Abstract
Schizophrenia and nicotine addiction are both highly heritable phenotypes. Because individuals with schizophrenia have a higher rate of smoking than those in the general population, one could hypothesize that genes associated with smoking might be over-represented in schizophrenia and thus help explain their increased smoking incidence. Although a number of genes have been proposed to explain the increased smoking risk in schizophrenia, none of them have been consistently linked to smoking and schizophrenia and thus difficult to explain the increased smoking in schizophrenia. A functional smoking-related nicotinic acetylcholine receptor α5 subunit gene (CHRNA5) nonsynonymous SNP rs16969968 (Asp398Asn) has recently been discovered and replicated. As such, we tested whether this variant contributes to smoking in schizophrenia in a sample of 313 schizophrenia patients and 525 controls. The Asp398Asn risk allele is significantly associated with smoking severity independently in schizophrenia patient smokers (p=0.001) and in control smokers (p=0.029). Furthermore, the same risk allele is significantly associated with schizophrenia in both Caucasian (p=0.022) and African American (p=0.006) nonsmoker schizophrenia patients compared to control nonsmokers. Intriguing, this SNP was not significantly associated with smoking status (smokers vs. nonsmokers) in either schizophrenia patients or controls. Therefore, our study identifies a genetic variant that is simultaneously linked to smoking and schizophrenia in the same cohort, but whether and how this SNP contributes to the increased smoking prevalence in schizophrenia patients requires additional studies.
Keywords: smoking, nicotine addiction, schizophrenia, nAChR, alpha5, comorbidity
Introduction
Smoking rate in schizophrenia is about three times higher than that in the general population (Hughes et al. 1986; Chapman et al. 2009). Most patients smoke prior to psychosis onset(Kelly and McCreadie, 1999; Diaz et al. 2008), suggesting an increased risk for smoking prior to psychosis onset. Schizophrenia and nicotine addiction are highly heritable: the heritability is 60-75% for nicotine dependence(Sullivan and Kendler, 1999) and 60-80% for schizophrenia(Cannon et al. 1998). Thus, these two discrete yet tightly coupled phenotypes are also highly heritable.
Indeed, a genetic attribute to this smoking-schizophrenia comorbidity has often been proposed. However, testing whether a genetic variant contributes to smoking increases in schizophrenia is more intricate than a simple case-control association test. A candidate variant could cause the increased smoking rate by either: 1) contributing to both smoking and schizophrenia in an additive pattern, leading to the increased smoking; or 2) contributing specifically to smoking in schizophrenia, distinct from other genetic variants for smoking. Accordingly, such a variant should show 1) association with smoking in controls, smoking in patients, and simultaneously also with schizophrenia diagnosis regardless of smoking, thus possibly accounting for the increased smoking risk; or 2) association specifically and more robustly with schizophrenia smokers.
It has been suggested that increased smoking in schizophrenia is mediated by the nicotinic acetylcholine receptor (nAChR) α7 subunit gene CHRNA7 (Freedman et al. 1996; Leonard et al, 2002). However, evidence for its association with smoking itself is weak (Faraone et al. 2004; Zammit et al. 2007; Sanders et al. 2008) and none of the GWAS on smoking has implicated CHRNA7(Thorgeirsson et al. 2008). CHRNA7 variants may be associated with schizophrenia and not with smoking in the disorder(Stephens et al, 2009). CHRNA4 and CHRNB2 have been associated with smoking (Li et al. 2005; Hutchison et al. 2007; Conti et al. 2008), although no association with schizophrenia or smoking status in schizophrenia has been identified with the exception of an association with smoking quantity (De, V et al. 2006; Voineskos et al. 2007). Dysbindin polymorphisms have been associated with both schizophrenia and smoking although association with smoking in schizophrenia patients was not reported (Voisey et al 2010). A dopamine-related gene NR4A3 was associated with smoking severity in schizophrenia smokers but not in controls (Novak et al. 2010). The lack of consistent association of these variants with smoking itself, let alone stronger association with smoking in schizophrenia, indicating that we have yet identified the key genetic variants, if exist, for the comorbidity.
The discovery of nicotinic acetylcholine receptor α5 subunit gene (CHRNA5) SNP rs16969968 (Asp398Asn) is compelling because the association between this or nearby SNPs with smoking related phenotypes is based on replicated genome-wide searches (Saccone et al. 2007; Thorgeirsson et al. 2008; Lips et al. 2010). The aspartic acid to asparagine substitution is functional such that the risk allele Asn reduces α4β2α5 receptor functions(Bierut et al. 2008). It provides an opportunity to test whether a gene robustly contributing to smoking may explain the increased smoking risk in schizophrenia. To test this hypothesis, we examined whether the Asp398Asn association with smoking severity is replicable in our control sample, whether it can be extended to a schizophrenia sample and finally, whether it is over-represented in schizophrenia.
Materials and Methods
Schizophrenia patients were recruited from the outpatient clinics of the Maryland Psychiatric Research Center (MPRC) and from community clinics from which we receive referrals. Controls were recruited from the Baltimore area using local media advertisement. All participants gave written consent using local Institutional Review Board approved consent forms. The Structured Clinical Interview for DSM IV was administered to determine DSM-IV Axis I diagnosis. Patient participants were individuals with DSM-IV schizophrenia. Control subjects were screened and excluded for Axis I psychosis diagnoses. The study included 313 unrelated schizophrenia patients and 525 healthy controls with genotyping information. A subgroup of these subjects (42 schizophrenia and 180 controls) and their genotyping information were used in a previous imaging study, although no schizophrenia-related analyses were conducted there(Hong et al. 2010). Genotyping of rs16969968 was performed using Taqman Assays-on-Demand (Applied Biosystems, Foster City, California) methods as previously described(Hong et al. 2008; Hong et al, 2010). Genotyping accuracy was determined by duplicate genotyping of 10% of the samples selected randomly. The error rate was <0.005.
Smokers (n=323, including schizophrenia patients and controls) were classified as individuals who smoked for over one year and were currently smoking. Fagerström Test for Nicotine Addiction (FTND) was used to measure nicotine addiction severity (0-10, 10 being most severe). Cigarettes per day (CPD) was included as an additional measure of smoking severity, given its use in previous GWAS studies. CPD data were not available for 2 smokers, while FTND data were not available for 16 smokers. Chronic cigarette exposure was estimated in pack-years. Age of regular smoking onset was also collected. Nonsmokers (n=379, including schizophrenia patients and controls) were defined by lifetime smoking of less than 100 cigarettes and currently not smoking. Past-smokers (n=67) were smokers who had quit smoking for any duration and were currently not smoking. Subjects’ smoking status was designated as “undetermined” (n=69) when we could not reliably classify current, past, or nonsmoking status when the reported smoking history was deemed unreliable. Subjects with past or undetermined smoking status were included in genotype comparisons of schizophrenia vs. control, but were excluded from smoking-related analyses. Smoking behavioral characteristics from both case and controls are given in Table 1.
Table 1.
Demographic and smoking related characteristics of the sample
| All schizophrenia |
All controls |
χ2 or F | p value |
|
|---|---|---|---|---|
| N | 313 | 525 | ||
| Gender (male: female) | 226:87 | 280:245 | 29.19 | 0.000 |
| Age | 38.9±0.55 | 36.1±0.56 | 10.97 | 0.001 |
| Race (Caucasian: African American: others) * | 155:149:9 | 271:211:43 | 2.06 | 0.163 |
| Current smoker : nonsmoker : past smoker # | 149:65:30 | 174:314:37 | 73.37 | 0.000 |
| FTND (current smokers) | 4.60±0.19 | 4.41±0.20 | 0.47 | 0.49 |
| Cigarettes per day (CPD) (current smoker) | 16.00±0.78 | 17.13±0.71 | 1.17 | 0.28 |
| Pack year (current smokers) | 13.95±1.34 | 15.42±1.10 | 0.73 | 0.39 |
| Age of regular smoking onset (current smoker) | 20.37±0.81 | 19.38±0.50 | 1.17 | 0.28 |
Statistics based on Caucasians vs. African Americans
Not including subjects with undetermined smoking status
We compared observed genotype frequencies with those expected under Hardy-Weinberg equilibrium using Haploview (Barrett et al. 2005). Effects of genotype on quantitative smoking related measures were examined using linear regressions based on an additive model (coded as 0, 1, and 2 for the number of “risk allele”). Genotype and allelic tests for association between schizophrenia cases and controls or between smokers and nonsmokers were performed using χ2 tests, separately in each ethnic subgroup to reduce bias from population stratification during case-control comparisons. Data were also re-examined using logistic regression where age and gender were entered as covariates and Wald’s χ2, odds ratio [OR as calculated by Exp(B)] and its 95% confidence interval (CI) were calculated. Power analysis for single-locus main effects was performed using the Quanto program (version 1.2.4; http://hydra.usc.edu/gxe/).
Results
Demographics
The sample includes 426 Caucasians (155 schizophrenia patients, 271 controls), 360 African-Americans (149 patients and 211 controls) and 52 from other ethnic backgrounds. The distribution of genotypes was consistent with those predicted under Hardy Weinberg Equilibrium in all ethnic and diagnostic subgroups. Schizophrenia cases and controls differed in age and gender (both p<0.001). There were no significant gender differences of the Asp398Asn genotype (χ2=2.71, p=0.26). Gender was not significantly associated with nicotine addiction severity (FTND: F=1.84, p=0.18), daily cigarettes (CPD: F=0.08, p=0.78) or pack years (F=0.06, p=0.80). Males became regular smokers at a younger age (19.17±0.48) than females (21.53±1.05, F=5.44, p=0.021). See Table 1 for these and other demographic and smoking related data.
Associations with schizophrenia status
The frequency of the Asp398Asp risk allele Asn was 37.1% in Caucasians and 5.6% in African Americans (combined schizophrenia patients and controls), similar to those reported in the NCBI SNP database (35.4% in Caucasians and 4.3% in African Americans). To reduce stratification bias, case-control analyses were carried out in separate ethnic groups. The frequency of the Asn allele was significantly higher in schizophrenia cases than controls in Caucasians (p=0.025), but not in African Americans (p=0.419, see Table 2 for allele frequency and number of subject information), and remained significant after Bonferroni correction for two comparisons (Caucasians and African Americans). As this effect could be biased by more smokers in schizophrenia patient, we re-analyzed case vs. control association by stratifying smoking. The overrepresentation of this allele in schizophrenia was significant in both Caucasians nonsmokers (p=0.022) and African American nonsmokers (p=0.006) (see Table 2 for allele frequency and number of subject information); both remained significant after Bonferroni correction for two comparisons. However, no significant relationship was found in smokers (Table 2). The data were also analyzed using logistic regression to account for age and gender. Here, the frequency of the Asn allele was significantly higher in cases than controls in both Caucasian nonsmokers (Wald’s χ2=3.87, OR=1.91 for one copy and 3.47 for two copies of Asn, 95%CI =1.01-11.99) and African American nonsmokers [χ2=8.77, OR=4.80, 95%CI=1.57-14.72 for one copy of Asn (no homozygote Asn in this group)]. Therefore, the overrepresentation of this smoking-related risk allele in schizophrenia is not due to more smokers in the schizophrenia cohort and is present in nonsmoking schizophrenia patients in two cohorts with different ethnic backgrounds.
Table 2.
Frequencies of the Asn allele in schizophrenia patients and control subjects by ethnic subgroups and by smoking vs. non-smoking status
| All subjects* (n=838) |
Non-smokers (n=379) |
Smokers (n=323) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnic groups |
Patient | Control | χ 2 | p | Patient | Control | χ 2 | p | Patient | Control | χ 2 | p |
| Caucasian | 0.406 (155) |
0.331 (271) |
4.98 | 0.025 | 0.481 (26) |
0.318 (157) |
5.22 | 0.022 | 0.403 (72) |
0.346 (94) |
1.14 | 0.286 |
| African- American |
0.064 (149) |
0.050 (211) |
0.65 | 0.419 | 0.105 (38) |
0.029 (121) |
7.50 | 0.006 | 0.040 (75) |
0.071 (77) |
1.42 | 0.233 |
| Other | 0.278 (9) |
0.116 (43) |
n/c | n/c | (1) | (36) | n/c | n/c | (2) | (3) | n/c | n/c |
All subjects regardless of smoking status, including smokers, nonsmokers, past-smokers, and those with smoking status undetermined. Number of subjects in each cell is in parenthesis. n/c: not calculated due to small numbers.
Association with smoking severity
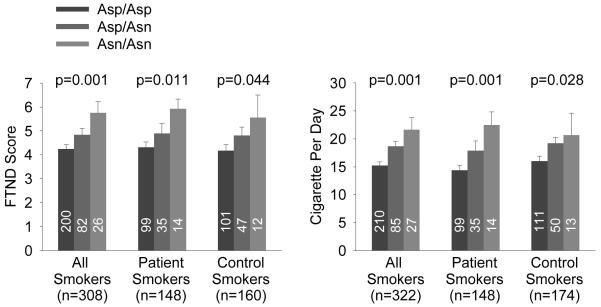
The Asp398Asn genotype was significantly associated with nicotine addiction severity as measured by FTND, and explained 3.3% (R2 change; F=10.44, p=0.001) of the addiction severity variance in the entire smoker sample and 2.5% (F=4.12, p=0.044) and 4.4% (F=6.67, p=0.011) of the variances in control smokers and patient smokers analyzed separately (Figure 1). Although more robust in patients, the R2 changes were not significantly different between the two groups (z=−0.43, p=0.67). The findings were similar for CPD, with genotype explaining 4.6% (F=15.60, p<0.001), 2.8% (F=4.86, p=0.029), and 7.3% (F=11.53, p=0.001) of the CPD variances in the combined, control, and patient samples, respectively. Again, genotype contribution to CPD was not significantly different between patients and controls (z=−0.85, p=0.40), although the relationship is numerically more robust in patients. After Bonferroni corrections for three calculations (combined, patient and control samples), genotype association with FTND and CPD were significant in the combined and patient sample, but not in the control sample alone.
Figure 1.
Asp398Asn genotype effect on nicotine addiction severity as measured by Fagerström Test for Nicotine Dependence (FTND) and cigarettes per day (CPD) in schizophrenia smokers and control smokers (mean±s.e.). Numbers in vertical bars are number of subjects in each genotype. The risk allele Asn is significantly associated with addiction severity and CPD, independently in patients and controls and in the combined sample. The significant associations with FTND and CPD remained after Bonferroni corrections for 3 calculations in the combined and the patient samples, but not in the control sample.
Asp398Asn was not associated with chronic cigarette exposure as measured by pack year in non-psychiatric controls (R2 change=0.2%, F=0.33, p=0.57), although the association was significant in patients (6.9%, F=10.39, p=0.002). Finally, on age to becoming a regular smoker, the risk allele was significantly associated with younger age of regular smoking onset in the combined sample (3.6%, F=6.25, p=0.012). Although the effect was in the same directions in both groups, it was not significant in patients (1.9%, F=1.80, p=0.18) but was nominally significant in controls (5.8%, F=4.47, p=0.038). This finding may be related to previous data showing that this SNP is associated with heavy smoking only in those with younger smoking onset(Weiss et al. 2008).
Association with smoking status
When comparing smokers vs. nonsmokers (allele frequencies in Table 2), Asp398Asn was not significantly associated with smoking status in the combined patient and control sample in Caucasians (χ2=0.24, p=0.621) or African Americans (χ2=0.64, p=0.425), consistent with previous data that have shown that this SNP or SNPs in LD is associated with smoking or addiction severity but is not associated with smoking status (Thorgeirsson et al, 2008; Lips et al, 2010). Logistic regression with age and gender as covariates showed similar findings in Caucasians (p=0.665) and African Americans (p=0.474). In separate diagnostic group, Asp398Asn was nominally overrepresented in smokers (χ2=3.92, p=0.048) compared with nonsmokers in Caucasian controls; but not overrepresented in nonsmokers compared with smokers in Caucasian patients (χ2=3.70, p=0.054). None of the above remained significant after Bonferroni correction. There were no significant findings in African American controls (χ2=0.40, p=0.529) or African American patients (χ2=0.95, p=0.329).
Posterior power calculations with Quanto for main effects of schizophrenia, assuming average rs16969968 genotype relative risk for schizophrenia to be similar to that for heavy smokers in several 15q25.1 SNPs, i.e., around 1.4 (Schlaepfer et al, 2008; Saccone et al, 2009), with MAFs at 37.1% in Caucasians and 5.6% in African Americans, and under an additive model, showed that the sample has 73% power for alpha of 0.05 for Caucasians but only 30% power for African Americans. Power is only 43% for Caucasian smokers and 29% for Caucasian nonsmokers under the assumption of the same genotype relative risk, so the lack of significant findings in smokers could be due to insufficient power. Power for smoking status was expected to be insufficient given the lack of significant findings in much larger samples (e.g., 13,945 smokers vs. 4,203 nonsmokers, p=0.60, in Thorgeirsson et al, 2008), unless this locus confers a much higher risk in schizophrenia smokers vs. schizophrenia nonsmokers as compared to control smokers vs. control nonsmokers. However, this does not appear to be the case (χ2=3.92, p=0.048 in non-schizophrenia samples and χ2=3.70, p=0.054 in schizophrenia samples, see above). Power for the quantitative trait FTND was estimated to be 40% for R2 change of 0.7% (Thorgeirsson et al, 2008) but 90% for the observed R2 change of 3.3% in the combined smoker groups.
Discussion
In this sample of 525 non-psychiatric control subjects, rs16969968 was associated with smoking severity as previously shown in multiple replications (Saccone et al, 2007; Bierut et al, 2008; Berrettini et al, 2008; Stevens et al. 2008; Thorgeirsson et al, 2008; Lips et al, 2010). We also expanded these findings in a sample of 313 schizophrenia patients. This SNP was also significantly associated with schizophrenia in Caucasians, and interestingly also in nonsmokers in two ethnic groups. However, the sample sizes were small and findings in schizophrenia smokers were not significant, therefore replication studies are needed. Nevertheless, a genetic contribution to the schizophrenia-smoking comorbidity has been repeatedly hypothesized, although we are not aware of evidence of any functional genetic variant that has been associated with nicotine addiction in controls, nicotine addiction in schizophrenia patients, and association with schizophrenia itself in the same cohort beside the data on rs16969968 described here.
However, these findings occur in a pattern that the overrepresentation in schizophrenia was not due to more risk allele in schizophrenia smokers, which could have been a more straightforward explanation to the increased smoking rate in schizophrenia. Rather, the increased risk for schizophrenia occurs in the Caucasian sample and in nonsmokers in both Caucasians and African Americans. The lack of significant association with smoking status in schizophrenia is perhaps not surprising: rs16969968 and other SNPs in LD have been repeatedly associated with smoking related phenotypes that are related to smoking quantity and severity (Berrettini et al. 2008; Thorgeirsson et al, 2008; Bierut et al, 2008; Grucza et al. 2008; Chen et al. 2009; Le Marchand et al. 2008; Sherva et al. 2008; Saccone et al, 2007; Amos et al. 2008). However, it is typically not associated with smoking status (i.e., not significant if compared smokers vs. nonsmokers) (Thorgeirsson et al, 2008; Lips et al, 2010).
While most linkage evidence on chromosome 15q in schizophrenia have been reported on the 15q13-14 region given the strong interest in CHRNA7, some studies have shown linkage signals with psychosis at 15q25, an area where the CHRNA5 is located(Gejman et al, 2001; Park et al. 2004; Vazza et al. 2007), suggesting that a schizophrenia link to this genomic region is also possible. The over-representation of a smoking-related risk allele in nonsmoking schizophrenia patients remains intriguing, although an explanation is not yet clear. Interestingly, another study on association of CHRNB3 polymorphisms in bipolar disorder and nicotine addiction found that the CHRNB3 association with nicotine dependence was not different between bipolar disorder patients and the general population; although two synonymous SNPs in CHRNB3, rs4952 and rs4953, were significantly associated with bipolar disorder even in nonsmokers (Hartz et al 2010). Petrovsky et al showed an association between CHRNA3 polymorphisms and prepulse inhibition in schizophrenia patients although schizophrenia case vs. control association was not significant (Petrovsky et al 2010). Winterer et al showed that rs16969968 was associated with working memory and other cognitive phenotypes that have been related to schizophrenia (Winterer et al 2010). We also note that rs16969968 was protective for cocaine dependence(Grucza et al, 2008), despite the fact that cocaine dependence is also associated with an increased smoking rate(Lai et al. 2000). Therefore, while evidence for the association of rs16969968 and other smoking-related variants with nicotine addiction in psychiatric illnesses is accumulating, the field has yet demonstrated clear evidence that any of the variants is contributing to the increased smoking in schizophrenia or other psychiatric conditions.
We started with a candidate gene shown to be consistently related to smoking and then examined whether it is also overrepresented in schizophrenia itself. Using such strategy, our data suggest that the CHRNA5 variant Asp398Asn is an important genetic variant in our consideration for the genetic basis of smoking in schizophrenia, and should be considered along with other candidate genes in future studies.
In summary, our study shows that Asp398Asn is significantly associated with the severity of smoking in schizophrenia patients similar to (and numerically more robust than) findings in multiple non-psychiatric samples. While a genetic attribute to the high risk of smoking has been repeatedly suggested, this is the first time that a functional genetic variant has been simultaneously linked to smoking and schizophrenia in the same cohort. However, the finding did not provide immediate evidence that Asp398Asn is contributing to the cause of the increased smoking prevalence in schizophrenia patients because this SNP was not significantly more frequent in schizophrenia smokers. Our study suggests that this SNP is significantly contributing to nicotine addiction severity in schizophrenia smokers. Whether and how this finding is related to the increased smoking rate in schizophrenia cannot be determined using the present evidence alone.
Acknowledgements
Support was received from the NIDA and NIAAA Intramural Research Programs, NIH grants DA027680, MH085646, MH077852, and the Maryland Cigarette Restitution Fund Program.
Reference List
- Amos CI, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat.Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol.Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- Chapman S, Ragg M, McGeechan K. Citation bias in reported smoking prevalence in people with schizophrenia. Aust.N.Z.J Psychiatry. 2009;43:277–282. doi: 10.1080/00048670802653372. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr.Genet. 2009 doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti DV, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol.Genet. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De L V, Voineskos S, Wong G, Kennedy JL. Genetic interaction between alpha4 and beta2 subunits of high affinity nicotinic receptor: analysis in schizophrenia. Exp Brain Res. 2006;174:292–296. doi: 10.1007/s00221-006-0458-y. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Velasquez DM, Susce MT, de Leon J. The association between schizophrenia and smoking: Unexplained by either the illness or the prodromal period. Schizophr Res. 2008;104:214–219. doi: 10.1016/j.schres.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Su J, Taylor L, Wilcox M, Van Eerdewegh P, Tsuang MT. A novel permutation testing method implicates sixteen nicotinic acetylcholine receptor genes as risk factors for smoking in schizophrenia families. Hum Hered. 2004;57:59–68. doi: 10.1159/000077543. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejman PV, Sanders AR, Badner JA, Cao Q, Zhang J. Linkage analysis of schizophrenia to chromosome 15. Am J Med Genet. 2001;105:789–793. doi: 10.1002/ajmg.1552. [DOI] [PubMed] [Google Scholar]
- Grucza RA, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol.Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Lin P, Edenberg HJ, Xuei X, Rochberg N, Saccone S, Berrettini W, Nelson E, Nurnberger J, Bierut LJ, Rice JP. Genetic association of bipolar disorder with the β3 nicotinic receptor subunit gene. Psychiatr Genet. 2010 Dec 28; doi: 10.1097/YPG.0b013e32834135eb. 2010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc.Natl.Acad.Sci.U.S.A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Wonodi I, Stine OC, Mitchell BD, Thaker GK. Evidence of missense mutations on the neuregulin 1 gene affecting function of prepulse inhibition. Biol.Psychiatry. 2008;63:17–23. doi: 10.1016/j.biopsych.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Allen DL, Filbey FM, Jepson C, Lerman C, Benowitz NL, Stitzel J, Bryan A, McGeary J, Haughey HM. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Arch Gen Psychiatry. 2007;64:1078–1086. doi: 10.1001/archpsyc.64.9.1078. [DOI] [PubMed] [Google Scholar]
- Kelly C, McCreadie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry. 1999;156:1751–1757. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- Lai S, Lai H, Page JB, McCoy CB. The association between cigarette smoking and drug abuse in the United States. J Addict Dis. 2000;19:11–24. doi: 10.1300/J069v19n04_02. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, Tiirikainen M, Wang H. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, et al. Association of promoter variants in the alpha-7 nicotine acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;12:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol.Genet. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Lips EH, et al. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39:563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, Zai CC, Mirkhani M, Shaikh S, Vincent JB, Meltzer H, Lieberman JA, Strauss J, Lévesque D, Kennedy JL, Le Foll B. Genes Brain Behav. 2010;9:910–917. doi: 10.1111/j.1601-183X.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- Park N, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol.Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Quednow BB, Ettinger U, Schmechtig A, Mössner R, Collier DA, Kühn KU, Maier W, Wagner M, Kumari V. Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers. Neuropsychopharmacology. 2010;35:1429–39. doi: 10.1038/npp.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol.Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet Part B. 2009;150:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AR, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SH, et al. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109:102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol.Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine.Tob.Res. 1999;1(Suppl 2):S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazza G, Bertolin C, Scudellaro E, Vettori A, Boaretto F, Rampinelli S, De Sanctis G, Perini G, Peruzzi P, Mostacciuolo ML. Genome-wide scan supports the existence of a susceptibility locus for schizophrenia and bipolar disorder on chromosome 15q26. Mol.Psychiatry. 2007;12:87–93. doi: 10.1038/sj.mp.4001895. [DOI] [PubMed] [Google Scholar]
- Voineskos S, De L V, Mensah A, Vincent JB, Potapova N, Kennedy JL. Association of alpha4beta2 nicotinic receptor and heavy smoking in schizophrenia. J Psychiatry Neurosci. 2007;32:412–416. [PMC free article] [PubMed] [Google Scholar]
- Voisey J, Swagell CD, Hughes IP, Connor JP, Lawford BR, Young RM, Morris P. A polymorphism in the dysbindin gene (DTNBP1) associated with multiple psychiatric disorders including schizophrenia. Behav Brain Funct. 2010;6:41. doi: 10.1186/1744-9081-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RB, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS.Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, Breitling LP, Nitz B, Raum E, Müller H, Gallinat J, Gal A, Heim K, Prokisch H, Meitinger T, Hartmann AM, Möller HJ, Gieger C, Wichmann HE, Illig T, Dahmen N, Rujescu D. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1448–58. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]
- Zammit S, Spurlock G, Williams H, Norton N, Williams N, O’Donovan MC, Owen MJ. Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use. Br J Psychiatry. 2007;191:402–407. doi: 10.1192/bjp.bp.107.036129. [DOI] [PubMed] [Google Scholar]