Abstract
Activation of β-adrenergic receptors (β-AR) drives proangiogenic factor production in several types of cancers. To examine β-AR regulation of breast cancer pathogenesis, β-AR density, signaling capacity, and functional responses to β-AR stimulation were studied in four human breast adenocarcinoma cell lines. β-AR density ranged from very low in MCF7 and MB-361 to very high in MB-231 and in a brain-seeking variant of MB-231, MB-231BR. Consistent with β-AR density, β-AR activation elevated cAMP in MCF7 and MB-361 much less than in MB-231 and MB-231BR. Functionally, β-AR stimulation did not markedly alter vascular endothelial growth factor (VEGF) production by MCF7 or MB-361. In the two high β-AR-expressing cell lines MB-231 and MB-231BR, β-AR-induced cAMP and VEGF production differed considerably, despite similar β-AR density. The β2-AR-selective agonist terbutaline and the endogenous neurotransmitter norepinephrine decreased VEGF production by MB-231, but increased VEGF production by MB-231BR. Moreover, β2-AR activation increased IL-6 production by both MB-231 and MB-231BR. These functional alterations were driven by elevated cAMP, as direct activation of adenylate cyclase by forskolin elicited similar alterations in VEGF and IL-6 production. The protein kinase A antagonist KT5720 prevented β-AR-induced alterations in MB-231 and MB-231BR VEGF production, but not IL-6 production.
Conclusions
β-AR expression and signaling is heterogeneous in human breast cancer cell lines. In cells with high β-AR density, β-AR stimulation regulates VEGF production through the classical β-AR-cAMP-PKA pathway, but this pathway can elicit directionally opposite outcomes. Furthermore, in the same cells, β-AR activate a cAMP-dependent, PKA-independent pathway to increase IL-6 production. The complexity of breast cancer cell β-AR expression and functional responses must be taken into account when considering β-AR as a therapeutic target for breast cancer treatment.
Keywords: Breast cancer, β-Adrenergic receptors, VEGF, IL-6, Norepinephrine, cAMP
Introduction
Sympathetic nervous system activation and release of the neurotransmitters norepinephrine (NE) and epinephrine (EPI) can promote solid tumor growth and metastases by activating β-adrenergic receptors (β-AR) expressed by tumor cells or host stromal cells [1–5]. β-AR are Gs-protein coupled receptors that activate adenylate cyclase to elevate intracellular 3′,5′-cyclic adenosine monophosphate (cAMP) and activate protein kinase A (PKA), leading to down-stream alterations in gene expression that regulate a variety of cellular functions. More recently, β-AR-activated effector molecules have been identified that diverge from the classical cAMP-PKA pathway [6–8].
Direct stimulation of β-AR expressed by tumor cells may play an important role in tumor pathogenesis. For example, exposure to a chronic stressor promoted in vivo angiogenesis and production of vascular endothelial growth factor (VEGF), a key proangiogenic protein. This effect was eliminated by silencing ovarian tumor cell β-AR expression, implicating tumor cell β-AR expression and signaling as an important facilitator of stress-induced tumor angiogenesis in vivo [1]. In vitro studies using tumor cell lines suggest that NE can promote tumor progression by a β-AR-driven proangiogenic pathway, featuring increased production of VEGF, interleukin-6 (IL-6), and matrix metalloproteinases. This pathway has been demonstrated in multiple tumor types and in normal cells [4, 9–13]. The direct impact of β-AR stimulation of breast tumor cells has not been well characterized, despite the potential for β-AR facilitation of angiogenic and metastatic pathways.
Here, we characterize β-AR expression and signaling capacity in a panel of human breast cancer cell lines and determine the impact of β-AR signaling on production of two proteins that drive tumor angiogenesis, VEGF and IL-6. The results suggest that heterogeneity in β-AR expression and function in breast cancer cells needs to be taken into account when considering β-blockade as a therapeutic strategy to inhibit tumor growth and/or angiogenesis in breast cancer.
Materials and methods
Cell lines
Human breast adenocarcinoma cell lines MDA-MB-231 (MB-231), MDA-MB-361 (MB-361), and MCF7 were purchased from American Tissue Type Collection (Manassas, VA). MDA-MB-231BR (MB-231BR), a brain-seeking variant of MB-231, was obtained from Dr. T. Yoneda (University of Texas Health Science Center, San Antonio, TX) [14]. All cell lines were employed experimentally within 3 months of acquiring and/or thawing and were regularly tested for the absence of mycoplasma contamination. All cell lines were grown in Dulbecco’s Modified Essential Medium (DMEM) containing 4.5 g/l glucose, L-glutamine, penicillin/streptomycin and 10% fetal calf serum (FCS). All cells were grown in T75 tissue culture flasks (Corning Inc., Corning, NY) to no greater than 70–80% confluence.
Reagents
(–)Isoproterenol hydrochloride (ISO), terbutaline hemi-sulfate (TERB), isobutylmethylxanthine (IBMX), forskolin, and KT 5720 were purchased from Sigma-Aldrich, St. Louis, MO. [125I]-cyanopindolol (125ICYP) was purchased from NEN Radiochemicals (Perkin Elmer Life and Analytical Sciences, Waltham, MA). All media, Hank’s Balanced Salt Solution (HBSS), and components were purchased from Gibco, Invitrogen Inc., Carlsbad, CA.
β-AR radioligand binding assay
The specific binding of 125ICYP to whole cells was used to quantify β-AR expression [15]. Tumor cells (1 × 106) resuspended in HBSS were incubated with varying concentrations of 125ICYP with or without the unlabeled β-AR-antagonist CGP-12177 (1 μM) in duplicate tubes. Incubations were carried out for 60 min in a shaking water bath at 37°C, sufficient time for equilibrium binding to occur. Following incubation, 3 ml of ice-cold hypotonic phosphate buffer (3.8 mM KH2O4, 16.2 mM Na2HPO4, and 4 mM MgSO4) was added to each tube for 10 min. The reaction mixtures were filtered under reduced pressure through Whatman glass fiber filters using an automatic harvester (Brandel Corp., Gaithersburg, MD). Filters were rinsed with 16 ml of ice-cold Tris–EGTA buffer to remove unbound radioligand, and radioactivity was determined in a LKB Clinigamma gamma counter. Specific binding is defined as the difference between binding of the radioligand at each concentration in the absence and in the presence of 1 μM CGP-12177 and was calculated for each ligand concentration using the specific activity of the radioligand. Specific 125ICYP (M) bound versus the amount of ligand added was plotted, and the maximal number of binding sites (Bmax) and receptor affinity (Kd) was determined using non-linear regression analysis (GraphPad Prism software, San Diego, CA). The maximal number of binding sites per cell is calculated from the Bmax based on simple stoichiometric assumptions (one molecule binds to one receptor site).
cAMP assay
Tumor cells (1 × 106) were incubated for 20 min at 37°C in a shaking water bath in HBSS containing 0.1% bovine serum albumin (BSA) and 100 μM isobutylmethylxanthine (IBMX), a phosphodiesterase inhibitor (HBSS/BSA/IBMX buffer). ISO, forskolin, or buffer alone was added to each tube, and the cells were incubated for 5–60 min at 37°C (final volume = 1 ml). To stop the reaction, 2 ml ice-cold HBSS/BSA/IBMX buffer was added to each tube. The cells were centrifuged two times for 10 min at 200×g and then resuspended in 1× Cell Lysis Buffer, provided in the cAMP ELISA kit. After subjecting the cells to freezing at −20°C, followed by boiling for 10 min in a 95°C heat block and repeating the sequence, the cells were centrifuged at 600×g at 4°C for 10 min to remove cellular debris. Supernatants were collected and stored at −80°C until analysis of cAMP content using the cAMP Parameter ELISA kit from R&D Systems (Minneapolis, MN) following the manufacturer’s instructions. Absorption was measured at 450 nm using a multiwell plate reader (Synergy HT, Biotek Instruments Inc, Winooski, VT). Curve fitting and sample concentration calculations were conducted with Gen5 software (Biotek).
VEGF and IL-6 production in vitro
Tumor cells were resuspended in Advanced DMEM containing 1% FCS, penicillin/streptomycin, and L-glutamax (Invitrogen). Cells were plated in duplicate at 5 × 104 cells per well in 24-well flat bottom tissue culture plates (Falcon, Becton-Dickinson, Franklin Lakes, NJ). At this concentration, tumor cells reached confluence by 96 h in culture. Cells were cultured at 37°C in a 5% CO2 humidified atmosphere and were allowed to adhere for 3 h before addition of adrenergic agonists or antagonists. The β2-selective blocker ICI 118,551 or the PKA inhibitor KT 5720 was added 30 min prior to β-AR agonists. VEGF or IL-6 concentration in cell-free supernatants was measured using human-specific VEGF or IL-6 Quantikine kits (R&D Systems) according to the manufacturer’s instructions. Absorption was measured at 450 nm using a multiwell plate reader. Curve fitting and sample concentration calculations were conducted with Gen5 software.
Cellular proliferation in vitro
Tumor cells were resuspended in Advanced DMEM containing 1% FCS, penicillin/streptomycin, and L-glutamax (Invitrogen) and plated at 1–2 × 104 cells per well in 96-well flat bottom tissue culture plates (Falcon, Becton-Dickinson) in triplicate, and allowed to adhere for 3 h at 37°C in a 5% CO2 humidified atmosphere before addition of adrenergic agonists or antagonists. For blocking experiments, antagonists were added 30 min prior to addition of agonists. Proliferation was measured using a fluorescent DNA binding dye (CyQuant NF Proliferation Assay kit, Invitrogen) according to manufacturer’s instructions. Fluorescent intensity was measured at excitation 490 nm and emission 520 nm using a multiwell plate reader equipped with the appropriate filters. Background fluorescence (in the absence of cells) was subtracted automatically from each well.
Statistical analysis
Statistically significant differences between groups were determined by analysis of variance (ANOVA) using GraphPad Prism software. Initially, two-way ANOVAs were conducted with experimental repetition and drug treatment as a variable. If experimental repetitions were not significantly different, the raw values or values normalized to medium alone were combined for further ANOVA. When experimental repetitions were significantly different from each other, individual experimental repetitions were analyzed separately with concentrations obtained from individual experimental repetitions. In experiments with time in culture as a factor, highly significant (P < 0.0001) effects of time in culture were observed. The results reported were analyzed at each time point by one-way ANOVA. When a significant main effect of drug treatment was identified (P < 0.05), Newman-Keuls post hoc test to compare between groups with P < 0.05 considered significant.
Results
β-AR expression and signaling capacity varies between breast adenocarcinoma cell lines
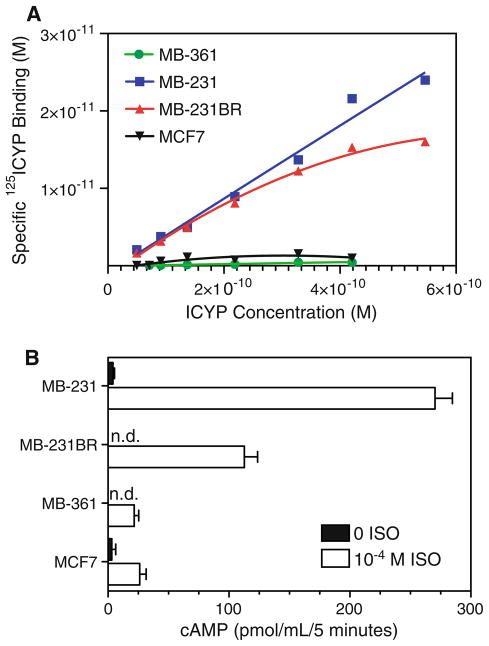
β-AR expression was measured in four human breast adenocarcinoma cell lines. MB-231 represents the metastatic, aggressive “triple-negative” phenotype and lacks expression of estrogen receptor-alpha (ER-a) and other hormone receptors [16]. MB-231BR, a variant of MB-231, metastasizes exclusively to the mouse brain [14]. MCF7 is less aggressive and is non-metastatic in mice. MB-361 was isolated from a human brain metastasis [16]. Standard radioligand binding was used to quantify β-AR cell surface density. Specific binding of the β-AR antagonist 125ICYP in each cell line is shown in Fig. 1a. The number of binding sites, calculated as described in “Materials and methods” section, ranged from approximately 150–300 sites per cell in MB-361 and MCF7 to ~ 11,000 to 14,000 sites per cell in MB-231 and MB-231BR (Table 1). High-affinity binding of ICYP (Kd) was observed, ranging from 1 × 10−11 to 9 × 10−11 M in all cell lines (Table 1).
Fig. 1.
Heterogeneity of β-AR expression in breast cancer cell lines. a Binding of the radiolabeled β-AR antagonist 125ICYP was measured in four human breast adenocarcinoma cell lines. Specific binding at each ligand concentration was calculated by subtracting radioligand binding in the presence of unlabeled antagonist CGP-12177 from total ICYP binding. The concentration of 125ICYP specifically bound at varying concentrations of ligand was calculated for each cell line as described in “Materials and methods” section. Results are representative of 1–3 experimental repetitions. b cAMP accumulation in response to β-AR stimulation. cAMP production was measured after stimulating 1 × 106 cells with HBSS buffer alone or 10−4 M ISO for 5 min. All reactions took place in the presence of 100 μM IBMX. Intracellular cAMP concentration was measured by ELISA. Results shown are mean ± SEM of 2–4 experimental repetitions. n.d. not detectable; level of detection = 1 pmol/ml
Table 1.
Human breast cancer cell lines: β-AR binding sites and affinity
| Cell line | Metastatic?a | Species | Bmax (M) (SEM) | Sites per cell (SEM) | Kd (M) |
|---|---|---|---|---|---|
| MB-231 | Yes | Human | 7.2 × 10−11 (2.9) | 13798 (4858) | 9.4 × 10−11 (4.9) |
| MB-231BR | Yes | Human | 6.1 × 10−11 (1.8) | 11027 (3991) | 7.8 × 10−11 (3.0) |
| MCF7 | No | Human | 0.14 × 10−11 (0.003) | 297 (13) | 1.1 × 10−11 (0.16) |
| MB-361 | Yes | Human | 0.089 × 10−11 | 160 | 3.9 × 10−11 |
Metastatic in mice
Next, we determined if β-AR density reflected signaling capacity as measured by β-AR-induced intracellular cAMP. The reaction was conducted in the presence of the phosphodiesterase inhibitor IBMX to prevent hydrolysis of cAMP and to minimize the possibility that potential variation in phosphodiesterase activity contributed to differences in β-AR-induced intracellular cAMP. cAMP accumulation was measured after 5 min incubation with 10−4 M isoproterenol (ISO), a non-selective β-AR agonist (Fig. 1b). MB-231 and MB-231BR produced much more cAMP relative to the moderate responses by MB-361 and MCF7, as predicted by β-AR expression. Moreover, the magnitude of the cAMP response was approximately 2.5-fold higher in MB-231 compared to MB-231BR, despite the fact that MB-231 and MB-231BR expressed equivalent numbers of β-AR per cell. Non-stimulated, basal cAMP levels were near the level of detection of the ELISA (1 pmol/ml) in MB-231BR and MB-361, but were somewhat elevated (5 pmol/ml) in MB-231 and MCF7 (Fig. 1b).
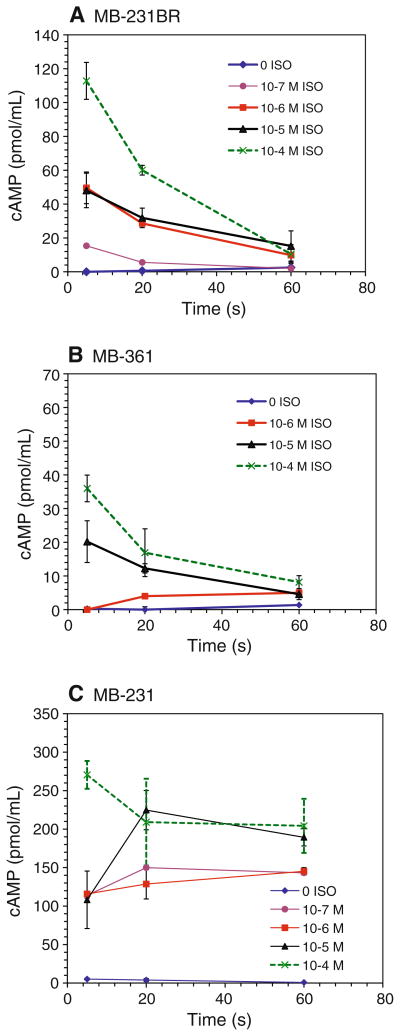
To determine if the differences in the β-AR-induced cAMP response between MB-231 and MB-231BR reflected altered kinetics, intracellular cAMP was measured in MB-231 and MB-231BR with varying concentrations of ISO at 5, 20, and 60 min. The cAMP response of the low β-AR-expressing cell line MB-361 was included for comparison. In MB-231BR, a rapid rise and decline in intracellular cAMP was observed at all ISO concentrations (≥0.1 μM) tested within the 60-min time period (Fig. 2a). Similarly, in MB-361, in response to high concentrations of ISO, cAMP rose rapidly and declined to near baseline by 60 min (Fig. 2b). In marked contrast, in MB-231, β-AR-induced cAMP remained elevated throughout the 60-min time period at all ISO concentrations tested (Fig. 2c). The functional implications of these differences in β-AR signaling capacity were next investigated in vitro.
Fig. 2.
Kinetics of cAMP production with β-AR activation. Varying concentrations of ISO was added to a MB-231BR, b MB-361, or c MB-231. Intracellular cAMP production was measured after stimulating 1 × 106 cells for 5, 20, or 60 min in the presence of 100 μM IBMX. cAMP concentration was determined by ELISA. Results shown are mean ± SEM of 2–5 experimental repetitions
β-AR stimulation and VEGF production
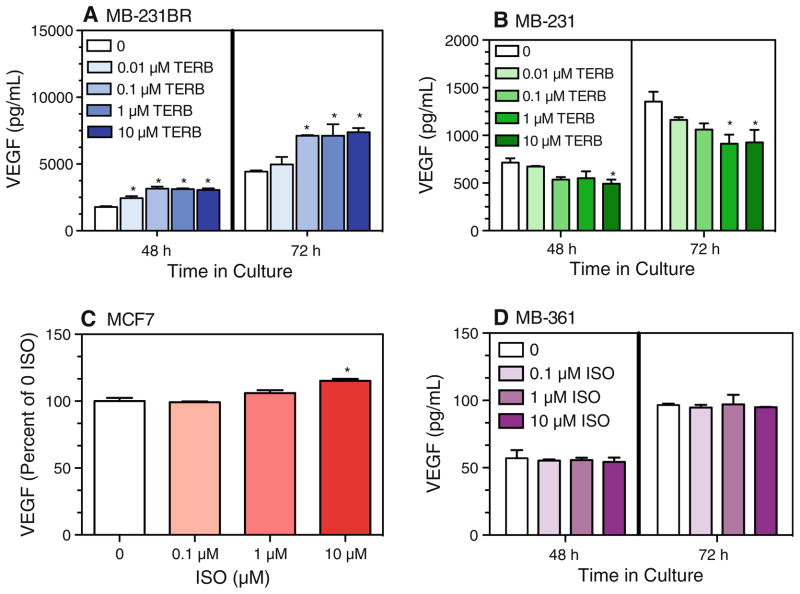
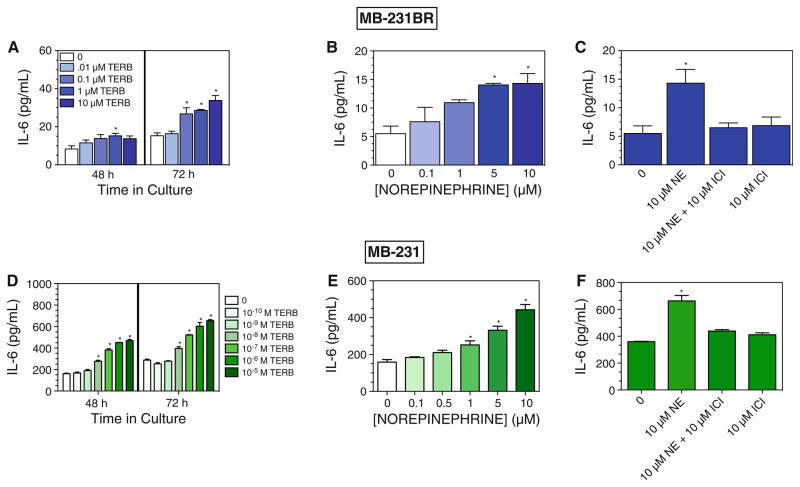
Several reports have demonstrated that β-AR activation of a variety of tumor cells in vitro increased VEGF [4, 10, 13]. Therefore, we predicted that β-AR stimulation would increase constitutive VEGF production by the breast cancer cell lines, and the magnitude of the response would reflect the magnitude of the β-AR-induced cAMP response. The β2-AR-selective agonist terbutaline (TERB) (≥0.1 μM) (Fig. 3a) and ISO (data not shown) increased VEGF production by MB-231BR at 48 and 72 h in culture. In contrast, β-AR stimulation with ≥0.1 μM TERB (Fig. 3b) and ISO (data not shown) inhibited VEGF production by MB-231. The highest concentration of ISO tested (10 μM) slightly increased production of VEGF by MCF7 (Fig. 3c) and did not alter VEGF production by MB-361 (Fig. 3d).
Fig. 3.
β-AR activation and VEGF production by breast cancer cell lines in vitro. The non-selective β-AR agonist ISO or the β2-AR-agonist TERB were added at varying concentrations to a MB-231BR, b MB-231, c MCF7, and d MB-361. Cell-free supernatants were harvested at the indicated time points, and VEGF concentration was determined by ELISA. For c MCF7, results shown are at 72 h in culture. Results shown are mean ± SEM from 2 to 4 experimental repetitions per cell line. a, b One-way ANOVA: main effects of TERB, P < 0.05 at each time point; c for MCF7, VEGF values differed between two experimental repetitions, therefore results from two experimental repetitions were normalized relative to 0 ISO. Baseline VEGF for the two MCF7 experiments at 72 h was 412 and 316 pg/ml. Main effect of drug treatment, P = 0.01. d For MB-361, no main effect of drug treatment. Asterisks indicate statistically significant versus 0 ISO at the respective time point in culture by Newman–Keuls post hoc analysis (P < 0.05)
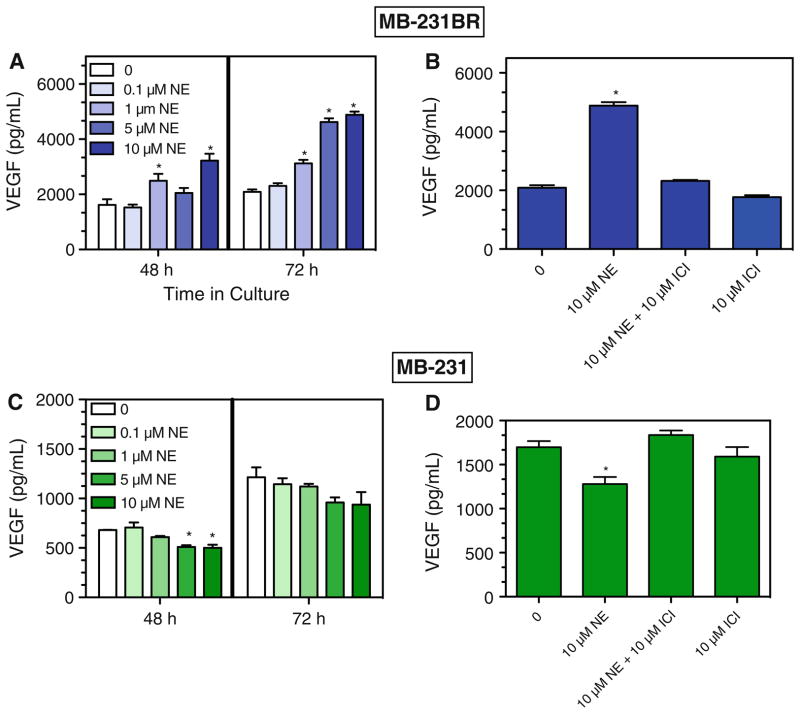
NE, an endogenous AR ligand, has mixed affinity for both β-AR and α-AR. NE increased VEGF production by MB-231BR at 48 and 72 h in culture (Fig. 4a). The NE-induced effect in MB-231BR was completely blocked by the β2-selective blocker ICI 118,551 (ICI) (Fig. 4b). In contrast, NE inhibited VEGF production by MB-231 (Fig. 4c), and the NE-induced inhibition was completely blocked by an equimolar concentration of ICI (Fig. 4d). The effectiveness of low concentrations of the β2-AR selective agonist TERB and the ability of the β2-AR selective blocker to block NE-induced effects indicate that β2-AR activation elicits opposite effects on VEGF production by MB-231 and MB-231BR.
Fig. 4.
NE-induced alterations in VEGF production are blocked by a β2-AR-specific antagonist. NE was added at varying concentrations to MB-231BR (a, b) and MB-231 (c, d). VEGF concentration was determined in cell-free supernatants at 48 h (a, c) or 72 h (a–d) in culture. The β2-AR blocker ICI was added 30 min prior to NE. Results shown are representative of 2–3 experimental repetitions. a–d By one-way ANOVA, main effects of NE at each time point, P < 0.05. In a and c, asterisks indicate statistical significance versus 0 ISO at the respective time point by Newman–Keuls (P < 0.05). In b and d, asterisks indicate group which is statistically different versus all other groups by Newman–Keuls analysis (P < 0.05). No other group differences were detected
β-AR-induced IL-6 production
To further investigate this divergent response, we next determined whether other tumor-produced cytokines are similarly altered by β-AR stimulation. IL-6 is a multifunctional, proinflammatory cytokine that has been implicated in tumor progression and metastasis [17, 18]. Under our low serum culture conditions, constitutive production of IL-6 is detectable in MB-231 and MB-231BR, but not by MCF7 or MB-361 (data not shown). β2-AR activation with TERB increased IL-6 production in MB-231BR (Fig. 5a) and MB-231 (Fig. 5d) at 48 and 72 h in culture. Moreover, the magnitude of the TERB response was greater in MB-231 compared to MB-231BR (at 72 h, 10 μM TERB compared to 0 drug, ~5× versus ~3×, respectively). NE also increased IL-6 in both MB-231 BR and MB-231, but at higher concentrations compared to TERB (Fig. 5b, e). ICI completely prevented the response to NE in MB-231BR (Fig. 5c) and in MB-231 (Fig. 5f). These results indicate that β2-AR activation increases IL-6 production by MB-231 and MB-231BR.
Fig. 5.
β-AR stimulation increases IL-6 production by MB-231BR and MB-231. TERB (a, d) or NE (b, e) were added at varying concentrations to (a–c) MB-231BR or MB-231 (d–f). The β2-AR blocker ICI was added 30 min prior to NE. Cell-free supernatants were harvested 48 h (a, d) or 72 h (a–f) later. IL-6 concentration was determined by ELISA. Results shown are representative of 2–3 experimental repetitions. In a–f, by one-way ANOVA, significant main effects of drug treatment were detected (P < 0.05). In (a, b, d, e), asterisks indicate significantly different versus 0 ISO at the respective time point in culture by Newman–Keuls (P \0.05). In c and f, asterisk indicates group which is significantly different versus all other groups by Newman–Keuls (P < 0.05). No other group differences were detected
β-AR stimulation and Cellular Proliferation
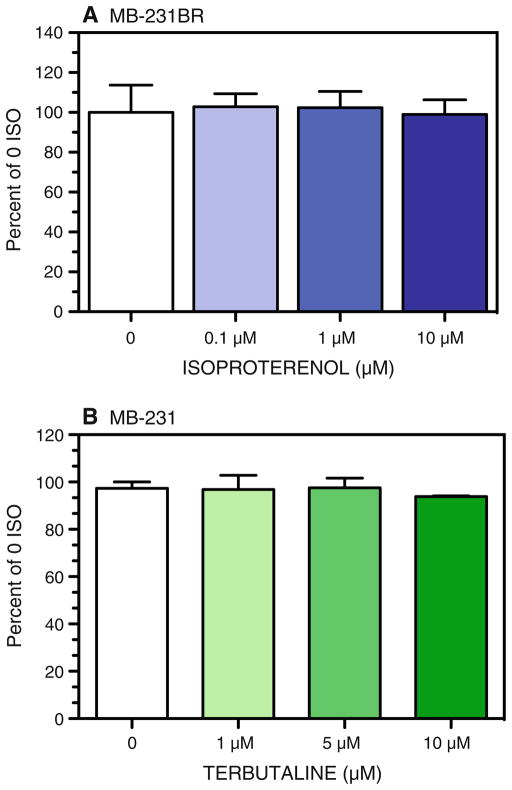
To determine if the β-AR-induced alterations in VEGF and IL-6 production are a function of altered cell number, we measured proliferation using a fluorescent DNA binding dye. ISO did not alter proliferation at any time point. Figure 6 shows the normalized results at 72 h in culture by MB-231BR (Fig. 6a) or MB-231 (Fig. 6b).
Fig. 6.
β-AR activation does not alter MB-231BR and MB-231 proliferation in vitro. Proliferation of a MB-231BR and b MB-231 was measured at 72 h in culture with and without the non-selective β-AR agonist ISO or the β2-AR selective agonist TERB. Results from individual experimental repetitions were normalized to percent of 0 ISO/TERB and averaged across 2–5 experimental repetitions. One-way ANOVA revealed no significant main effects of drug treatment
Regulation of VEGF and IL-6 production by the adenylate cyclase-cAMP-PKA pathway
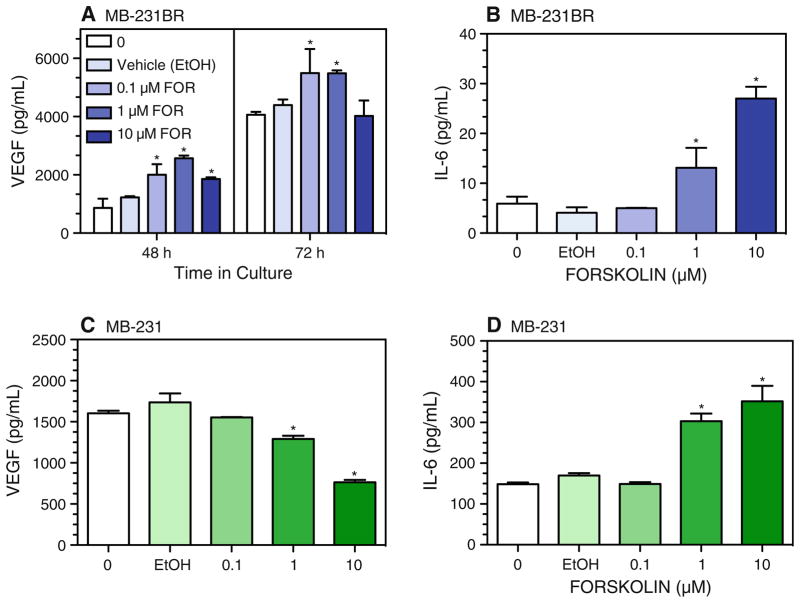
We next tested the role of effectors involved in the classical β-AR-adenylate cyclase-cAMP pathway in the β-AR-mediated VEGF and IL-6 responses. Forskolin directly activates adenylate cyclase to elevate cAMP independently of β-AR. In MB-231BR, forskolin (0.1 and 1 μM) increased VEGF production, but at 10 μM, forskolin-induced VEGF production was attenuated at 48 h and eliminated at 72 h in culture (Fig. 7a). In MB-231, VEGF production was reduced at 48 h (data not shown) and at 72 h (Fig. 7c). In contrast, IL-6 production was increased by forskolin at 48 h (data not shown) and at 72 h in culture in both MB-231BR (Fig. 7b) and MB-231 (Fig. 7d). These forskolin-induced alterations in VEGF and IL-6 production are similar qualitatively to those elicited by β-AR stimulation in both cell lines.
Fig. 7.
Forskolin-induced alterations in VEGF and IL-6 production. MB-231BR (a, b) and MB-231 (c, d) were incubated with varying concentrations of forskolin, the EtOH vehicle for 10 μM forskolin, or medium only for 48 h (a) or 72 h (a–d). VEGF or IL-6 concentration in cell-free supernatants was measured by ELISA. Results are representative of two experimental repetitions per cell line. In (a–d), one-way ANOVA at each time point revealed main effects of drug treatment, P < 0.0001. Asterisks indicate different versus 0 drug at the respective time point by Newman–Keuls post hoc analysis (P < 0.05)
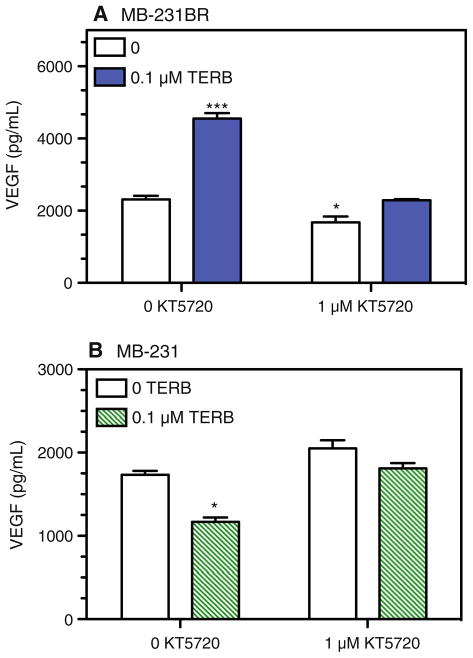
To determine if cAMP activation of PKA plays a role in the β-AR-induced responses in MB-231 and MB-231BR, PKA activity was inhibited by KT 5720, a blocker with high specificity for PKA. KT 5720 (1 μM) completely blocked TERB-induced alterations in VEGF production by MB-231BR (Fig. 8a) and by MB-231 (Fig. 8b). We noted that PKA blockade in the absence of β-AR stimulation slightly inhibited constitutive VEGF production by MB-231BR (Fig. 8a). These results demonstrate a role for β-AR-induced PKA activation in MB-231 and MB-231BR VEGF production.
Fig. 8.
PKA inhibition prevents TERB-induced alterations in VEGF production by MB-231BR and MB-231. a MB-231BR and b MB-231 were stimulated with 0.1 μM TERB. KT 5720 (1 μM) was added 30 min prior to addition of TERB. Supernatants were harvested 72 h after initiation of culture. Results are representative of at least two experimental repetitions. In a and b, significant main effects of drug treatment were analyzed by Newman–Keuls post hoc analysis. In a, *** indicates different versus all other groups, P < 0.001; * indicates group which is significantly different versus 0 KT 5720, 0 TERB group. In b, * indicates group which is significantly different from all other groups, with no other group differences detected
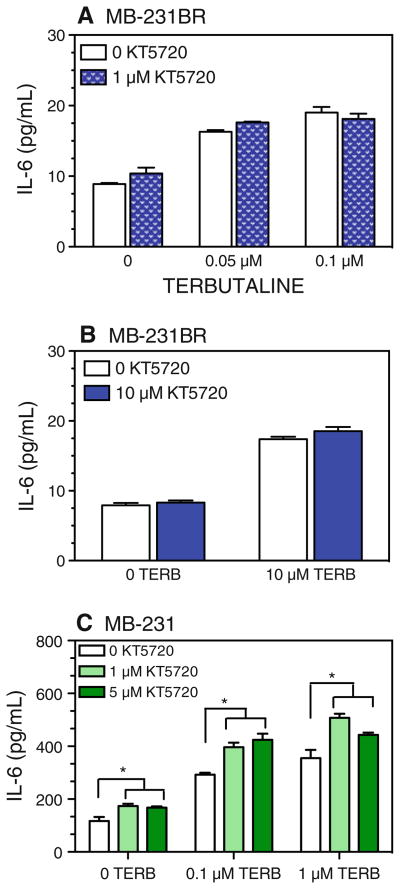
In terms of IL-6 production, KT 5720 did not alter the β-AR-induced increase in IL-6 production by MB-231BR in response to low or high concentrations of TERB (Fig. 9a, b). In MB-231, KT 5720 (≤5 μM) alone increased IL-6 production (Fig. 9c), suggesting that constitutively activated PKA in MB-231 inhibits IL-6 production. In the presence of TERB, increasing concentrations of KT 5720 did not block the response to 0.1 or 1 μM TERB, but instead augmented the TERB-induced increase in IL-6 production by MB-231 (Fig. 9c), the magnitude being similar to the KT 5720-induced enhancement in the absence of TERB. These results suggest that the β-AR-induced increase in IL-6 production is not dependent on PKA activation. Furthermore, it appears that constitutive PKA activation in MB-231 dampens IL-6 production. Together, these results demonstrate that β-AR-induced alterations in constitutive production of VEGF, but not IL-6, are dependent on PKA activation.
Fig. 9.
PKA inhibition does not block TERB-induced IL-6 production by MB-231BR and MB-231. MB-231BR (a, b) and MB-231 (c) were stimulated with varying concentrations of TERB and/or KT 5720. KT 5720 was added 30 min prior to addition of TERB. Supernatants were harvested 72 h after initiation of culture. Results are representative of at least two experimental repetitions for each experimental condition. By two-way ANOVA, in a and b, a main effect of TERB (P < 0.001) was detected with no significant effects of KT 5720 or interaction between TERB and KT 5720. In c, main effects of TERB (P < 0.001) and KT 5720 (P < 0.001), and a significant TERB by KT 5720 interaction (P = 0.0063) were detected. Asterisks indicates significantly different versus the corresponding 0 KT 5720 group by Newman–Keuls post hoc analysis (P < 0.05)
Discussion
Despite the evidence that β-AR expressed by breast, ovarian, and other tumor cell types can modulate tumor growth and metastasis, few reports have evaluated the connection between tumor cell β-AR expression, signaling capacity, and functional capacity in breast cancer. In two human breast adenocarcinoma cell lines (MCF7 and MB-361) with low β-AR expression and signaling capacity, β-AR stimulation elicited little or no change in production of VEGF, a key driver of tumor angiogenesis. In MB-231, a model of aggressive breast cancer, we confirmed high β-AR density [19] and also demonstrated that MB-231BR, a variant of MB-231, retained high β-AR expression. However, the β-AR-induced cAMP response by MB-231 was greater and prolonged compared to MB-231BR. Functionally, β-AR stimulation elicited directionally opposite changes in VEGF production in MB-231 and MB-231BR, while production of IL-6 was increased by β-AR stimulation in both breast cancer cell lines. Elevating cAMP independently of β-AR elicited alterations in VEGF and IL-6 production that mimic the respective responses to β-AR, indicating that cAMP is a key mediator of the functional responses produced by β-AR stimulation. Finally, in MB-231 and MB-231BR, PKA activation was necessary for β-AR-induced alterations in VEGF, but not IL-6 production, indicating that β-AR in MB-231 and MB-231BR regulates VEGF and IL-6 production through divergent pathways with cAMP as a central mediator. These results imply that the impact of direct tumor cell β-AR stimulation, or its antagonism, will vary considerably from individual to individual, depending on tumor cell β-AR density and signaling capacity.
Heterogeneity of β-AR signaling capacity
In all four human breast cancer cell lines, the peak β-AR-induced cAMP response generally reflected β-AR density, demonstrating coupling between β-AR, Gs, and adenylate cyclase. Furthermore, MB-231BR and MB-361 displayed typical β-AR desensitization, with a rapid peak and decline in cAMP production upon exposure to ISO. In MB-231, however, the magnitude and prolonged duration of β-AR-induced cAMP is evidence of impaired β-AR feedback mechanisms. We measured cAMP induction in the presence of the phosphodiesterase inhibitor IBMX, eliminating the possibility that differences in phosphodiesterase expression may contribute to differences in cAMP response kinetics. Preliminary evidence from our laboratory points to impaired ligand-induced β-AR desensitization in MB-231 (data not shown). The prolonged cAMP response in MB-231 compared to MB-231BR has profound functional significance, as discussed below.
β-AR-induced alterations in VEGF production are dependent on β-AR density, cAMP, and PKA activation
In the four breast cancer cell lines, high β-AR density predicted β-AR-induced alterations, but not directionality, in VEGF production. In the high β-AR-expressing cell lines, MB-231 and MB-231BR, we demonstrated that the opposing effects of β-AR activation were mediated by the same adrenergic receptor subtype, the β2-AR. One possible explanation for the differences in the β-AR-induced VEGF responses is divergence from the classical adenylate cyclase-cAMP-PKA pathway as has been described in other cells [6–8]. However, the β-AR-induced alterations in VEGF were mimicked by cAMP elevation and required PKA, implying that effectors downstream of PKA lead to the differential β-AR regulation of VEGF in MB-231 and MB-231BR.
Our working hypothesis is that PKA can phosphorylate multiple substrates with the capacity to either facilitate or inhibit VEGF production in breast cancer cells. The forskolin response in MB-231BR provides evidence for cAMP-mediated fine-tuning of VEGF production, with attenuation of forskolin-induced VEGF production at high-forskolin concentrations (Fig. 7a). Note, however, that such an inhibitory mechanism does not appear to be active with high concentrations of TERB in MB-231BR, perhaps due to the ability of MB-231BR to rapidly down regulate β-AR-induced cAMP production (Fig. 2a). We speculate that the cAMP inhibitory pathway in MB-231 predominates due to the inability to down regulate β-AR-induced cAMP production. Elucidation of the molecular pathways involved in fine-tuning VEGF synthesis by breast tumor cells specifically identification of PKA substrates responsible for regulating VEGF in MB-231 and MB-231BR may yield new therapeutic targets to inhibit tumor cell VEGF production and subsequent tumor angiogenesis. This is particularly important for aggressive breast cancer phenotypes, such as the “triple negative” phenotype that MB-231 models, for which effective therapies are limited.
It is interesting to speculate that the capacity for β-AR stimulation to elevate VEGF production by MB-231BR may have contributed to the selection pressure that produced this brain-seeking variant of MB-231. However, MB-361, isolated from a human brain metastasis, exhibits very low β-AR expression, a correspondingly low cAMP response to β-AR stimulation, and no change in VEGF production with β-AR stimulation. Therefore, it is apparent that β-AR-induced VEGF production observed in MB-231BR is not a general feature of breast adenocarcinomas isolated from the brain. However, since both MB-231 and MB-231BR express high levels of β-AR and are metastatic in mice, activation of high-density β-AR expressed by breast cancer cells may represent a mechanism to promote metastasis to secondary sites in breast cancer.
β-AR-induced IL-6 production in MB-231 and MB-231BR is dependent on cAMP, but independent of PKA activation
IL-6 has been shown to participate in the epithelial–mesenchymal transition in human breast cancer cells and is associated with advanced breast tumor stage and poor prognosis [17, 18, 20, 21]. β-AR-induced IL-6 production has been observed in a variety of normal cell types [8, 22] and tumor cell types [10, 23, 24], even in the absence of a pro-inflammatory stimulus [6]. IL-6 production by MB-361 and MCF7 was undetectable under our culture conditions, and β-AR stimulation did not induce IL-6 production in these cell lines (data not shown). MB-231 and MB-231BR produce detectable IL-6. Its production in both cell lines is elevated by β2-AR stimulation, and the effect is mimicked by a non-βAR-induced elevation of cAMP, similar to VEGF. However, unlike VEGF, PKA inhibition did not significantly block β-AR-induced IL-6 production, indicative of a largely PKA-independent response to β-AR stimulation. The cAMP pathway driving IL-6 production also appears to lack an inhibitory component in response to high-cAMP concentrations, unlike VEGF [for example, compare the forskolin-induced IL-6 response to VEGF in MB-231BR (Fig. 7a vs. b)]. These results are indicative of a divergence in the β-AR-induced pathway immediately downstream of cAMP.
We also noted that in the absence of β-AR stimulation, PKA inhibition increased IL-6 production in MB-231, but not in MB-231BR (Fig. 9), suggesting that constitutive PKA activation in MB-231 may inhibit IL-6 production. A low level of constitutive PKA activation is consistent with the elevated basal cAMP detected in MB-231 (Fig. 1b). Understanding the intracellular pathway underlying the IL-6 response to β-AR stimulation is critical to blocking production of this proinflammatory cytokine to inhibit tumor development.
Increased β-AR VEGF and IL-6 production is not associated with increased cellular proliferation
β-AR activation did not alter MB-231 or MB-231BR proliferation in vitro. Other investigators have shown reduced MB-231 proliferation with exposure to β-AR agonists in vitro [19, 25]. In our hands, MB-231 proliferation was inhibited 10–15% at the highest concentration of ISO tested (10 μM), but this effect was not blocked by βAR antagonists (data not shown). Carie and Sebti, utilizing an atypical β-AR agonist (ARA-211) reported reduced MB-231 proliferation in the presence of ARA-211, and this effect was blocked by a β-blocker [25]. Interestingly, ARA-211 administration in vivo strongly inhibited tumor growth, and even induced tumor regression, but the β-AR agonist ISO was much less effective. Functional differences between agonists may indicate that signal strength or spatial/temporal aspects of the cAMP response may also contribute to functional heterogeneity to β-AR stimulation in breast cancer cells.
Our inability to demonstrate increased β-AR-induced VEGF production in vitro by MB-231 was unexpected. Thaker et al. showed that chronic stress exposure increased VEGF production in vivo in MB-231 tumors through a β-AR-mediated mechanism [1], but a direct effect of tumor cell β-AR stimulation in vivo was not tested with MB-231, leaving open the possible involvement of VEGF-expressing stromal cells (such as macrophages) in the stress response. In the context of primary breast tumors, β-AR-expressing cells of the tumor stroma may also contribute to β-AR-induced alterations in tumor pathogenesis and most likely involve multiple mechanisms, including altered angiogenesis and/or immunosuppression in vivo [3]. If breast tumor cells express no or low β-AR, as shown here with MCF7 and MB-361, β-AR-mediated alterations in tumor pathogenesis may still be expected. Our in vitro results demonstrate the potential for direct β-AR stimulation of high β-AR-expressing tumor cells altering proangiogenic factor production in vivo. Our goals are to determine the role of β-AR stimulation in breast tumor pathogenesis in vivo and to determine the relative contribution of the variety of β-AR-expressing cells to β-AR stimulation in primary breast tumors.
Conclusions
The existence of a β-AR-driven proangiogenic pathway in tumor cells suggests that pharmacological β-AR blockade, commonly used in the treatment of cardiovascular disease, may effectively block undesirable effects of stress exposure and subsequent release of NE. In order to pursue β-AR blockade as a therapeutic option, it is important to understand the ubiquity of β-AR expression, signaling, and function in breast tumors. We have demonstrated heterogeneity in β-AR expression and signaling capacity, and only in the high β-AR-expressing cell lines did the endogenous neurotransmitter NE markedly alter VEGF and IL-6 production. Furthermore, in high β-AR expressing cell lines, differences in β-AR signaling capacity can lead to disparate functional effects, especially with regard to VEGF production. These results suggest that blocking β-AR on tumor cells may reduce stress-induced production of proangiogenic factors, but caution must be used in employing β-AR blockers. For instances, depending on β-AR density and signaling capacity, NE release may inhibit tumor cell VEGF production, and therefore β-AR blockade may increase VEGF production. Future studies will explore the response of a variety of breast cancer types to β-AR stimulation in vivo so that more accurate predictions can be made regarding responsiveness of breast tumors to β-AR stimulation and the potential impact of β-AR blockade.
Acknowledgments
This work was supported by Department of Defense IDEA Award (W81XWH-10-01-008), National Institutes of Health (1 R21 CA152777-01), and by the Breast Cancer Coalition of Rochester to KSM, Department of Defense Era of Hope Scholar Research Award (W81XWH-09-1-0405), National Institutes of Health Director’s New Innovator Award (1 DP2 OD006501-01), and Pew Scholar in the Biomedical Sciences Award to EBB, and Department of Defense Predoctoral Training Award (W81XWH-10-1-0058) to MJS. MJS is a trainee in the Medical Scientist Training Program funded by NIH T32 GM07356. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or National Institutes of Health. We thank Kathryn Fitzgerald, Khawarl Liverpool, and Michael Storonsky for their excellent technical assistance.
Abbreviations
- β-AR
Beta-adrenergic receptor(s)
- cAMP
3′,5′-Cyclic adenosine monophosphate
- HBSS
Hank’s balanced salt solution
- BSA
Bovine serum albumin
- FCS
Fetal calf serum
- IBMX
Isobutylmethylxanthine
- ICI
ICI 118,551 hydrochloride
- 125ICYP
125I-cyanopindolol
- IL-6
Interleukin 6
- ISO
Isoproterenol
- NE
Norepinephrine
- PKA
Protein kinase A
- TERB
Terbutaline
- VEGF
Vascular endothelial growth factor
- ANOVA
Analysis of variance
Contributor Information
Kelley S. Madden, Email: Kelley_Madden@urmc.rochester.edu, Department of Biomedical Engineering, University of Rochester Medical Center, Goergen Hall, RC Box 270168, Rochester, NY 14627, USA
Mercedes J. Szpunar, Email: Mercedes_Szpunar@urmc.rochester.edu, Department of Pathology, University of Rochester Medical Center, 601 Elmwood Ave., Rochester, NY 14642, USA
Edward B. Brown, Email: Edward_Brown@urmc.rochester.edu, Department of Biomedical Engineering, University of Rochester Medical Center, Goergen Hall, RC Box 270168, Rochester, NY 14627, USA
References
- 1.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 2.Raju B, Haug SR, Ibrahim SO, Heyeraas KJ. Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience. 2007;149(3):715–725. doi: 10.1016/j.neuroscience.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 3.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tang-kanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 5.Shakhar G, Ben-Eliyahu S. In vivo β-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- 6.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19(2):251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Pullar CE, Isseroff RR. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119(Pt 3):592–602. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 8.Yin F, Wang YY, Du JH, Li C, Lu ZZ, Han C, Zhang YY. Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol. 2006;40(3):384–393. doi: 10.1016/j.yjmcc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Weil J, Benndorf R, Fredersdorf S, Griese DP, Eschenhagen T. Norepinephrine upregulates vascular endothelial growth factor in rat cardiac myocytes by a paracrine mechanism. Angiogenesis. 2003;6(4):303–309. doi: 10.1023/B:AGEN.0000029411.76494.33. [DOI] [PubMed] [Google Scholar]
- 10.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23(2):267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredriksson JM, Lindquist JM, Bronnikov GE, Nedergaard J. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta-adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J Biol Chem. 2000;275(18):13802–13811. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- 12.Banfi C, Cavalca V, Veglia F, Brioschi M, Barcella S, Mussoni L, Boccotti L, Tremoli E, Biglioli P, Agostoni P. Neurohormonal activation is associated with increased levels of plasma matrix metalloproteinase-2 in human heart failure. Eur Heart J. 2005;26(5):481–488. doi: 10.1093/eurheartj/ehi073. [DOI] [PubMed] [Google Scholar]
- 13.Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9(12):4514–4521. [PubMed] [Google Scholar]
- 14.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16(8):1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 15.Engel G, Hoyer D, Berthold R, Wagner H. (±)[125Iodo]cyanopindolol, a new ligand for β-adrenoceptors: identification and quantification of subclasses of β-adrenoceptors in guinea pig. Naun Schmiedebergs Arch Pharmacol. 1981;317:277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- 16.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006. 10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. IL-6 triggers malignant features in mammo-spheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer. 2007;110(9):1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 19.Slotkin TA, Zhang J, Dancel R, Garcia SJ, Willis C, Seidler FJ. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2000;60(2):153–166. doi: 10.1023/a:1006338232150. [DOI] [PubMed] [Google Scholar]
- 20.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103(5):642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohrbach S, Engelhardt S, Lohse MJ, Werdan K, Holtz J, Muller-Werdan U. Activation of AP-1 contributes to the beta-adrenoceptor-mediated myocardial induction of interleukin-6. Mol Med. 2007;13(11–12):605–614. doi: 10.2119/2007-00071.Rohrbach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, Jennings N, Arevalo J, Lutgendorf SK, Gallick GE, Sanguino AM, Lopez-Berestein G, Cole SW, Sood AK. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282(41):29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65(23):10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carie AE, Sebti SM. A chemical biology approach identifies a beta-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2 pathway. Oncogene. 2007;26(26):3777–3788. doi: 10.1038/sj.onc.1210172. [DOI] [PubMed] [Google Scholar]