GMP synthesis pathway. Both effects of the stringent response cause a reduction in the GTP pool. CodY-regulated genes are also expressed during slow exponential growth (Slack et al. 1991; Wray et al. 1997); the stringent response may be an important mechanism for reducing the GTP pool under these conditions.
The role of amino acid mixtures, such as CAA, in provoking CodY-dependent repression cannot be fully mimicked by individual amino acids (Atkinson et al. 1990; P. Serror and A.L. Sonenshein, unpubl.). In the case of the dpp operon, only branched-chain amino acids (Val, Ile, and Leu; BCAA), separately or in combination, had a significant stimulating effect on CodY activity (P. Serror, unpubl). Interestingly, in L. lactis, the CodY-dependent repression of genes involved in protein degradation is strongly dependent on the presence of BCAA (Guédon et al. 2001). If L. lactis CodY has the same properties as the B. subtilis CodY, the BCAA may act by affecting the intracellular pool of GTP, perhaps via the stringent response.
Although CodY clearly has motifs in common with small GTPases and binds GTP, the highly purified protein has no GTPase activity (data not shown). Small GTPases usually have high affinity for GTP. The low apparent affinity of CodY is consistent, however, with its putative role in sensing intracellular GTP pools. Whereas the activity of the GTPases is modulated by cleavage of bound GTP, activation of CodY depends on the high steady-state concentration of GTP found in rapidly growing exponential-phase cells. The G1 motif of CodY (GGERLGTL) lacks the lysine residue found at the seventh position of the G1 consensus sequence (Table 3). In small GTPases, this conserved lysine has been shown to interact with the α- and β-phosphates of guanine nucleotides (Saraste et al. 1990; Bourne et al. 1991); its absence may contribute to the low affinity of CodY for GTP. Interestingly, the codY16 mutation changes the conserved glycine in the sixth position of the G1 motif of CodY to asparagine and causes strong derepression of dpp during exponential growth (Slack et al. 1995). Preliminary experiments indicate that the CodY16 protein has a greatly decreased affinity for GTP (D. Negusse and M. Ratnayake-Lecamwasam, unpubl.).
The CodY G3 motif (DRVG) spans the last three residues of helix I and the first residue of the turn of the CodY helix-turn-helix (HTH) motif, which has been previously implicated in DNA-binding (Serror and Sonenshein 1996a). This potential overlap between GTP- and DNA-binding motifs is unusual. Although the presence of GTP has only a small effect on the affinity of CodY for DNA, it is possible that the binding of GTP alters the interaction of CodY with target sites in such a way as to allow it to act as a repressor. Such a model would be formally analogous to the effect of ATP on DnaA protein (Speck et al. 1999).
Expression of CodY-regulated and CodY-independent stationary-phase and early-sporulation genes is also modulated by proteins (e.g., AbrB, SinR, Soj) that are responsive to cell population density signals or other early-stationary-phase conditions (Bai et al. 1993; Cervin et al. 1998; Quisel et al. 1999). Moreover, CodY is not the only GTP-binding B. subtilis protein implicated in gene regulation. Obg is an essential protein (Trach and Hoch 1989) required for activation of the Spo0A transcription factor at the onset of sporulation (Kok et al. 1994; Vidwans et al. 1995) and may also be a GTP-sensing regulator of stationary-phase gene expression. However, Obg has not yet been implicated as a direct regulator of sporulation genes in response to GTP availability. Obg also appears to be necessary for the activation of the stress-induced transcription factor ςB (Scott et al. 2000).
Guanine nucleotides may be generally sensed by bacteria as indicators of nutritional sufficiency. In E. coli, growth-rate-dependent control appears to be mediated by the availability of GTP (and ATP), which acts on RNA polymerase at the initiation step of transcription from rRNA promoters (Gaal et al. 1997). In Myxococcus xanthus, conversion of GTP to (p)ppGpp is necessary for differentiation, but the effect is independent of any change in the GTP pool; the requirement for RelA during differentiation in this organism is not bypassed by treatment with decoyinine (Singer and Kaiser 1995).
Materials and methods
Bacterial strains, plasmids, and growth conditions
The B. subtilis strains used in this study are described in Table 4. Strain KWB1 encoding CodY-His6 was constructed as follows: The 3′ region of the CodY gene was amplified by PCR, using B. subtilis JH642 chromosomal DNA as the template DNA, with OKW1 (5′GCATGGATCCATACTGCTTTCCCTGTTGAG-3′) and OKW3 (5′CGCGAAGCTTTTAATGATGATGATGAT GATGAGATTTTAGATTTTCTAATTCAATTAGG-3′) as upstream and downstream primers, respectively. The OKW3 primer includes a sequence that would add a 6× His-tag (indicated in boldface) to the C-terminus of CodY. The PCR-amplified DNA was digested with restriction endonucleases BamHI and HindIII (cleavage sites italicized) and ligated (Sambrook et al. 1989) to the BamHI and HindIII-digested pJPM1 vector (Mueller et al. 1992), creating pKW1. Integration of pKW1 into the B. subtilis strain JH642 chromosome by homologous recombination resulted in strain KWB1. CodY-His6 was fully functional in vivo as judged by repression of a dppP-lacZ fusion.
Table 4.
Bacillus subtilis strains used in this study
| Strain
|
Genotype
|
Source/reference
|
|---|---|---|
| FJS107 | trpC2 SPβs | Slack et al. 1993 |
| JH642 | trpC2 pheA1 | J.A. Hoch |
| KWB1 | trpC2 pheA1 codY ::pKW1 (cat) | JH642 X pKW1 |
| MRLB10 | trpC2 ΔamyE::(φdpp′-lacZ neo) ΔrelA::mls | PS59 X DNA TW30 |
| PS29 | trpC2 gid::spc | Slack et al. 1995 |
| PS37 | trpC2 gid::spc ΔcodY | Serror and Sonenshein 1996b |
| PS56 | trpC2 ΔamyE::(φdpp′-lacZ neo) abrB::cat::tet | P. Serror (unpub.) |
| PS59 | trpC2 ΔamyE::(φdpp′-lacZ neo) | FJS107 X DNA PS56 |
| PS83 | trpC2 ΔamyE::(φdpp′-lacZ neo) abrB::cat::tet gid::spc ΔcodY | PS56 X DNA PS37 |
| PS164 | trpC2 ΔamyE::(φdpp′-lacZ neo) gid::spc ΔcodY | PS37 X DNA PS56 |
| TW30 | trpC2 pheA1 ΔrelA::mls | Wendrich and Marahiel 1997 |
Determination of CodY abundance in B. subtilis
B. subtilis strains were grown in 500 mL of DS medium (Fouet and Sonenshein 1990) or S7 medium (Freese et al. 1979b) supplemented as indicated with 0.5% glucose and 0.1% CAA. At the indicated time points, 35-mL samples were removed; cells were collected by centrifugation, washed with 10 mL of solution A (Serror and Sonenshein 1996a), and stored overnight at −80°C. Cell pellets were suspended in 2 mL of solution A and subjected to six 30-sec cycles of sonication, with 15-sec rest periods between cycles. Unbroken cells and cell debris were removed by centrifugation at 13,000 rpm for 15 min at 4°C. The total protein concentrations of the supernatant fluids were determined by using the Bio-Rad protein assay reagent, and 10 μg of total protein of each sample was analyzed by Western blotting, as described below.
SDS-PAGE and Western blotting
Protein samples were mixed with an equal volume of 2× SDS-PAGE-loading buffer, boiled for 3 min, loaded on 12% denaturing polyacrylamide gels, and subjected to electrophoresis at a constant voltage of 100V for 2 h. The GIBCO BRL Benchmark prestained protein ladder provided markers of molecular weight. For Western blotting, proteins were electrotransferred to Immobilon-P membranes (Millipore) and immunoblotted (Craig et al. 1997) using rabbit antibody to CodY prepared by Biodesign International.
Treatment of cells with decoyinine
B. subtilis strains were grown in S6 medium (Freese et al. 1979b), supplemented with 50 μg tryptophan per mL, 50 μg phenylalanine per mL, 0.1% CAA, and 0.5% glucose (S6C medium). When the cells reached an optical density (600 nm) of 0.3 to 0.4, decoyinine (dissolved in 1 N KOH) was added to a final concentration of 500 μg/mL to the appropriate cell cultures. An equivalent volume of 1 N KOH was added to control cell cultures. At various time intervals after the addition of decoyinine, 0.5–1 mL samples were harvested and assayed for β-galactosidase activity as described by Slack et al. (1991). In some experiments, the cell cultures were incubated for 20 h, and the sporulation efficiency of each strain was determined as the fraction of cells resistant to incubation for 10 min at 80°C.
Purification of 6× His-tagged CodY from B. subtilis
B. subtilis strain KWB1 was grown in 4 L of DS medium to an OD600 of ∼1.0 and harvested by centrifugation at 5000 rpm and for 10 min at 4°C. The cell pellets were washed with 15 mL of solution A (Serror and Sonenshein 1996a) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and stored at −70°C. The cell pellets were resuspended in 10 mL of buffer S (200 mM KCl, 50 mM Tris-Cl at pH 7.5, 10% glycerol, 0.1% Nonidet P-40, 1 mM PMSF, 2 μM Pepstatin-A [PEP-A], 0.5 mM DTT, 0.2 mM EDTA) and passed three times through a French pressure cell at 12,000 psi. The cell lysates were then subjected to sonication using a Branson Sonifier Cell Disrupter 200 for 3 to 4 cycles of 30 sec each, with 15-sec rest periods between each cycle. The cleared lysates were centrifuged at 13,000 rpm for 20 min at 4°C to remove cell debris. The supernatant fluids were collected and genomic DNA and ribosomes were precipitated by the addition of streptomycin sulfate (0.01 g/mL), with stirring for 10 min at 4°C. The lysates were then centrifuged at 13,000 rpm and for 15 min at 4°C and the supernatant fluids were dialyzed against 1 L of buffer S without DTT or EDTA for 1 h at 4°C. Following a protocol provided by S. Gross, the dialysates were mixed with Talon (Clontech) metal (Co+) affinity resin (15 mL of cell lysate/0.5 mL Talon) that had been equilibrated (10 min at 4°C, with tumbling) with buffer I (150 mM KCl, 20 mM Tris-Cl at pH 8.0, 5 mM β-mercaptoethanol, 10% glycerol, 1 mM PMSF, 2 μM PEP-A, 0.1% Nonidet P-40) and incubated for 20 min at 4°C, with tumbling. The resin and bound protein were collected by centrifugation at 3000 rpm for 3 min at 4°C and washed sequentially with 15 mL each of buffer I adjusted to 0.5 M KCl, 5 mM imidazole and buffer I containing 125 mM KCl, 5 mM imidazole. After loading the resin into a small column, proteins were eluted with buffer I containing 125 mM KCl and 50–100 mM imidazole. Fractions (0.5 mL) were collected and mixed with 5 μL of 100 mM DTT, and 100 mM EDTA. All fractions were subjected to SDS-PAGE and the presence of CodY was determined by Western-blot analysis. Fractions found to contain CodY were pooled and in some cases dialyzed briefly against 2 L of solution A at pH 7.5 (Serror and Sonenshein 1996a) at 4°C. The preparations of CodY-His6 used here were typically 75%–85% pure.
Ultraviolet-induced cross-linking of [α-32P] GTP to CodY
One μg of CodY protein (3 μM) purified to near homogeneity from cell extracts of a CodY-overexpressing E. coli strain (Serror and Sonenshein 1996a) was incubated with 50 mM Tris-Cl at pH 7.5, 100 mM KOAc, 0.1 mM [α-32P] GTP (NEN; 3 μCi/nmol) for 20 min on ice in a 10 μL-reaction volume. In some experiments, unlabelled nucleotides were included in the incubation. The reactions were then exposed to six cycles of UV light (1200 J/min) in a UV Stratalinker 1800 (Stratagene). Samples were immediately added to an equal volume of 2× SDS-PAGE loading buffer (50 mM Tris-Cl at pH 6.8, 2.5% SDS, 0.1% bromophenol blue, 10% glycerol; Sambrook et al. 1989), incubated for 3 min at 80°C, and then subjected to SDS-PAGE in a 12% denaturing polyacrylamide gel for 90 min at 100V. The gel was washed three times in distilled water (15 min per wash), dried, and exposed to Kodak X-OMAT AR film at −70°C or to a PhosphorImager screen (Molecular Dynamics) overnight at room temperature.
In vitro transcription
DNA templates for in vitro runoff transcription were prepared by PCR amplification of pFS48 (dpp; Slack et al. 1993) or pPH9 (veg; Le Grice et al. 1986). Promoter DNA (40 –80 nM) was incubated at 37°C in a 10-μl reaction containing 40 mM Tris-Cl at pH 8.0, 5 mM MgCl2, 1 mM MnCl2, 0.05 mM EDTA at pH 8.0, 0.05 M KCl, 0.05 mg BSA per mL, 2.5% glycerol, 0.2 mM each of ATP, GTP, and CTP, 0.05 mM [α32P] UTP (NEN; 5 μCi/nmol), 2U RNasin (Promega), 2 mM DTT, 19–37 nM B. subtilis RNA polymerase (Whipple and Sonenshein 1992) and varying amounts of CodY protein. After 15 min, 4 μL of formamide-loading buffer (Sambrook et al. 1989) was added. After incubation for 10 min at 80°C, samples (5 μL) were subjected to polyacrylamide gel electrophoresis on an 8 M urea/5% polyacrylamide gel in TBE (0.09 M Tris-borate, 0.002 M EDTA at pH 8.0). The dried gel was exposed to a PhosphorImager screen overnight. The expected runoff transcripts for dpp and veg are 155 n and 104 n, respectively.
Acknowledgments
We thank B. Belitsky, D. RayChaudhuri, C. Squires, and A. Wright for their critical reading; S. Gross for sharing his His-tagged protein-purification protocol; C. Delorme and colleagues for allowing us to cite their unpublished results; and T.M. Wendrich and M.A. Marahiel for providing strain TW30. We are very grateful to D. RayChaudhuri and A. Wright for their expert advice and to N. Mani for help with the in vitro transcription assays. This work was supported by U.S. Public Health Service research grant GM42219.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL linc.sonenshein@tufts.edu; FAX (617) 636-0337.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.874201.
References
- Altschul F, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MR, Wray LV, Fisher SH. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J Bacteriol. 1990;172:4758–4765. doi: 10.1128/jb.172.9.4758-4765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai U, Mandic-Mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein–protein interaction. Genes & Dev. 1993;7:139–148. doi: 10.1101/gad.7.1.139. [DOI] [PubMed] [Google Scholar]
- Bolotin A, Mauger S, Malarme K, Ehrlich SD, Sorokin A. Low-redundancy sequencing of the entire Lactococcus lactis IL403 genome. Antonie Van Leeuwenhoek. 1999;76:27–76. [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Burkholder WF, Grossman AD. Regulation of the initiation of endospore formation in Bacillus subtilis. In: Brun Y, Shimkets L, editors. Prokaryotic development. Washington, D.C.: ASM Press; 2000. pp. 151–166. [Google Scholar]
- Cashel M, Gentry D, Hernandez V, Vinella D. The stringent response. In: Neidhardt F, et al., editors. Escherichia coli and Salmonella. Washington, D.C.: ASM Press; 1996. pp. 1458–1496. [Google Scholar]
- Cervin MA, Lewis RJ, Brannigan JA, Spiegelman GB. The Bacillus subtilis regulator SinR inhibits spoIIG promoter transcription in vitro without displacing RNA polymerase. Nucleic Acids Res. 1998;26:3806–3812. doi: 10.1093/nar/26.16.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JE, Ford MJ, Blaydon DC, Sonenshein AL. A null mutation in the Bacillus subtilis aconitase gene causes a block in Spo0A-phosphate-dependent gene expression. J Bacteriol. 1997;179:7351–7359. doi: 10.1128/jb.179.23.7351-7359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbouille M, Gardan R, Arnaud M, Rapoport G. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J Bacteriol. 1999;181:2059–2066. doi: 10.1128/jb.181.7.2059-2066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman DW, Rosenkrantz MS, Sonenshein AL. Relationship between aconitase gene expression and sporulation in Bacillus subtilis. J Bacteriol. 1987;169:3068–3075. doi: 10.1128/jb.169.7.3068-3075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymann C, Mittenhuber G, Hecker M. The stringent response, ςH-dependent gene expression and sporulation in Bacillus subtilis. Mol Gen Genet. 2001;264:913–931. doi: 10.1007/s004380000381. [DOI] [PubMed] [Google Scholar]
- Ferson AE, Wray LV, Jr, Fisher SH. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol Microbiol. 1996;22:693–701. doi: 10.1046/j.1365-2958.1996.d01-1720.x. [DOI] [PubMed] [Google Scholar]
- Fisher SH, Rohrer K, Ferson AE. Role of CodY in regulation of the Bacillus subtilis hut operon. J Bacteriol. 1996;178:3779–3784. doi: 10.1128/jb.178.13.3779-3784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet A, Sonenshein AL. A target for carbon source–dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990;172:835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E, Heinze JE, Galliers EM. Partial purine deprivation causes sporulation of Bacillus subtilis in the presence of excess ammonia, glucose, and phophate. J Gen Microbiol. 1979a;115:193–205. doi: 10.1099/00221287-115-1-193. [DOI] [PubMed] [Google Scholar]
- Freese EB, Vasantha N, Freese E. Induction of sporulation in developmental mutants in Bacillus subtilis. Mol Gen Genet. 1979b;170:67–74. doi: 10.1007/BF00268581. [DOI] [PubMed] [Google Scholar]
- Gaal T, Bartlett MS, Ross W, Turnbough CL, Jr, Gourse RL. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- Guédon, E., Serror, P., Ehrlich, S.D., Renault, P., and Delorme, C. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched chain amino acids in Lactococcus lactis Mol. Microbiol. (in press). [DOI] [PubMed]
- Kok J, Trach KA, Hoch JA. Effects on Bacillus subtilis of a conditional lethal mutation in the essential GTP-binding protein Obg. J Bacteriol. 1994;176:7155–7160. doi: 10.1128/jb.176.23.7155-7160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazazzera BA, Kurtser IG, McQuade RS, Grossman AD. An autoregulatory circuit affecting peptide signaling in Bacillus subtilis. J Bacteriol. 1999;181:5193–5200. doi: 10.1128/jb.181.17.5193-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice SF, Shih CC, Whipple F, Sonenshein AL. Separation and analysis of the RNA polymerase binding sites of a complex Bacillus subtilis promoter. Mol Gen Genet. 1986;204:229–236. doi: 10.1007/BF00425503. [DOI] [PubMed] [Google Scholar]
- Lopez J, Marks C, Freese E. The decrease in guanine nucleotides initiates sporulation in Bacillus subtilis. Biochim Biophys Acta. 1979;587:238–252. doi: 10.1016/0304-4165(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Lopez JM, Dromerick A, Freese E. Response of guanosine 5′-triphopshate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J Bacteriol. 1981;146:605–613. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiopoulos C, Mueller JP, Slack FJ, Murphy CG, Patankar S, Bukusoglu G, Sonenshein AL. A Bacillus subtilis dipeptide transport system expressed early during sporulation. Mol Microbiol. 1991;5:1903–1913. doi: 10.1111/j.1365-2958.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Mirel DB, Estacio WF, Mathieu M, Olmsted E, Ramirez J, Marquez-Magana LM. Environmental regulation of Bacillus subtilis sigma(D)-dependent gene expression. J Bacteriol. 2000;182:3055–3062. doi: 10.1128/jb.182.11.3055-3062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani T, Heinze J, Freese E. Induction of sporulation in Bacillus subtilis by decoyinine or hadacidin. Biochim Biophys Acta. 1977;77:1118–1125. doi: 10.1016/s0006-291x(77)80094-6. [DOI] [PubMed] [Google Scholar]
- Mueller JP, Bukusoglu G, Sonenshein AL. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: Control of gsiA by the ComP–ComA signal transduction system. J Bacteriol. 1992;174:4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F, Ingraham J, Schaechter M. Physiology of the bacterial cell. A molecular approach. Sunderland, Massachusetts: Sinauer Associates, Inc.; 1990. p. 98. [Google Scholar]
- Nygaard P. Purine and pyrimidine salvage pathways. In: Sonenshein AL, et al., editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: American Society of Microbiology; 1993. pp. 359–378. [Google Scholar]
- Ochi K, Kandala J, Freese E. Initiation of Bacillus subtilis sporulation by the stringent reponse to partial amino acid deprivation. J Biol Chem. 1981;256:6866–6875. [PubMed] [Google Scholar]
- Quisel JD, Lin DC, Grossman AD. Control of development by altered localization of a transcription factor in B. subtilis. Mol Cell. 1999;4:665–672. doi: 10.1016/s1097-2765(00)80377-9. [DOI] [PubMed] [Google Scholar]
- Ratnayake-Lecamwasam M. “Synthesis and regulation of CodY, a nutrient-sensitive repressor of early stationary phase genes, in Bacillus subtilis.” Ph.D. thesis. Medford, Massachusetts: Tufts University; 2001. [Google Scholar]
- RayChaudhuri D, Park JT. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular cloning. A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Saraste M, Sibbald PR, Wittinghofer A. The P-loop—A common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Scott JM, Ju J, Mitchell T, Haldenwang WG. The Bacillus subtilis GTP binding protein Obg and regulators of the sigma(B) stress response transcription factor cofractionate with ribosomes. J Bacteriol. 2000;182:2771–2777. doi: 10.1128/jb.182.10.2771-2777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serror P, Sonenshein AL. Interaction of CodY, a novel Bacillus subtilis DNA-binding protein, with the dpp promoter region. Mol Microbiol. 1996a;20:843–852. doi: 10.1111/j.1365-2958.1996.tb02522.x. [DOI] [PubMed] [Google Scholar]
- ————— CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol. 1996b;178:5910–5915. doi: 10.1128/jb.178.20.5910-5915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Kaiser D. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes & Dev. 1995;9:1633–1644. doi: 10.1101/gad.9.13.1633. [DOI] [PubMed] [Google Scholar]
- Slack FJ, Mueller JP, Strauch MA, Mathiopoulos C, Sonenshein AL. Transcriptional regulation of a Bacillus subtilis dipeptide transport operon. Mol Microbiol. 1991;5:1915–1925. doi: 10.1111/j.1365-2958.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Slack F, Mueller J, Sonenshein AL. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack F, Serror P, Joyce E, Sonenshein AL. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol Microbiol. 1995;15:689–702. doi: 10.1111/j.1365-2958.1995.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Sonenshein AL. Metabolic regulation of sporulation and other stationary-phase phenomena. In: Smith I, et al., editors. Regulation of prokaryotic development. Washington, D.C.: Am. Soc. Microb. Press; 1989. pp. 109–130. [Google Scholar]
- ————— . Endospore-forming bacteria: An overview. In: Brun Y, Shimkets L, editors. Prokaryotic development. Washington, D.C.: ASM Press; 2000. pp. 133–150. [Google Scholar]
- Speck C, Weigel C, Messer W. ATP- and ADP-DnaA protein, a molecular switch in gene regulation. EMBO J. 1999;18:6169–6176. doi: 10.1093/emboj/18.21.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch MA. Regulation of Bacillus subtilis gene expression during transition from exponential growth to stationary phase. Prog Nucleic Acids Res Mol Biol. 1993;46:121–153. doi: 10.1016/s0079-6603(08)61020-x. [DOI] [PubMed] [Google Scholar]
- Trach K, Hoch JA. The Bacillus subtilis spo0B stage 0 sporulation operon encodes an essential GTP-binding protein. J Bacteriol. 1989;171:1362–1371. doi: 10.1128/jb.171.3.1362-1371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidwans SJ, Ireton K, Grossman AD. Possible role for the essential GTP-binding protein Obg in regulating the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3308–3311. doi: 10.1128/jb.177.11.3308-3311.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendrich TM, Marahiel MA. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol Microbiol. 1997;26:65–79. doi: 10.1046/j.1365-2958.1997.5511919.x. [DOI] [PubMed] [Google Scholar]
- Whipple FW, Sonenshein AL. Mechanism of initiation of transcription by B. subtilis RNA polymerase at several promoters. J Mol Biol. 1992;223:399–414. doi: 10.1016/0022-2836(92)90660-c. [DOI] [PubMed] [Google Scholar]
- Wray LV, Jr, Ferson AE, Fisher SH. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J Bacteriol. 1997;179:5494–5501. doi: 10.1128/jb.179.17.5494-5501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]