Abstract
Hypothesis
Self-reported use of complementary and alternative medicine (CAM) has been shown to increase following a cancer diagnosis, and breast cancer survivors are the heaviest users among cancer survivors. The aim of this study was to determine whether the prevalence estimate of CAM use varied according to classification of CAM. We used a comprehensive system to classify CAM users and test differences in demographic, lifestyle, quality of life, and cancer characteristics among them.
Study Design and Methods
Participants were 2562 breast cancer survivors participating in the Women's Healthy Eating and Living (WHEL) Study, aged 28-74 years. A structured telephone interview assessed CAM use, questioning about specific CAM practices, and whether use was related to cancer. We examined CAM use in relation to demographics, health behaviors, and quality of life.
Results
Approximately 80% of the women used CAM for general purposes but only 50% reported CAM use for cancer purposes. Visual imagery, spiritual healing, and meditation were the most frequently used practices for cancer purposes. CAM use, defined as consulting a CAM practitioner and regular use, was significantly related to younger age, higher education, increased fruit & vegetable intake, and lower body mass index (p < .05). CAM users who had seen a practitioner were also more likely to report poor physical and mental health than non-CAM users (p < .05). CAM use was not associated with changes in physical and mental health between study baseline and 1-year follow-up.
Conclusion
This study addressed important differences in the classification of CAM use among breast cancer survivors. Future studies need to further test the potential benefits and risks associated with CAM use.
INTRODUCTION
Complementary and alternative medicine (CAM) use has grown dramatically and is commonly practiced among cancer patients, but the study of CAM use remains challenged by inconsistencies in methodology across research studies. The National Center for Complementary and Alternative Medicine (NCCAM) has defined CAM as a group of diverse medical systems, practices, and products that are not presently considered to be part of conventional medicine1. The majority of the literature has examined CAM use broadly, simply categorizing breast cancer patients as CAM users or nonusers2-7. This binary classification lacks the specificity necessary to inform either future research or clinical practice8 since all CAM users are considered to be similar to one another. This method could introduce bias into CAM research, as CAM use estimates range from 16.5% to 84.0% in breast cancer patients2, 9. Studies that use this binary measure cannot account for variation among CAM users in the purpose of use (cancer versus general purposes), the frequency or duration of use, or the method of administration (self versus practitioner) 2-7. Further, correlates of CAM use differ when CAM use is classified in a binary method versus examining each therapy separately10-11. The binary method showed that CAM users are younger, female, and better educated than nonusers 12-15. However, studies that have examined CAM practices individually found that significant correlates varied by CAM modality, and that younger age was the only consistent correlate10. It has also been suggested that the predictors of CAM use for cancer purposes may be different than those for general CAM use 9. In addition, it is important for clinicians to understand and distinguish the different types of CAM users in order to identify any potential interactions there may be between conventional and CAM treatments, 16 or to communicate with patients who may be considering using a CAM therapy in place of conventional treatments 17-18.
A recent study has proposed a model to classify an individual's total exposure level to CAM; the model considers the modality, whether a CAM practitioner had been consulted, and the frequency of the consultation 19. This system may provide a more valid assessment of CAM use because it addresses the divergent classifications of CAM and allows for the inclusion of various CAM treatments across different population. Further, the model distinguishes between patients who only have used self-help CAM modalities or just a casual visit and those patients who have employed a comprehensive course of CAM treatments involving a CAM practitioner.
The present study investigates CAM use in 2562 breast cancer survivors participating in the Women's Healthy Eating and Living (WHEL) Study 20. We use a comprehensive classification system, based on the pattern of use, to estimate the prevalence of CAM use: to examine any differences in demographic, lifestyle, and cancer characteristics among CAM users; and to determine whether breast cancer survivors with low quality of life were more likely to use CAM and whether use of CAM was associated with improvements in quality of life.
METHODS
Participants
This project was part of a large multi-site clinical trial investigating the efficacy of diet intervention to reduce risk for breast cancer recurrence (the WHEL Study). The WHEL Study enrolled 3088 participants at seven clinical sites between 1995 and 2000: Institutional Review Boards approved the protocol, and participants provided written, informed consent. Details of the study protocol are described elsewhere 21. Major eligibility criteria included diagnosis within the past 4 years of primary operable invasive stage I (≥ 1 cm), II, or IIIA breast carcinoma 22; age 18-70 years at diagnosis; no current or planned chemotherapy; no evidence of recurrent disease or new breast cancer since completion of initial treatment; and no other cancer in the past 10 years. The intervention promoted a diet rich in vegetables, fruit, and fiber and low in fat; and did not address the use of CAM such as dietary supplements (neither encouraged nor restricted) 20. This secondary data analysis only includes the WHEL participants who completed the study's CAM assessment (n=2562) an average of 5 years after study enrollment. There were no significant sample differences between those who completed the CAM assessment and the entire WHEL sample.
Measures
CAM usage
The CAM assessment was administered over the telephone between January 2003 and May 2004. The assessment followed the NCCAM domain structure, listing 16 popular CAM practices (Table 1), and provided an opportunity for participants to also report additional CAM practices. Interviewers asked whether each CAM practice had ever been used. A positive response elicited these follow-up questions: purpose for use (cancer versus non-cancer); when it was used (during or after cancer therapy); the frequency of use (occasional versus regular); whether they consulted a CAM practitioner; and whether they discussed CAM use with a physician.
Table 1.
Prevalence of self-reported CAM use for both cancer and non-cancer purposes among a cohort of 2562 breast cancer survivors enrolled in the WHEL Study.
| Ever Used CAM | Purpose of Use | ||
|---|---|---|---|
| CAM Types | % Users, N (n=2047) | % Cancera, N (n=1265) | % Non-Cancer, N (n=782) |
| Alternative Medicine System | |||
| Acupuncture | 21.4 (549) | 36.6 (201) | 63.4 (348) |
| Homeopathic Medicine | 13.1 (336) | 36.3 (122) | 63.7 (214) |
| Naturopathic Medicine | 10.1 (258) | 58.5 (151) | 41.5 (107) |
| Mind-Body Medicine | |||
| Biofeedback | 8.8 (225) | 23.1 (52) | 76.9 (173) |
| Visual Imagery | 30.1 (772) | 79.0 (610) | 21.0 (162) |
| Meditation/Relaxation | 41.6 (1066) | 58.7 (626) | 41.3 (440) |
| Yoga | 33.9 (868) | 29.8 (259) | 70.2 (609) |
| Chanting/Music Therapy | 13.5 (347) | 58.5 (203) | 41.5 (144) |
| Spiritual Healing | 31.3 (801) | 73.2 (586) | 26.8 (215) |
| Qigong/Tai chi | 14.1 (362) | 28.7 (104) | 71.3 (258) |
| Body-based Medicine | |||
| Chiropractic Medicine | 31.5 (806) | 8.3 (67) | 91.7 (739) |
| Massage Therapy | 43.2 (1108) | 30.0 (332) | 70.0 (776) |
| Energy Medicine | |||
| Crystals | 2.5 (65) | 35.4 (23) | 64.6 (42) |
| Magnets | 8.1 (207) | 16.4 (34) | 83.6 (173) |
| Reiki | 8.5 (219) | 51.6 (113) | 48.4 (106) |
| Therapeutic Touch | 6.8 (173) | 59.5 (103) | 40.5 (70) |
Cancer purposes included for cancer specifically, treatment side-effects or both.
The CAM assessment excluded biologically-based therapies (special diets, dietary supplement use), because half of the participants were following the WHEL intervention diet, and dietary supplement use was already being examined in detail separately in the WHEL Study. Hence, the analyses will only include the modalities included in four CAM domains (alternative medical systems, mind-body medicine, body-based medicine, and energy medicine) (See Tables 1 and 2).
Table 2.
Frequency of self-reported CAM use for cancer purposes among a cohort of 1265 breast cancer survivors enrolled in the WHEL Study.
| Frequency of CAM Use for Cancer Purposes | ||||
|---|---|---|---|---|
| | ||||
| CAM Types | n=1265 | Only Experimented | Occasional | Regular |
| Alternative Medicine System | ||||
| Acupuncture | 201 | 14.9% | 13.9% | 71.1% |
| Homeopathic Medicine | 122 | 13.1% | 14.8% | 69.7% |
| Naturopathic Medicine | 151 | 15.2% | 11.3% | 73.5% |
| Mind-Body Medicine | ||||
| Biofeedback | 52 | 11.5% | 38.5% | 50.0% |
| Visual Imagery | 610 | 7.5% | 23.1% | 67.7% |
| Meditation/Relaxation | 626 | 5.1% | 23.0% | 71.9% |
| Yoga | 259 | 14.7% | 23.9% | 61.4% |
| Chanting/Music Therapy | 203 | 8.9% | 18.2% | 72.9% |
| Spiritual Healing | 586 | 3.8% | 10.2% | 86.0% |
| Qigong/Tai chi | 104 | 21.2% | 29.8% | 49.0% |
| Body-based Medicine | ||||
| Chiropractic Medicine | 67 | 10.4% | 17.9% | 71.6% |
| Massage Therapy | 332 | 8.7% | 30.1% | 61.1% |
| Energy Medicine | ||||
| Crystals | 23 | 13.0% | 26.1% | 60.9% |
| Magnets | 34 | 2.9% | 20.6% | 76.5% |
| Reiki | 113 | 31.0% | 33.6% | 35.4% |
| Therapeutic Touch | 103 | 18.4% | 36.9% | 44.7% |
Demographic and medical information
Participants reported demographic and medical information at study enrollment. Medical information was confirmed by reviewing the patient's medical record. Body mass index (BMI, weight [kg]/height [m2]) was computed based on measurements of height and weight using standard protocols 21.
Smoking status and physical activity
At baseline, participants completed a questionnaire on their personal habits, which included standard questions on smoking history and a 9-item physical activity assessment 23. The frequency, duration and intensity of physical activity were converted into metabolic equivalents (METs). Total energy expenditure was obtained by weighting time spent per week by METs: mild, moderate, and vigorous activity were weighted as 3, 5, and 8 METS, respectively 24. Walking was weighted 2 to 6 METs according to intensity. As per Holmes 25, we chose 540 MET-min/wk as the recommended level of physical activity for breast cancer survivors 26.
Comorbidity assessment
At baseline, participants completed a health status questionnaire that inquired about diagnosed diseases and conditions. We combined specific diseases into general systems (such as cardiovascular disease) to avoid the potential for double counting and to limit the problem of small sample sizes. The categories included the following:
Diabetic conditions: any hypoglycemia, prediabetic/diabetes requiring insulin, and diabetes not requiring insulin.
Cardiovascular conditions: any high cholesterol requiring pills, high blood pressure, angina, peripheral arterial disease, or other heart related problems.
Digestive conditions: any stomach or duodenal ulcer, diverticulitis, ulcerative colitis, Crohn's disease, pancreatitis, intestinal or polyp removal, irritable bowel syndrome, or malabsorption syndrome.
Arthritis
Quality of Life
Participants completed the SF-36 health-related quality of life questionnaire at baseline and 1 year. This analysis included the physical health (PH) and mental health (MH) summary scores of the SF-36. The PH summary score is composed of the indicators for physical functioning, general health, bodily pain, and role limitations due to physical problems; while the MH score consists of the mental health index, role limitations due to emotional problems, social functioning, and vitality. PH and MH scores range from 0 to 100 (higher score, better health). This analysis categorizes women in the lowest two quintiles for PH and MH as “low,” based on an analysis showing that WHEL participants in the lower two quintiles of physical health score had a worse prognosis than those in the upper three quintiles 27.
Analytic Methods
Descriptive statistics were calculated for demographic characteristics and lifestyle and treatment variables. Participants were evaluated according to CAM use (ever used) and whether that use had been for cancer (cancer and/or treatment side effects) or non-cancer purposes. Among the modalities used for cancer, we further investigated the pattern of CAM use, which included the modality, the frequency of use, and whether a CAM practitioner had been consulted. This categorization was completed for the 1265 women who reported using CAM for cancer purposes, and resulted in the following five classes of CAM use: 1) Regular use and consulting a CAM practitioner, 2) Occasional use and consulting a CAM practitioner, 3) Occasional or regular basis without practitioner consultation, 4) Only experimented with CAM therapies and 5) Only used spiritual healing/prayer. We used one-way ANOVAs and chi-square tests to examine the differences on demographic characteristics, as well as health behaviors, treatment variables, and health status. Finally, chi-square tests and linear regressions were used to examine whether CAM use was associated with the changes (either categorical [low versus high] or mean score differences) in physical and mental health between the baseline and 1 year assessment.
RESULTS
Baseline Characteristics of CAM survey respondents (n=2562)
Participants had a mean age at baseline of 53 years, and the majority was white and had health insurance. Over half were college graduates and 70% were married. Slightly over half the sample had Stage II breast cancer at diagnosis and 70% reported having had chemotherapy prior to study entry. Participants reported an average intake of 3 vegetable servings and 2.5 fruit servings per day, 53% had more than 540 MET-min/wk of physical activity and only 4% of the sample was current smokers. The average BMI was 27.1 (SD=6.07), and 30% of the sample were considered obese. (Data not shown).
CAM Prevalence & Descriptive Data
Table 1 shows the distribution of CAM use for each modality and the purpose of use. Most participants (80%) reported use of at least one CAM therapy, with an average of 3.3 types of therapies used. CAM use, defined as use of any therapy within each of the NCCAM categories, revealed categorical differences in usage. Among CAM users, 83% reported using mind-body interventions (e.g., meditation, yoga, spiritual healing) and 69% reported using body-based methods (e.g., chiropractic, massage). Thirty-nine percent reported having used a whole medical system (e.g., homeopathic medicine, acupuncture) and 24.2% had used an energy-based therapy (e.g., Reiki). Massage therapy, meditation/relaxation and yoga were the most prevalent modalities overall.
Several mind-body medicine practices were popular for cancer purposes: 79.0% of visual imagery users, 73.2% of spiritual healing users, and 58.7% of meditation users reported using these practices for cancer. On the other hand, massage therapy and chiropractic were the most commonly reported CAM practices for non-cancer purposes. Overall, the prevalence of CAM use for cancer purposes was 50%, while the prevalence of CAM use for general purposes was 80%.
Further examination of those women who used CAM for cancer purposes showed that the majority of CAM users had used it regularly (Table 2). For each of the CAM therapies, regular users accounted for at least 50% of the users with few exceptions. Additionally, the percentage of regular users was highest among those who reported using spiritual healing, naturopathic medicine and chanting/music therapy.
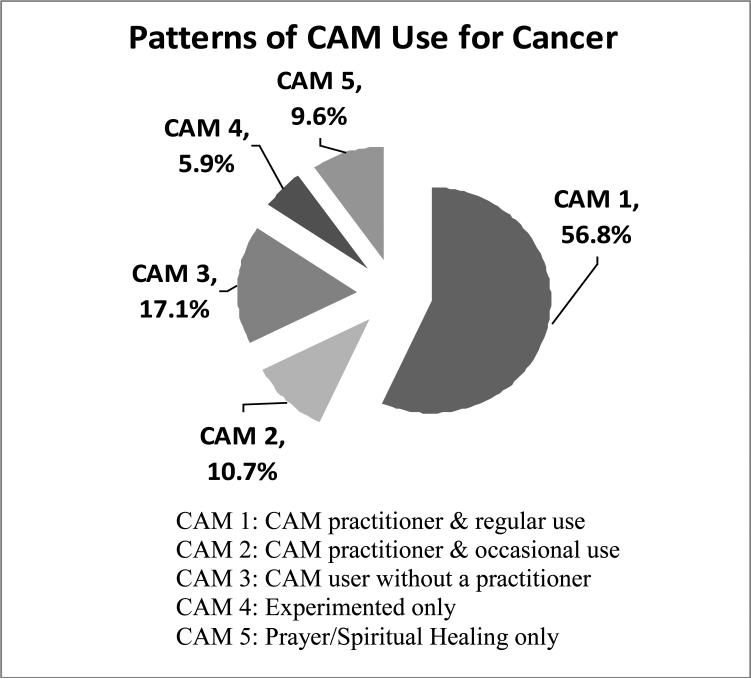
Among women who reported at least one type of CAM use for cancer, we found five different classes of users (Figure 1): 56.8% reported regular use and consulting a CAM practitioner, 10.7% reported occasional use and consulting a CAM practitioner and 17.1% reported use on either an occasional or regular basis without practitioner consultation. Those who had only experimented with CAM therapies (5.9%) or had only used spiritual healing/prayer (9.6%) accounted for the rest of the sample. For the following comparisons, we combined the CAM users who had not seen a practitioner into one category in order to have an adequate sample size for group comparisons.
Figure 1.
Percentages according to each class of CAM users in a cohort of breast cancer survivors who reported CAM use for cancer purposes and were enrolled in the WHEL Study (n=1265).
Table 3 compares three groups of CAM users to non-CAM users on demographic characteristics, health behaviors, tumor and treatment characteristics, and health status. We found that CAM users differed significantly from non-CAM users; all three groups of CAM users had a lower mean age than that of the non-users (p < .001). Further, CAM users were more likely to be white, have a college education, and less likely to be obese (p < .001. CAM use was also related to a greater consumption of fruits and vegetables and more physical activity (p < .001). Additionally, CAM users were less likely to have a co-morbid condition (such as arthritis /or cardiovascular diseases) than non-CAM users (p < .05). Further comparisons among the groups of CAM users showed similar trends in the covariates. Regular CAM users who had consulted a practitioner were more likely to be younger, white, have higher education, have a lower BMI and have fewer co-morbid conditions (p-trends < .05). Also, we found that regular CAM users who had consulted a practitioner were more likely to have had chemotherapy following their breast cancer diagnosis than non-CAM users (p < .003). However, CAM use was not related to radiation or anti-estrogen therapies, or to any cancer characteristics such as tumor stage, tumor grade, or receptor status.
Table 3.
Comparison of sample characteristics according to CAM use in a cohort of breast cancer survivors enrolled in the WHEL Study (n=1780).
| No CAM use | CAM without a practitioner (class 3 to 5) | CAM with a practitioner & occasional use (class 2) | CAM with a practitioner & regular use (class 1) | p-value | |
|---|---|---|---|---|---|
| | |||||
| Sample Size (n) | 515 | 412 | 135 | 718 | |
| Demography | |||||
| Age (mean (SD)) | 54.1 (9.33) | 52.8 (8.8) | 52.7 ( 8.13) | 51.3 (8.15) | 0.001 |
| Ethnicity (% ) White | 78.4 | 85.9 | 89.6 | 88 | 0.001 |
| African American | 8.9 | 4.6 | 5.9 | 4.6 | |
| Hispanic | 6.6 | 3.2 | 2.2 | 1.9 | |
| Asian/Pacific Islander | 4.5 | 4.4 | 2.2 | 3.8 | |
| Education (% College & Beyond) | 48.7 | 54.9 | 60 | 64.8 | 0.001 |
| Obese (%) (BMI > 30 kg/m2) | 31.1 | 23.1 | 23.7 | 18.2 | 0.001 |
| Health Behaviors | |||||
| Fruit/Vegetables (servings/day) | 4.9 (2.85) | 5.3 (2.77) | 5.8 (2.97) | 5.7 (2.77) | 0.001 |
| Adequate Physical Activity (%) | 44.4 | 54 | 55.9 | 63.6 | 0.001 |
| Current Smokers (%) | 3.9 | 3.2 | 2.2 | 4.1 | 0.68 |
| Health Status | |||||
| Co-morbid Conditions (% with 1 or more) | 46.7 | 44.1 | 39.2 | 38.3 | 0.05 |
| Diabetic Conditions | 3.9 | 7.1 | 4.3 | 4 | 0.16 |
| Cardiovascular Diseases | 25.6 | 23.7 | 18.9 | 18.7 | 0.05 |
| Digestive Diseases | 8.2 | 9.7 | 10.9 | 9.5 | 0.81 |
| Arthritis | 23.2 | 18.1 | 22.6 | 14.7 | 0.01 |
| Osteoporosis | 7 | 5.7 | 6.6 | 7.4 | 0.82 |
| Tumor and Treatment Characteristics | |||||
| Tumor Stage (%) I | 40.0 | 36.9 | 36.3 | 36.4 | 0.84 |
| II | 54.6 | 58.5 | 58.5 | 58.9 | |
| IIIA | 5.4 | 4.6 | 5.2 | 4.7 | |
| Tumor Grade (%) Poor | 34.2 | 34.2 | 42.2 | 37.3 | 0.69 |
| Moderate | 41.0 | 40.0 | 39.3 | 38.4 | |
| Well | 15.7 | 17.5 | 11.9 | 16.6 | |
| Unknown | 9.1 | 8.3 | 6.7 | 7.7 | |
| Estrogen Receptor (ER) positive | 73.0 | 76.5 | 73.3 | 76.9 | 0.38 |
| Chemotherapy (%) | 67.4 | 73.3 | 77.8 | 76.3 | 0.003 |
| Radiation (%) | 61.2 | 58.9 | 63.0 | 66.3 | 0.07 |
| Anti-estrogen therapy (% current users) | 63.9 | 62.4 | 64.4 | 61.3 | 0.78 |
* calculated as weight in kilograms divided by the square of height in meters
†defined as >=540 met-min/wk
For each group, we examined the participants who had low physical health (PH) and mental health (MH) scores at baseline. CAM users who reported regular use and consulted a CAM practitioner reported poorer physical (p < .02) and mental health (p < .002) compared to non-CAM users (Table 4). The percentage of participants with improved PH and MH scores at the 1-year follow-up did not differ significantly between any of the CAM users and the non-CAM users (Table 4). Approximately one-third of each group (users and non-CAM users) improved their PH and MH scores (from low to adequate) between baseline and 1 year.
Table 4.
Comparisons of baseline SF-36 physical and mental health scores and comparisons of changes in physical and mental health from baseline to 1-year follow-up according to CAM use in a cohort of breast cancer survivors enrolled in the WHEL Study (n=1780).
| Baseline Physical Health | Year 1 Physical health | Baseline Mental Health | Year 1 Mental health | ||||
|---|---|---|---|---|---|---|---|
| | |||||||
| % Low (n) | % (n) | % Low (n) | % (n) | ||||
| | |||||||
| Sample Size (n)a | No Change | Improved | No Change | Improved | |||
| No CAM use | 410 | 31.2 (128) | 60.2 (77) | 39.8 (51) | 32.0 (131) | 57.3 (75) | 42.7 (56) |
| CAM without consultation of a practitioner (class 3-5) | 354 | 36.2 (128) | 66.4 (85) | 33.6 (43) | 37.2 (132) | 54.5 (72) | 45.5 (60) |
| CAM with a practitioner & Occasional Use (class 2) | 112 | 42.9 (48) | 70.8 (34) | 29.2 (14) | 44.6 (50) | 66.0 (33) | 34.0 (17) |
| CAM with a practitioner & Regular Use (class 1) | 576 | 42.3 (243) | 62.1 (151) | 37.9 (92) | 40.6 (234) | 61.1 (143) | 38.9 (91) |
| | |||||||
| Chi-square p-value | 0.002 | 0.496 | 0.024 | 0.441 | |||
Sample size for each category was reduced due to missing data in the physical and mental health scores.
Additionally, we examined the mean baseline scores for PH and MH across the groups and found statistically significant differences, with non-users of CAM having slightly higher mean baseline scores than the classes of CAM users (i.e. 78-79 versus 74-75). However, the changes seen between baseline and year 1 were not statistically different between these two groups. The mean increases for PH and MH scores were slightly higher among the CAM users (mean (SD) [PH=0.9 (14.3); MH= 0.5 (14.7)] than among the non-CAM users (mean (SD) [PH=0.2 (12.8), MH= -0.6 (13.7], but these changes were not significantly different.
DISCUSSION
This study explored CAM use among a large cohort of breast cancer survivors and addressed important differences in the categorization of CAM use. CAM use prevalence varied considerably by the classification criteria. CAM use (practiced more frequently or involving a practitioner) was significantly related to younger age, higher education, more fruit & vegetable servings, more physical activity, and lower BMI. Additionally, CAM users were more likely to report poor physical and mental health than non-CAM users, but improvements in physical and mental health were not associated with CAM use.
We found large differences in prevalence of CAM when we examined use according to the purpose of use and when we classified CAM in order to capture the exposure or intensity of use. Approximately 80% of the sample reported CAM use when defined as ever using any CAM modality, but prevalence dropped to 50% when CAM use was restricted to cancer purposes. Among CAM users for cancer purposes, we also found variation when frequency and consultation with a CAM practitioner was considered. The final estimate of CAM use was 28%, which included women who used at least one CAM modality with regularity and consulted a CAM practitioner. This wide variation was also observed in a Norwegian study of cancer survivors, which originally reported classifying CAM according to exposure 19. They found that CAM use varied from 72% (any use including prayer) to 11% (seen a CAM practitioner at least 4 times). Additionally, these investigators compared estimates across studies and found that the classification of CAM was directly correlated with prevalence: a more inclusive definition resulted in higher prevalence estimates 9, 28-32. Not only do CAM use classifications influence prevalence estimates, but they also impact the reported characteristics of CAM users and their differences from non-CAM users 13.
We examined characteristics associated with each class of CAM user. Not only did we find that CAM users differed significantly from non-CAM users on demographic characteristics, health behaviors and health status, we also found significant differences among the classes of CAM users. Women who had seen a practitioner and used CAM regularly were the youngest; the most highly educated, and had the lowest BMI. This group also engaged in other health behaviors to a higher degree. They reported nearly six fruit and vegetable servings per day (on average) and the majority of them reported adequate daily physical activity. Other studies have shown age and education to be consistently related to CAM use 13, 31, 33 but few have examined health behaviors such as diet and physical activity. There were also differences in the use of chemotherapy following diagnosis across the class of CAM use with the non-users being the least likely to have taken chemotherapy. Our findings suggest the CAM use in this sample was complementary in nature and not being used as an alternative to conventional treatments. We also showed that CAM use was associated with having a co-morbid condition. CAM users who had seen a practitioner and used CAM regularly had lower levels of cardiovascular diseases and/or arthritis. Previous studies have not examined co-morbid conditions with CAM use, so it is unknown whether a causal relationship exists between the two factors 34.
CAM users who had consulted a practitioner (class 1 & 2) reported worse physical and mental health than CAM users who had not seen a practitioner and non-users at baseline. Since the reported reasons for using CAM are associated with quality of life, this finding is plausible in the context of breast cancer. Reported reasons for CAM use among breast cancer survivors have included: to strengthen the immune system, to reduce pain and fatigue, to alleviate treatment side-effects, and to have a direct anti-cancer effect 15, 32. Further, the investigation of changes in physical and mental health between baseline and one year indicated that CAM users had improvements in quality of life, but the magnitude of those changes was not enough to show statistical differences among the groups. However, our findings should be interpreted with caution since this study was not designed to test the efficacy of CAM modalities and the CAM usage questionnaire was administered an average of 5 years after the breast cancer diagnosis. Earlier studies that have tested CAM efficacy have shown a positive effect on quality of life for CAM users 35-39. It is important to note that CAM use was defined differently in our study compared to others, which could account for the variation in findings.
The proposed study has limitations that should be considered. WHEL Study participants may not be representative of all breast cancer survivors. They are predominantly white and highly educated, which could have inflated the estimates of CAM use. Also, not all WHEL Study participants completed the CAM survey, since it was administered during the follow-up period. This could have introduced some recall bias as well as sampling bias given that some of the correlates of early recurrence are also associated with CAM use. Additionally, our analysis of quality of life was exploratory given that we have not established temporality. In contrast, this study had several strengths, such as a large sample size and adequate CAM data to allow for a comprehensive classification system. This study examined characteristics among different types of CAM users that have not been examined previously in breast cancer survivors.
CAM is not a homogenous field; rather, it is a categorical term that covers a broad range of more than 100 healing philosophies, approaches, and therapeutic modalities that allopathic medicine does not commonly accept, use, or make available. But as CAM becomes increasingly popular among breast cancer patients, it is critical to address the potential benefits and risks associated with its use 40. This study used a classification system for CAM users that has important implications for practitioners and researchers. Physicians could better ensure patient safety if they were aware of CAM use and any potential interactions with conventional treatment 17. For researchers, this classification system could provide a reliable way to compare CAM use among studies and across various populations. Future research should consider adopting this system to more accurately assess use and, therefore, test CAM effectiveness on breast cancer outcomes.
Footnotes
DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- 1.NCCAM . What is Complementary and Alternative Medicine? U.S. Department of Health and Human Services, National Institutes of Health, National Center for Complementary and Alternative Medicine; Bethesda: 2000. CAM Basics. [Google Scholar]
- 2.Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health. 2007;7:4. doi: 10.1186/1472-6874-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999 Jun 3;340(22):1733–1739. doi: 10.1056/NEJM199906033402206. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter CL, Ganz PA, Bernstein L. Complementary and alternative therapies among very long-term breast cancer survivors. Breast Cancer Res Treat. 2009 Jul;116(2):387–396. doi: 10.1007/s10549-008-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassileth BR, Vickers AJ. High prevalence of complementary and alternative medicine use among cancer patients: implications for research and clinical care. J Clin Oncol. 2005 Apr 20;23(12):2590–2592. doi: 10.1200/JCO.2005.11.922. [DOI] [PubMed] [Google Scholar]
- 6.Ernst E, Schmidt K, Baum M. Complementary/Alternative therapies for the treatment of breast cancer. A systematic review of randomized clinical trials and a critique of current terminology. Breast J. 2006 Nov-Dec;12(6):526–530. doi: 10.1111/j.1524-4741.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein MS. Complementary and alternative medicine: Its emerging role in oncology. Journal of Psychosocial Oncology. 2003;21(2):1–21. [Google Scholar]
- 8.Lee ST. Evidence-based complementary medicine--challenges and future directions. Ann Acad Med Singapore. 2000 Jan;29(1):1–2. [PubMed] [Google Scholar]
- 9.Matthews AK, Sellergren SA, Huo D, List M, Fleming G. Complementary and alternative medicine use among breast cancer survivors. Journal of Alternative and Complementary Medicine. 2007 Jun;13(5):555–562. doi: 10.1089/acm.2007.03-9040. [DOI] [PubMed] [Google Scholar]
- 10.Buettner C, Kroenke CH, Phillips RS, Davis RB, Eisenberg DM, Holmes MD. Correlates of use of different types of complementary and alternative medicine by breast cancer survivors in the nurses’ health study. Breast Cancer Research and Treatment. 2006 Nov;100(2):219–227. doi: 10.1007/s10549-006-9239-3. [DOI] [PubMed] [Google Scholar]
- 11.Gerber B, Scholz C, Reimer T, Briese V, Janni W. Complementary and alternative therapeutic approaches in patients with early breast cancer: a systematic review. Breast Cancer Research and Treatment. 2006 Feb;95(3):199–209. doi: 10.1007/s10549-005-9005-y. [DOI] [PubMed] [Google Scholar]
- 12.Richardson MA, Masse LC, Nanny K, Sanders C. Discrepant views of oncologists and cancer patients on complementary/alternative medicine. Supportive Care in Cancer. 2004 Nov;12(11):797–804. doi: 10.1007/s00520-004-0677-3. [DOI] [PubMed] [Google Scholar]
- 13.Shumay DM, Maskarinec G, Gotay CC, Heiby EM, Kakai H. Determinants of the degree of complementary and alternative medicine use among patients with cancer. Journal of Alternative and Complementary Medicine. 2002 Oct;8(5):661–671. doi: 10.1089/107555302320825183. [DOI] [PubMed] [Google Scholar]
- 14.Sparber A, Bauer L, Curt G, et al. Use of complementary medicine by adult patients participating in cancer clinical trials. Oncol Nurs Forum. 2000 May;27(4):623–630. [PubMed] [Google Scholar]
- 15.Ashikaga T, Bosompra K, O'Brien P, Nelson L. Use of complimentary and alternative medicine by breast cancer patients: prevalence, patterns and communication with physicians. Supportive Care in Cancer. 2002 Oct;10(7):542–548. doi: 10.1007/s00520-002-0356-1. [DOI] [PubMed] [Google Scholar]
- 16.Tascilar M, de Jong FA, Verweij J, Mathijssen RH. Complementary and alternative medicine during cancer treatment: beyond innocence. Oncologist. 2006 Jul-Aug;11(7):732–741. doi: 10.1634/theoncologist.11-7-732. [DOI] [PubMed] [Google Scholar]
- 17.Saxe GA, Madlensky L, Kealey S, Wu DP, Freeman KL, Pierce JP. Disclosure to physicians of CAM use by breast cancer patients: findings from the Women's Healthy Eating and Living Study. Integr Cancer Ther. 2008 Sep;7(3):122–129. doi: 10.1177/1534735408323081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiger WA, Smith M, Boon H, Richardson MA, Kaptchuk TJ, Eisenberg DM. Advising patients who seek complementary and alternative medical therapies for cancer. Ann Intern Med. 2002 Dec 3;137(11):889–903. doi: 10.7326/0003-4819-137-11-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kristoffersen AE, Fonnebo V, Norheim AJ. Use of complementary and alternative medicine among patients: classification criteria determine level of use. J Altern Complement Med. 2008 Oct;14(8):911–919. doi: 10.1089/acm.2008.0127. [DOI] [PubMed] [Google Scholar]
- 20.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. Jama. 2007 Jul 18;298(3):289–298. doi: 10.1001/jama.298.3.289. PMCID: 2083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002 Dec;23(6):728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 22.American Joint Committee on Cancer: Manual for Staging of Cancer. 4th ed. Springer-Verlag; New York, NY: 2002. [Google Scholar]
- 23.WHI. Women's Health Initiative [June 17, 2010];WHI Personal Habits Questionnaire. http://www.whiscience.org/data/forms/F34v2.pdf.
- 24.Lof M, Hannestad U, Forsum E. Comparison of commonly used procedures, including the doubly-labelled water technique, in the estimation of total energy expenditure of women with special reference to the significance of body fatness. Br J Nutr. 2003 Nov;90(5):961–968. doi: 10.1079/bjn2003975. [DOI] [PubMed] [Google Scholar]
- 25.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005 May 25;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 26.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007 Jul 18;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saquib N, Pierce JP, Saquib J, et al. Poor physical health predicts additional breast cancer events and mortality in breast cancer survivors. Psycho-oncology. doi: 10.1002/pon.1742. ( http://www3.interscience.wiley.com/journal/5807/home?CRETRY=1&SRETRY=0) Early view. [DOI] [PMC free article] [PubMed]
- 28.Molassiotis A, Scott JA, Kearney N, et al. Complementary and alternative medicine use in breast cancer patients in Europe. Supportive Care in Cancer. 2006 Mar;14(3):260–267. doi: 10.1007/s00520-005-0883-7. [DOI] [PubMed] [Google Scholar]
- 29.Nagel G, Hoyer H, Katenkamp D. Use of complementary and alternative medicine by patients with breast cancer: observations from a health-care survey. Support Care Cancer. 2004 Nov;12(11):789–796. doi: 10.1007/s00520-004-0675-5. [DOI] [PubMed] [Google Scholar]
- 30.Nahleh Z, Tabbara IA. Complementary and alternative medicine in breast cancer patients. Palliat Support Care. 2003 Sep;1(3):267–273. doi: 10.1017/s1478951503030256. [DOI] [PubMed] [Google Scholar]
- 31.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000 Jul;18(13):2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 32.Tagliaferri M, Cohen I, Tripathy D. Complementary and alternative medicine in early-stage breast cancer. Semin Oncol. 2001 Feb;28(1):121–134. doi: 10.1016/s0093-7754(01)90049-1. [DOI] [PubMed] [Google Scholar]
- 33.Navo MA, Phan J, Vaughan C, et al. An assessment of the utilization of complementary and alternative medication in women with gynecologic or breast malignancies. J Clin Oncol. 2004 Feb 15;22(4):671–677. doi: 10.1200/JCO.2004.04.162. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt G, Sikorskii A, Wills CE, Su H. Complementary and alternative medicine use, spending, and quality of life in early stage breast cancer. Nurs Res. Jan-Feb;59(1):58–66. doi: 10.1097/NNR.0b013e3181c3bd26. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson S, Barnes K, Storey L. Massage for symptom relief in patients with cancer: systematic review. J Adv Nurs. 2008 Sep;63(5):430–439. doi: 10.1111/j.1365-2648.2008.04712.x. [DOI] [PubMed] [Google Scholar]
- 36.Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: A systematic review. Journal of Clinical Oncology. 2006 Dec;24(34):5457–5464. doi: 10.1200/JCO.2006.08.3725. [DOI] [PubMed] [Google Scholar]
- 37.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003 Jul-Aug;65(4):571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 38.Danhauer SC, Tooze JA, Farmer DF, et al. Restorative yoga for women with ovarian or breast cancer: findings from a pilot study. J Soc Integr Oncol. 2008 Spring;6(2):47–58. [PubMed] [Google Scholar]
- 39.Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007 Oct 1;25(28):4387–4395. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 40.Robotin MC, Penman AG. Integrating complementary therapies into mainstream cancer care: which way forward? Med J Aust. 2006 Oct 2;185(7):377–379. doi: 10.5694/j.1326-5377.2006.tb00614.x. [DOI] [PubMed] [Google Scholar]