Abstract
Background
Azithromycin is a macrolide antibiotic that is active against several periodontal pathogens. Macrolides are taken up and concentrated inside gingival fibroblasts, which could influence their pharmacokinetics. This study tested the hypothesis that steady-state levels of azithromycin are higher and more sustained in gingival crevicular fluid (GCF) than in serum.
Methods
Four healthy subjects received an initial dose of 500 mg azithromycin followed by 250 mg doses on each of the next 2 days. Serum and GCF samples were obtained 2 hours after the last dose (day 2) and on days 4 and 7. GCF samples were collected from maxillary posterior sites with paper strips. The strips were pooled and eluted with high purity water. After extraction, the azithromycin content of the serum samples and GCF eluates was determined with an agar diffusion bioassay.
Results
On days 2, 4 and 7, the concentrations of azithromycin in blood serum were 0.22 ± 0.02, 0.08 ± 0.02 and 0.04 ± 0.01 μg/ml, respectively. The concentrations in GCF were 8.82 ± 1.25, 7.90 ± 1.72 and 7.38 ± 1.15 μg/ml, respectively. Mean GCF levels were significantly higher than mean serum levels (P ≤ 0.02, paired t-test).
Conclusion
The findings demonstrate that the pharmacokinetic profiles of azithromycin are different in GCF and serum. At steady state, azithromycin concentrations in GCF were higher and more sustained than those in serum. Based on previous studies, the levels observed in GCF were above the MIC for Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia.
Keywords: Anti-infective agents, Periodontitis, Tissue distribution
INTRODUCTION
Elimination of bacterial plaque from root surfaces is a major objective of periodontal therapy. Although this usually halts progression of attachment loss, this is a challenging goal in patients who are infected by invasive subgingival bacteria. Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia, which can invade pocket epithelium,1–4 are particularly difficult to eliminate by scaling and root planing. Use of adjunctive antibiotics is a logical approach for eradicating these pathogens and enhancing the response to scaling and root planing.5 Azithromycin is a macrolide antibiotic that inhibits a variety of subgingival bacteria.6–9 Clarithromycin, a closely related macrolide, is actively transported and concentrated inside oral epithelial cells and gingival fibroblasts.10 Antibiotic uptake by host cells could provide several benefits in the treatment of periodontitis. Elevated macrolide concentrations inside oral epithelial cells could facilitate the killing of invasive pathogens. Since gingival fibroblasts are a relatively large cellular compartment of the gingiva,11 macrolide accumulation by these cells could allow them to function as drug reservoirs that enhance and sustain therapeutic concentrations at that site. Previous studies have shown that azithromycin and clarithromycin can attain higher steady-state levels in gingival tissue than in serum and suggest that tissue levels are increased in the presence of inflammation.12,13 Azithromycin concentrations in gingiva reportedly persist for up to 14 days after systemic administration.14
Gingival crevicular fluid (GCF) originates from the vessels of the gingival plexus, within the gingival connective tissue. It seeps through gingival connective tissue and passes through the junctional epithelium prior to entering the gingival crevice.11 Following systemic administration, we hypothesize that steady-state azithromycin concentrations in GCF are higher and more sustained than the corresponding concentrations in blood. The present study tested this hypothesis, using methods adapted to work with small samples of GCF.
MATERIALS AND METHODS
Subject population
Four healthy adult volunteers (3 males and one female, with a mean age of 30 years) with no clinical periodontal attachment loss were recruited from the student population of the Ohio State University College of Dentistry. Written informed consent was obtained prior to their participation. The study protocol and subject recruitment procedures were reviewed and approved by the Institutional Review Board at the Ohio State University.
Study design
One week prior to administration of azithromycin, all participants had their teeth cleaned and received oral hygiene instruction. To obtain steady-state levels of azithromycin in periodontal tissues, participants were given an initial dose of 500 mg with subsequent 250 mg doses at 24 and 48 hours. Using an established protocol,15 GCF samples were collected and pooled from twelve maxillary posterior interproximal sites (the mesiofacial and mesiolingual aspects of all maxillary first and second premolars and first molars) immediately before the first dose of azithromycin (day 0), two hours after the last dose on day 2, and on days 4 and 7 after the initial dose. These sites were selected because they were readily accessible and easy to protect from contamination by saliva. Prior to GCF collection, the collection sites were isolated with cotton rolls, supragingival plaque was removed (if present), and the site was gently dried with air. GCF was collected with paper strips‡ positioned at the orifice of the crevice for 30 seconds. GCF volumes were determined with a calibrated gingival fluid measurement device§ and pooled. The Gingival Index (GI)16 and Plaque Index (PI)17 were assessed at the collection sites at every study visit. In addition, blood samples were obtained by venipuncture on days 2, 4 and 7. Blood serum and pooled GCF samples were stored at −20°C in sealed vials.
Sample analysis
Prior to analysis, GCF was eluted from the pools of paper strips with 200 μl of ultrapure water, using a method previously described.15 The efficiency of elution, as assessed with [3H]-labeled macrolide,|| was 67.4% ± 2.1% (data not shown). GCF eluates and blood serum samples (200 μl) were treated with 40 μl of 0.5 g/ml Na2CO3 and extracted three times with 1 ml diethyl ether. The extracts were dried, reconstituted in acetonitrile, and applied to sterile paper disks.¶ After complete evaporation of the acetonitrile, the azithromycin content of the disks was determined with an agar diffusion bioassay, using Kocuria rhizophila# as the indicator organism.14 The assay was calibrated with 2 to 18 ng of authentic azithromycin.** For GCF samples, calculations for azithromycin content incorporated a correction for the observed elution efficiency.
Statistical analysis
The paired t-test was used to evaluate differences in azithromycin concentration in GCF and blood. Based on a projected difference of 4 μg/ml in the mean azithromycin concentrations in GCF and blood serum and a pooled standard deviation of 1.5, the estimated number of subjects required to achieve a power of 0.80 with an alpha of 0.05 in a paired t-test was 4. Repeated measures analysis of variance (ANOVA) was used to examine the statistical significance in changes in azithromycin concentration, GCF azithromycin content and GCF volume over the course of the study. The Holm-Sidak test was used for post-hoc comparisons. In all statistical analyses, the statistical unit was the subject rather than the site.
RESULTS
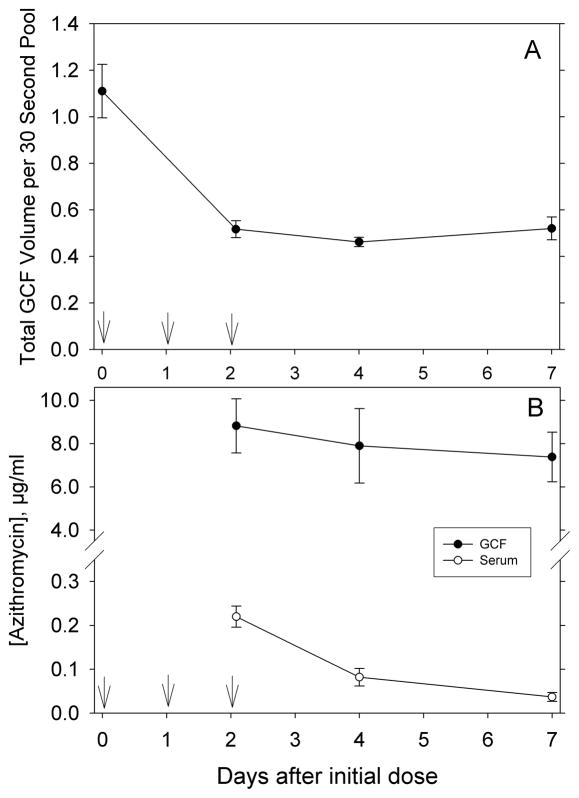
Consistent with maintenance of gingival health, the median PI and GI values were 0 throughout the course of the study (Table 1). After administration of azithromycin, there was a persistent and statistically significant reduction in the pooled volume of GCF collected from the study sites (P < 0.001, repeated measures ANOVA, power of test = 0.99, Figure 1A). The pooled volumes on days 2, 4 and 7 were significantly lower than the baseline level (P < 0.05, Holm-Sidak test). On day 2, the azithromycin concentrations in GCF and blood serum were 8.82 ± 1.25 and 0.22 ± 0.02 μg/ml, respectively. Over the next five days, these concentrations decreased to 7.38 ± 1.15 and 0.04 ± 0.01 μg/ml, respectively (Figure 1B). Although concentrations in GCF did not decrease significantly between day 2 and day 7, there was a significant decrease in serum concentrations on days 4 and 7 (P < 0.05, Holm-Sidak test). Mean GCF levels were significantly higher than mean serum levels on days 2, 4 and 7 (P ≤ 0.02, paired t-test, power of tests ≥ 0.84). The rate of azithromycin infusion into the gingival crevice, as assessed by the amount recovered per 30 second pooled GCF sample, did not change significantly between days 2 and 7 (Table 1).
Table 1.
Clinical and Pharmacological Observations During the Study
| Days After Initial Azithromycin Dose | Gingival Index* | Plaque Index* | GCF Azithromycin Content (ng per 30 second pooled sample)† |
|---|---|---|---|
| 0 | 0 (0 to 0) | 0 (0 to 0) | Not determined |
| 2 | 0 (0 to 0) | 0 (0 to 0) | 3.63 ± 0.39 |
| 4 | 0 (0 to 0) | 0 (0 to 0) | 3.41 ± 0.53 |
| 7 | 0 (0 to 0) | 0 (0 to 0) | 3.26 ± 0.56 |
Data are presented as *median and range observed in 4 subjects or †mean ± SEM. Within each column, there were no statistically significant differences between the observed values.
Figure 1.
Changes in GCF volume and azithromycin concentrations observed during the study. Vertical arrows indicate times when azithromycin was administered. A: Gingival crevicular fluid volumes collected during the study. The data represent the mean (± SEM) pooled GCF volume collected for 30 seconds from 12 maxillary premolar and first molar sites. B: Azithromycin concentrations in GCF and blood serum. The mean concentrations observed in GCF were significantly higher than those in serum on days 2, 4 and 7 (P ≤ 0.02, paired t-test).
DISCUSSION
The findings support the hypothesis that steady-state azithromycin concentrations in GCF are significantly higher and more sustained than those in serum. Azithromycin concentrations in GCF were more than 40-fold higher throughout the course of the study, and they decreased at a slower rate than the levels in serum. Azithromycin levels in GCF decreased by approximately 20% between the second and seventh days, while the levels in serum decreased by 80% during the same period. This may be attributed to the low degree of azithromycin binding to plasma proteins in combination with active accumulation of azithromycin by cells in peripheral tissues.18,19 Gingival connective tissue contains a large volume of fibroblasts, which could serve as reservoirs for maintaining high local levels of azithromycin. Oral epithelial cells and polymorphonuclear leukocytes may also accumulate azithromycin at this site.10, 20 Our findings are consistent with a previous report that azithromycin concentrations in gingival tissue are up to 25-fold higher than the corresponding concentrations in blood.21 Macrolides are not the only antimicrobial agents that have a propensity to concentrate in GCF. Tetracyclines (e.g., doxycycline) and fluoroquinolones (e.g., ciprofloxacin) reportedly attain GCF concentrations that are several-fold higher than their concentrations in serum.15, 22–24 The pharmacological properties of all these agents are presumably influenced by their ability to be taken up, sequestered and released by fibroblasts, leukocytes, and other types of cells.10, 20
Azithromycin concentrations observed in GCF are substantially higher than the minimal inhibitory concentration (MIC) previously reported for several periodontal pathogens, including Aggregatibactor actinomycetemcomitans (0.25–2.0 μg/ml),6 Porphyromonas gingivalis (0.125–1 μg/ml),7, 8 Prevotella intermedia (0.03–1 μg/ml), and Peptostreptococcus micros (0.5–1 μg/ml).8 Moreover, azithromycin concentrations remained above the MICs for these pathogens over the entire five day observation period. While many antibiotics can be classified as having either a concentration dependent killing effect or a time dependent killing effect, bacterial killing by azithromycin is not solely dependent on either model.25 The time that target organisms are exposed to azithromycin concentrations above the MIC appears to be the best index of efficacy.26 Azithromycin also exhibits a prolonged post-antibiotic effect on inhibition of bacterial regrowth.26 Thus, pathogens found within periodontal tissues or in periodontal pockets that have been treated to disrupt subgingival biofilm should be vulnerable to inhibition by azithromycin. Pathogens living in native subgingival biofilm could be more difficult to inhibit at the azithromycin concentrations observed in this study. However, studies with an in vitro periodontal biofilm model suggest that azithromycin can penetrate the biofilm surface and partially dissolve the biofilm.27 Azithromycin also appears to dissolve biofilm associated with diffuse panbronchiolitis.28
A limited number of randomized, placebo-controlled clinical trials have suggested that azithromycin is a useful adjunct to scaling and root planing (SRP) in the treatment of periodontitis. In a study of patients with aggressive periodontitis, the combination of SRP plus azithromycin resulted in a higher percentage of teeth with attachment gain ≥ 1 mm and a greater reduction in probing depths than SRP alone.29 In patients with chronic periodontitis, the combination of SRP plus azithromycin yielded a significantly greater reduction of probing depths for pockets initially ≥4 mm than SRP alone.30 A later study of non-surgical treatment of chronic periodontitis in smokers also demonstrated that adjunctive use of azithromycin with SRP resulted in enhanced pocket depth reduction and clinical attachment gain at moderate (4 to 6 mm) and deep (>6 mm) periodontal sites when compared to SRP alone.31 Moreover, a recent study of subjects with Porphyromonas gingivalis-associated chronic periodontitis demonstrated that, when compared with SRP alone, SRP combined with azithromycin yielded significantly enhanced pocket depth reduction and attachment gain and a significant decrease in the detection of Porphyromonas gingivalis.32
Due to their anti-inflammatory activity, macrolides have been used in immunomodulatory therapy for chronic inflammatory lung diseases.33 Azithromycin also appears to produce anti-inflammatory effects in gingiva, as evidenced by its ability to reduce GCF volume and the GCF content of pro-inflammatory cytokines IL-1β, IL-8 and TNF-α.34 The mechanism for these effects appears to involve modulation of nuclear factor-κB (NF-κB) and activator protein-1 (AP-1).35 Since GCF volume is strongly correlated with histological signs of gingival inflammation,36 the significant reduction of GCF volume observed between days 2 and 7 in this study is consistent with the previous report. The apparent anti-inflammatory effects of azithromycin could represent an additional benefit when applied to treatment of inflammatory periodontal diseases. In conclusion, the results demonstrate that systemic administration of azithromycin produces relatively high and sustained levels in GCF and provide a rationale for further clinical evaluation of its adjunctive benefits in the treatment of periodontitis.
Acknowledgments
Source of Support: NIDCR grant R21 DE018804 to John D. Walters
This study was supported by USPHS research grant R21 DE018804 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Periopaper™, Oraflow, Smithtown, NY
Periotron 6000, IDE Interstate, Amityville, NY
American Radiolabeled Chemicals, St. Louis, MO
BD Biosciences, Sparks, MD
ATCC 9341, American Type Culture Collection, Manassas, VA
US Pharmacoepeia, Rockville, MD
The authors have no conflicts of interest to declare.
References
- 1.Christersson LA, Albini B, Zambon JJ, Wikesjo UM, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- 2.Duncan MJ, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorn BR, Leung KL, Progulske-Fox A. Invasion of human oral epithelial cells by Prevotella intermedia. Infect Immun. 1998;66:6054–6057. doi: 10.1128/iai.66.12.6054-6057.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro activity of azithromycin compared with that of erythromycin against Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1992;36:1241–1243. doi: 10.1128/aac.36.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajukanta R. In vitro antimicrobial susceptibility of Porphyromonas gingivalis to azithromycin, a novel macrolide. Oral Microbiol Immunol. 1993;8:325–326. doi: 10.1111/j.1399-302x.1993.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein EJ, Citron DM, Merriam CV, Warren Y, Tyrrell K. Activities of telithromycin (HMR 3647, RU 66647) compared to those of erythromycin, azithromycin, clarithromycin, roxithromycin, and other antimicrobial agents against unusual anaerobes. Antimicrob Agents Chemother. 1999;43:2801–2805. doi: 10.1128/aac.43.11.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sefton AM, Maskell JP, Beighton D, et al. Azithromycin in the treatment of periodontal disease. Effect on microbial flora. J Clin Periodontol. 1996;23:998–1003. doi: 10.1111/j.1600-051x.1996.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 10.Chou CH, Walters JD. Clarithromycin transport by gingival fibroblasts and epithelial cells. J Dent Res. 2008;87:777–781. doi: 10.1177/154405910808700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 12.Burrell RC, Walters JD. Distribution of systemic clarithromycin to gingiva. J Periodontol. 2008;79:1712–1718. doi: 10.1902/jop.2008.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blandizzi C, Malizia T, Lupetti A, et al. Periodontal tissue disposition of azithromycin in patients affected by chronic inflammatory periodontal diseases. J Periodontol. 1999;70:960–966. doi: 10.1902/jop.1999.70.9.960. [DOI] [PubMed] [Google Scholar]
- 14.Gomi K, Yashima A, Iino F, et al. Drug concentration in inflamed periodontal tissues after systemically administered azithromycin. J Periodontol. 2007;78:918–923. doi: 10.1902/jop.2007.060246. [DOI] [PubMed] [Google Scholar]
- 15.Conway TB, Beck FM, Walters JD. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J Periodontol. 2000;71:1448–1452. doi: 10.1902/jop.2000.71.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Silness J, Loe H. Periodontal Disease in Pregnancy. Ii. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 18.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 19.Gladue RP, Snider ME. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob Agents Chemother. 1990;34:1056–1060. doi: 10.1128/aac.34.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladue RP, Bright GM, Isaacson RE, Newborg MF. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989;33:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malizia T, Tejada MR, Ghelardi E, et al. Periodontal tissue disposition of azithromycin. J Periodontol. 1997;68:1206–1209. doi: 10.1902/jop.1997.68.12.1206. [DOI] [PubMed] [Google Scholar]
- 22.Gordon JM, Walker CB, Murphy JC, Goodson JM, Socransky SS. Tetracycline: levels achievable in gingival crevice fluid and in vitro effect on subgingival organisms. Part I. Concentrations in crevicular fluid after repeated doses. J Periodontol. 1981;52:609–612. doi: 10.1902/jop.1981.52.10.609. [DOI] [PubMed] [Google Scholar]
- 23.Pascale D, Gordon J, Lamster I, Mann P, Seiger M, Arndt W. Concentration of doxycycline in human gingival fluid. J Clin Periodontol. 1986;13:841–844. doi: 10.1111/j.1600-051x.1986.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 24.Lavda M, Clausnitzer CE, Walters JD. Distribution of systemic ciprofloxacin and doxycycline to gingiva and gingival crevicular fluid. J Periodontol. 2004;75:1663–1667. doi: 10.1902/jop.2004.75.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain R, Danziger LH. The macrolide antibiotics: a pharmacokinetic and pharmacodynamic overview. Curr Pharm Des. 2004;10:3045–3053. doi: 10.2174/1381612043383322. [DOI] [PubMed] [Google Scholar]
- 26.Van Bambeke F, Tulkens PM. Macrolides: pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents. 2001;18 (Suppl 1):S17–23. doi: 10.1016/s0924-8579(01)00406-x. [DOI] [PubMed] [Google Scholar]
- 27.Tamura A, Ara T, Imamura Y, Fujii T, Wang PL. The effects of antibiotics on in vitro biofilm model of periodontal disease. Eur J Med Res. 2008;13:439–445. [PubMed] [Google Scholar]
- 28.Nagino K, Kobayashi H. Influence of macrolides on mucoid alginate biosynthetic enzyme from Pseudomonas aeruginosa. Clin Microbiol Infect. 1997;3:432–439. doi: 10.1111/j.1469-0691.1997.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 29.Haas AN, de Castro GD, Moreno T, et al. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35:696–704. doi: 10.1111/j.1600-051X.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith SR, Foyle DM, Daniels J, et al. A double-blind placebo-controlled trial of azithromycin as an adjunct to non-surgical treatment of periodontitis in adults: clinical results. J Clin Periodontol. 2002;29:54–61. doi: 10.1034/j.1600-051x.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 31.Mascarenhas P, Gapski R, Al-Shammari K, et al. Clinical response of azithromycin as an adjunct to non-surgical periodontal therapy in smokers. J Periodontol. 2005;76:426–436. doi: 10.1902/jop.2005.76.3.426. [DOI] [PubMed] [Google Scholar]
- 32.Oteo A, Herrera D, Figuero E, O’Connor A, Gonzalez I, Sanz M. Azithromycin as an adjunct to scaling and root planing in the treatment of Porphyromonas gingivalis-associated periodontitis: a pilot study. J Clin Periodontol. 37:1005–1015. doi: 10.1111/j.1600-051X.2010.01607.x. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Boado YS, Rubin BK. Macrolides as immunomodulatory medications for the therapy of chronic lung diseases. Curr Opin Pharmacol. 2008;8:286–291. doi: 10.1016/j.coph.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Ho W, Eubank T, Leblebicioglu B, Marsh C, Walters J. Azithromycin decreases crevicular fluid volume and mediator content. J Dent Res. 2010;89:831–835. doi: 10.1177/0022034510368650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350:977–982. doi: 10.1016/j.bbrc.2006.09.132. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]