Abstract
Mechanical loading is an important factor regulating cartilage metabolism maintained by chondrocytes. However, some of its underlying mechanisms remain poorly understood. In this study, we employed a chondrogenic cell line ATDC5 to investigate roles of P2Y2 and GRK2 in chondrocyte mechanotransduction. We first confirmed the expression of chondrocyte markers in differentiated ATDC5 cells. We then exposed both differentiated and undifferentiated ATDC5 cells to oscillatory fluid flow, and found that differentiated ATDC5 cells responded to oscillatory fluid flow by increasing COX-2 and aggrecan expressions. More importantly, fluid flow induced ERK1/2 response in differentiated cells was increased more than 10 times compared to that in undifferentiated cells. Furthermore, we found that P2Y2 mRNA and protein levels in differentiated ATDC5 cells were significant higher than those in undifferentiated cells. In contrast, GRK2 protein levels in differentiated cells were significantly lower than those in undifferentiated cells. Finally, overexpressions of P2Y2 and GRK2 in differentiated ATDC5 cells result in a 34% increase and a 21% decrease of the ERK1/2 phosphorylation respectively in response to oscillatory fluid flow, suggesting important roles of P2Y2 and GRK2 in chondrocyte mechanotransduction.
Keywords: Chondrocyte, Mechanotransduction, P2Y2, GRK2, ERK1/2
INTRODUCTION
Chondrocytes are responsible for the synthesis and maintenance of extracellular matrix in cartilages; and their activities are regulated by mechanical stimulation.1 Yet, how chondrocytes sense mechanical signals and convert them to intracellular events remains elusive. The in vivo mechanical milieu of chondrocytes is complex, involving tension, compression, shear, fluid flow and hydrostatic pressure. However, if we consider the pericellular environment of chondrocytes, oscillatory fluid shear stress is a major mechanical stimulus influencing chondrocyte bioactivity.2 Studies have shown steady fluid flow is able to significantly increase chondrocyte MAPK activation and nitric oxide release.3,4 Thus, it is necessary to study the effect of more physiological oscillatory fluid flow on regulation of chondrocyte functions.
Previously studies demonstrated that chondrocytes release ATP into their pericellular space in response to mechanical stimulation.5,6 ATP release activates intracellular calcium immobilization,7,8 downregulates nitric oxide release9 and enhances proteoglycan synthesis,1,9 suggesting that ATP receptors, P2 purinergic receptors, may be involved in chondrocyte mechanotransduction. Some studies suggest that one ATP receptor, P2Y, may be involved in chondrocyte mechanotransduction.8,10 We have previously demonstrated that one subtype of P2Y receptors, P2Y2, is important in osteoblastic mechanotransduction.11 However, the role of P2Y2 in chondrocyte mechanotransduction has not been investigated.
The P2Y2 receptor is a G-protein coupled receptor (GPCR). Almost every GPCR undergoes desensitization, and despite their diversity, all cells use a universal desensitizing mechanism.12 Desensitization of GPCR involves one family of proteins, G-protein-coupled receptor kinase (GRK). GRK specifically phosphorylates activated GPCR, and initiates the recruitment of additional proteins, termed arrestins, that aid receptor desensitization.12 Desensitization of GPCR, is an important characteristic of the mechanosensing apparatus in bone.13 One subtype of GRKs, GRK2, is expressed in musculoskeletal system.14 However, the role of GRK2 in regulating chondrocyte desensitizing in response to oscillatory fluid flow is unknown.
In this study, we employed ATDC5, a murine inducible chondrogenic cell line,15 to test the hypothesis whether P2Y2 receptor and GRK2 are involved in chondrocyte mechanotransduction. Oscillatory fluid flow was selected as our mechanical loading signal in vitro since loading and unloading create fluid shear stress on chondrocytes in an oscillatory pattern. We examined ERK1/2 phosphorylation to assess chondrocyte response to oscillatory fluid flow because ERK1/2 phosphorylation regulates a variety of chondrocyte activities, including migration, proliferation and differentiation,16 and is elevated following mechanical loading (compression and fluid flow).17 First, we identified and compared oscillatory fluid flow responses in differentiated and undifferentiated ATDC5 cells. Then, we overexpressed P2Y2 receptor and GRK2 in differentiated chondrocyte-like ATDC5 cells, and investigated the roles of P2Y2 of GRK2 in fluid flow induced ERK1/2 responses in chondrocytes. Our observations suggest that both P2Y2 and GRK2 have important roles in oscillatory fluid flow induced ERK1/2 response.
METHODS
Cell culture and induction of chondrogenic differentiation
ATDC5 cells were cultured in growth media composed of DMEM/F-12 (Life Technologies), 5% fetal bovine serum (FBS; Hyclone), 1% penicillin and streptomycin (P/S) (Life Technologies) and maintained in a humidified incubator at 37 °C with 5% CO2. Cell differentiation was induced by addition of 10mg/ml selenium (Sigma), 10μg/ml transferring (Sigma) and 10μg/ml insulin (Sigma) into the cell culture medium for 21 days.
Oscillatory fluid flow
After differentiation, ATDC5 cells were subcultured in growth media on glass slides (7cm × 3.5cm) for 2 days at a density of 5000 cells/cm2. Twelve hours prior to fluid flow, the media were changed to flow media containing DMEM/F-12, 0.5% FBS and 1% P/S. Oscillatory fluid flow was applied to cells at 10 dynes/cm2 peak stress level with 1Hz frequency as described in our previous publications.18,19
RT–PCR analysis
Cells were lysed and homogenized with a QIAshredder mini column (QIAGEN). Total RNA was extracted with Qiagen RNeasy mini kit. cDNA was prepared from 1μg total RNA using the iScript Kit (Bio-rad). PCR amplification was performed in a 25μl reaction with 1μl cDNA reaction using Qiagen PCR kit according to the manufacturer’s protocol. For comparison between undifferentiated and differentiated cells, RT-PCR was performed and the products were analyzed by agarose gel electrophoresis. For the quantification of gene expression in response to fluid flow, real time PCR was performed using the ABI PRISM 7900 sequence detection system 9 (Applied Biosystems). All primers for RT-PCR designed by software Primer Express (Applied Biosystems) are listed in Table 1.
Table 1.
Primers for RT-PCR
| Gene | Primer |
|---|---|
| Aggrecan | Forward primer: cta tgg tga caa gga cga gt Reverse primer: ctg gaa ggt gaa ttt ctct g |
| Collagen II | Forward primer: agg cag aca gta cct tga ga Reverse primer: ttg gga tca atc cag tag tc |
| Collagen X | Forward primer: ctc aaa tac cct ttc tgc tg Reverse primer: cct ctt act gga atc cct tt |
| SOX 9 | Forward primer: acg gaa cag act cac atc tc Reverse primer: ctc tcg ctt cag atc aac tt |
| COX-2 | Forward primer: gcg gtt acc act tca aac t Reverse primer: ctc ctg gtc ttc aat gtt ga |
| P2Y2 | Forward primer: acc tgc cgg ctg tct aca ttt Reverse primer: ccc gaa gat cca gtc agt ctt g |
Western immunoblotting
Immediately after fluid flow, ATDC5 cells, attached to slides, were frozen at −80°C and lysed at a later time with RIPA buffer, supplemented with 0.2mM Na3VO4 (Sigma) and a protease inhibitor cocktail (Calbiochem). Total protein concentrations of cell lysates were measured with BCA™ protein assay kit (Pierce). 15μg of protein from each sample was resolved by SDS-PAGE and transferred to PVDF membranes. The membrane was then probed with primary antibodies specific to phosphorylated ERK1/2, total ERK1/2 (Cell Signaling), GRK2 (Upstate), COX-2 (Calbiochem) or P2Y2 (Alomone). Visualization of immunoreactive proteins was achieved by employing an ECL film detection system (Pierce). Densitometric analysis was carried out with Quality One image analysis software (Bio-Rad).
Transient transfection
To examine the roles of P2Y2 and GRK2 in ATDC5 mechanotransduction pathways, full-length cDNA for mouse P2Y2 purinergic receptors (from ATCC) was subcloned into a pcDNA3.1 vector (Invitrogen). Full length mouse cDNA GRK2 was a gift from Dr. Richard T. Premont (Duke University). P2Y2 and GRK2 plasmids were transfected into ATDC5 cells with FuGENE 6 transfection reagent kit (Roche) according to manufacturer’s protocol.
Data Analysis
All data were analyzed with GraphPad Prism software (GraphPad Software). Data are expressed as means ± standard error. To compare observations from two groups, two-sample Student’s t-test was employed. A value of p< 0.05 was considered statistically significant.
RESULTS
Induction of ATDC5 chondrogenic differentiation
ATDC5 is a murine inducible chondrogenic cell line.15 We first examined the gene expression of four chondrogenic markers, aggrecan, type II collagen, type X collagen and Sox-9, in cells cultured in either growth or differentiation media for 7, 14, 21 and 28 days. As reported previously,15 the mRNA levels of all markers were elevated in cells cultured in differentiation media relative to those cultured in growth media (data not shown). Additionally, alcian blue staining, an indicator of the presence of sulfated glycosaminoglycans (sGAG) in matrix, was not present in cells cultured in growth media or differentiation media after 7 days. However, sGAG production was present in culture maintained in differentiation media after 21 days (data not shown). Our data demonstrated that the differentiated ATDC5 are chondrocyte-like cells.
Effect of oscillatory fluid flow on ATDC5
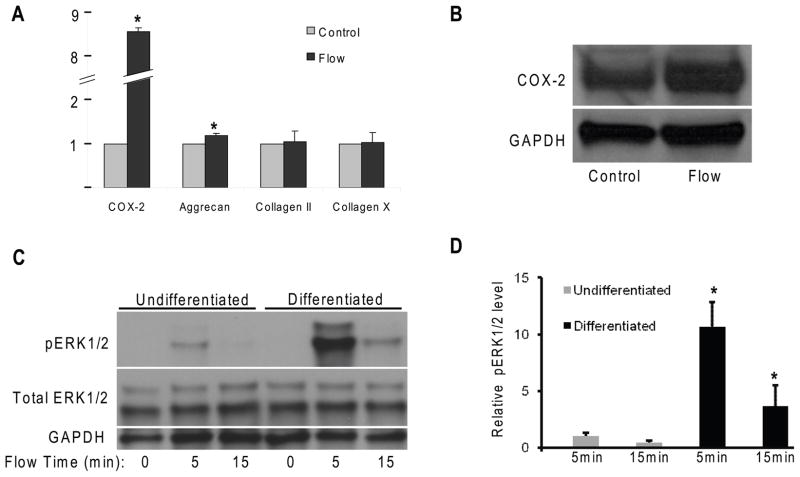
To study mechanotransduction in chondrocytes, 21-day differentiated chondrocyte-like ATDC5 cells were exposed to oscillatory fluid flow (10 dynes/cm2, 1 Hz) for 2 hours, and twenty-four hours after stimulation mRNA was extracted. Cells maintained under static conditions served as controls. Real time RT-PCR was performed to examine COX-2, aggrecan, type II collagen and type X collagen gene expression. COX-2 and aggrecan gene expressions were significantly elevated (750% and 15%, respectively) following oscillatory fluid flow (Fig. 1A). The directional changes in COX-2 protein are similar to that of mRNA (Fig. 1B). However, changes in type II collagen and type X collagen gene expression were not observed.
Figure 1.
(A) Real time RT-PCR shows the changes of mRNA levels of COX-2, aggrecan, collagen II, and collagen X in differentiated ATDC5 cells 24 hours after 2-hour oscillatory fluid flow (10 dynes/cm2, 1 Hz) stimulation. (B) Western blotting analysis of COX-2 protein expression 24 hours after 2-hour oscillatory fluid flow stimulation. (C) ERK1/2 phosphorylation in undifferentiated and differentiated ATDC5 cells in response to oscillatory fluid flow at 5 and 15 minutes. (D) Bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 3–5 times.
Employing a western blotting approach with antibodies specific for phosphorylated and total ERK1/2, we identified changes in ERK1/2 phosphorylation in differentiated chondrocyte-like ATDC5 cells in response to oscillatory fluid flow (10 dynes/cm2, 1 Hz). In the absence of flow there was minimal ERK1/2 phosphorylation (Fig. 1C,D). However, significant increases (more than 10 and 3.5 folds, respectively) in ERK1/2 phosphorylation in differentiated chondrocyte-like cells were observed at 5 and 15 minutes in response to oscillatory fluid flow. In contrast, oscillatory fluid flow moderately or did not significantly alter ERK1/2 phosphorylation in undifferentiated cells, at 5 and 15 minutes, respectively. Like GAPDH, total ERK1/2 was unchanged in response to fluid flow (Fig, 1C). Thus, we quantified all ERK1/2 phosphorylation normalized to GAPDH.
ATDC5 cells response to ATP
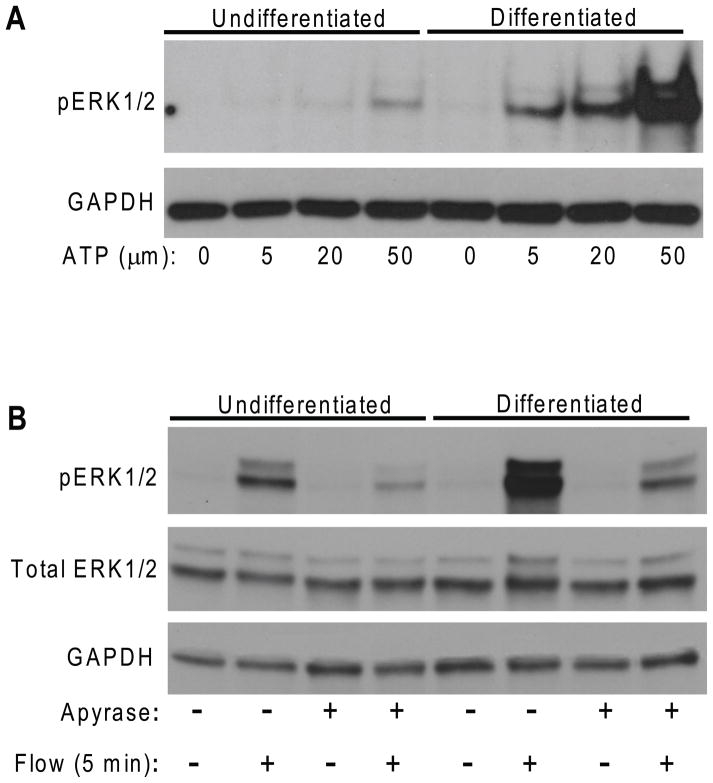
Studies have shown that chondrocytes, under mechanical stimuli, release ATP, which in turn can initiate MAPK activation and various downstream responses in chondrocytes.5,7,8 However, direct evidence of the involvement of purinergic receptors in chondrocyte mechanotransduction has not been established. To address this issue, we directly added ATP at three different concentrations (5μm, 20μm and 50μm) to both differentiated and undifferentiated ATDC5 cultures for 5 minutes. We observed that the addition of exogenous ATP to differentiated chondrocyte-like cells enhanced ERK1/2 phosphorylation (more than 10 folds) over undifferentiated cells (Fig. 2A). In addition, to confirm the involvement of ATP in flow response, apyrase (10 units/ml), an enzyme that rapidly hydrolyzes ATP to yield AMP,11 was added into the flow media and the flow induced increases in ERK1/2 phosphorylation at 5 minutes were significantly inhibited (Fig. 2B).
Figure 2.
(A) Increases in ERK1/2 phosphorylation in undifferentiated and differentiated ATDC5 cells were observed in response to 5μM, 20μM and 50μM of ATP stimulations. (B) Apyrase (10 units/ml) significantly inhibited flow induced ERK1/2 phosphorylation at 5 minutes in both undifferentiated and differentiated ATDC5 cells.
Effects of P2Y2 purinergic receptors and GRK2
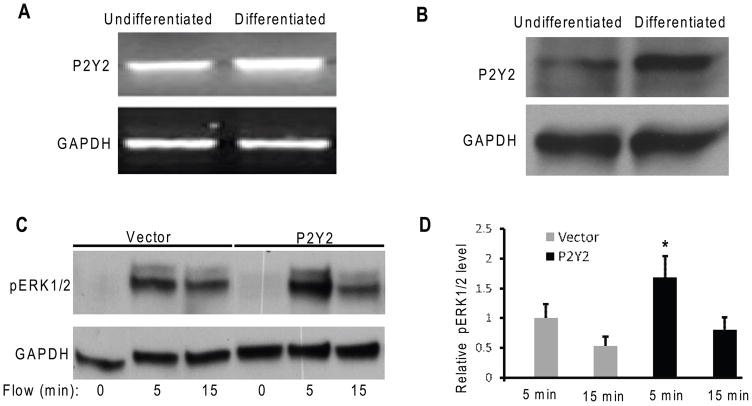
Since the addition of exogenous ATP induced ERK1/2 phosphorylation in chondrocyte-like ATDC5 cells, ATP receptors may be involved in ERK1/2 activation. To explore this possibility, we examined P2Y2 mRNA and protein expression in differentiated and undifferentiated ATDC5 cells by RT-PCR and western blotting, respectively. Our results demonstrated that P2Y2 mRNA and protein levels in differentiated cells were significantly increased relative to those in undifferentiated cells (Fig. 3A,B).
Figure 3.
(A) 35 cycles of RT-PRC show P2Y2 mRNA expression level in differentiated ATDC5 cells was about 2.76 fold higher relative to that in undifferentiated cells. (B) Western blotting analysis shows P2Y2 protein level in differentiated ATDC5 cells was about 3 fold higher than that in undifferentiated cells. (C) Increases in ERK1/2 phosphorylation in ATDC5 cells transfected with pcDNA3-P2Y2 (P2Y2) or empty vector pcDNA3 (Vector) were observed at 5 and 15 minutes in response to oscillatory fluid flow. (D) Bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 3–5 times.
To identify the impact of P2Y2 overexpression in chondrocyte mechanosensitivity, we transfected differentiated ATDC5 cells with either P2Y2 plasmid DNA or empty control vector. We found that after only 5 minute fluid flow stimulation cells overexpressing P2Y2 significantly increased the ERK1/2 phosphorylation relative to empty vector controls (Fig 3C,D).
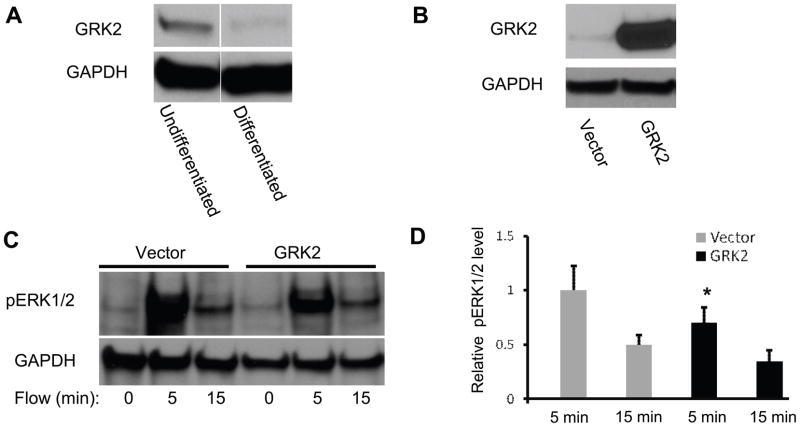
To examine the role of GRK2 in chondrocytes, we first examined the expression of GRK2 in both differentiated and undifferentiated ATDC5 cells. GRK2 expression was significant decreased in differentiated cells compared to undifferentiated cells (Fig. 4A). After overexpressing GRK2 in differentiated cells (Fig 4B), we exposed cells to oscillatory fluid flow (10 dynes/cm2, 1 Hz). Western blot analysis of total protein lysate suggests that ERK1/2 phosphorylation induced by oscillatory fluid flow was significantly inhibited by GRK2 overexpression (Fig. 4C,D).
Figure 4.
(A) Western blotting analysis of undifferentiated and differentiated ATDC5 cells, blotted with anti-GRK2 monoclonal antibody. (B) Western blotting analysis of differentiated ATDC5 cells transfected with pcDNA3-GRK2 (GRK2) and empty vector pcDNA3 (Vector), blotted with anti-GRK2 monoclonal antibody. (C) Increases in ERK1/2 phosphorylation in ATDC5 cells transfected with pcDNA3-GRK2 (GRK2) or empty vector pcDNA3 (Vector) were observed at 5 and 15 minutes in response to oscillatory fluid flow. (D) Bar graph representation of ERK1/2 phosphorylation quantified by scanning densitometry normalized to GAPDH. (*, p<0.05) Each bar represents the mean ± S.E. and each experiment was repeated on 3–5 times.
DISCUSSION
In this study, we demonstrated that differentiated ATDC5 cells express the chondrocyte markers, and secrete sulfated glycosaminoglycans. Our results are consistent with previous studies suggesting ATDC5 is an inducible chondrogenic cell line.20,21 In addition, we examined the effects of oscillatory fluid flow on differentiated ATDC5 cells, and our results demonstrate that differentiated ATDC5 cells (after 21-day treatment in differentiated media), like other chondrocyte cell lines or primary chondrocytes, respond to mechanical loading induced fluid flow by increasing mRNA levels of COX-2 and aggrecan, and ERK1/2 phosphorylation.17,22,23 Our results suggest that ATDC5 is a suitable chondrocyte cell model to study mechanotransduction. In contrast to a previous study in which bovine chondrocytes were exposed to mechanical loading for 3 days,17 we did not observe an increase in mRNA levels of type II and type X collagen. This may be due to our short duration (2 hours) of mechanical loading.
In chondrocytes, ERK1/2 activation is required for chondrocyte transcription and cell proliferation in response to shear or compression.17,24 Our studies demonstrate oscillatory fluid flow also increases ERK1/2 phosphorylation in differentiated ATDC5 cells, suggesting fluid flow as a mechanical signal may impact chondrocyte proliferation, survival, differentiation and metabolism. Interestingly, in undifferentiated cells we only detected moderate changes in ERK1/2 phosphorylation, suggesting that undifferentiated cells are less sensitive to mechanical loading, and differentiated ATDC5 cells have increased mechanosensitivity. This finding is consistent with the previous report about bone cells.25 In addition, the difference in ERK1/2 responses between differentiated and undifferentiated ATDC5 cells may be due to differences in pericellular matrix compositions. Previous studies have demonstrated that the interaction between pericellular matrix and integrins plays a critical role in transmission of mechanical force for chondrocytes.26–28 Our results show that differentiated cells secreted an abundance of pericellular matrix proteins. Thus, the greater ERK1/2 activation was observed in differentiated cells following fluid flow. Furthermore, the different ERK1/2 responses in differentiated and undifferentiated ATDC5 cells were observed following the addition of exogenous ATP, suggesting, in addition to pericellular matrix, the different expressions of ATP receptors (such as P2Y2 receptor) and their regulators (such as GRK2) may contribute to the enhanced mechanosensitivity in differentiated cells. Our apyrase experiments further confirmed that ATP and ATP receptors are involvement in flow induced ERK1/2 response in chondrocytes.
Previous studies have shown that, unlike other P2 receptors, P2Y2 receptors are present only in cells within the superficial zone of cartilage in vivo,29 where chondrocytes experience fluid shear stress resulted from synovial fluid. The increased expression of P2Y2 receptor mRNA and protein levels in differentiated ATDC5 comes with the increased ERK1/2 response to fluid flow, suggesting that P2Y2 may play an important role in enhancing chondrocyte mechanosensitivity. We have previously showed that P2Y2 plays an important role in Osteoblast mechanotransduction.11 Other studies also suggest P2Y2 has implications with mechanical stimulation in chondrocytes.5,8 Thus, our result of overexpressing of P2Y2 receptors for the first time demonstrated that P2Y2 receptors are directly involved in oscillatory fluid flow induced ERK1/2 phosphorylation. Together with observations from exogenous ATP studies, this suggests oscillatory fluid flow increases ERK1/2 phosphorylation via P2Y2 receptors, which is consistent with a previous study in human endometrial stromal cells.30
P2Y2 receptor, as a G-protein coupled receptor, is regulated by GRKs. GRK2 deficient mice are more sensitive to mechanical stimulation than wild-type mice after intraplantar lambda-carrageenan injection,31 and GRK has been suggested to be involved in desensitizing P2Y purinergic receptors in human platelets.32 Thus, we examined GRK2 protein levels in ATDC5 cells and found that GRK2 levels are significantly higher in undifferentiated ATDC5 cells relative to differentiated cells, suggesting that undifferentiated cells have a greater capacity to desensitize their GPCRs. This may be one reason why ERK1/2 phosphorylation is not significantly changed following fluid flow in undifferentiated cells. In addition, GRK2 overexpression in differentiated cells decreases ERK1/2 phosphorylation following fluid flow, suggesting that GRK2 has a role in regulating ERK1/2 phosphorylation following fluid flow. Interestingly, exogenous ATP moderately increased ERK1/2 phosphorylation in undifferentiated cells, which does not correlate well with elevated P2Y2 mRNA and protein levels in these cells, suggesting P2Y2 is not the sole player here. Nevertheless, it is likely that the desensitization of P2Y2 by GRK2 may contribute to the moderate ERK1/2 response in undifferentiated cells since these cells have high levels of GRK2 which can desensitize the ATP receptor to induce responses. However, whether GRK2 directly desensitizes P2Y2 receptors, resulting in decreased ERK1/2 phosphorylation, needs further investigation.
In summary, we employed ATDC5 chondrocyte cell line to study mechanotransduction, and showed an increased mechanosensitivity of ATDC5 chondrocyte cells during the differentiation. More importantly, we for the first time demonstrated that P2Y2 purinergic receptors and GRK2 are directly involved in mechanical loading induced ERK1/2 response, suggesting important roles of P2Y2 and GRK2 in chondrocyte mechanotransduction.
Acknowledgments
This work was supported by National Institutes of Health Grants AR054851 (to J.Y.) and AR054915 (to R.R. Gomes). We greatly appreciate the gift of mouse GRK2 in pcDNA3 plasmid from Dr. Richard T. Premont (Duke University).
References
- 1.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 2.Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191 (Pt 1):1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung CT, Henshaw DR, Wang CC, et al. Mitogen-activated protein kinase signaling in bovine articular chondrocytes in response to fluid flow does not require calcium mobilization. J Biomech. 2000;33:73–80. doi: 10.1016/s0021-9290(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 4.Das P, Schurman DJ, Smith RL. Nitric oxide and G proteins mediate the response of bovine articular chondrocytes to fluid-induced shear. J Orthop Res. 1997;15:87–93. doi: 10.1002/jor.1100150113. [DOI] [PubMed] [Google Scholar]
- 5.Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41:567–575. [PubMed] [Google Scholar]
- 6.Graff RD, Lazarowski ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 2000;43:1571–1579. doi: 10.1002/1529-0131(200007)43:7<1571::AID-ANR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Pingguan-Murphy B, El-Azzeh M, Bader DL, Knight MM. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol. 2006;209:389–397. doi: 10.1002/jcp.20747. [DOI] [PubMed] [Google Scholar]
- 8.Elfervig MK, Graff RD, Lee GM, et al. ATP induces Ca(2+) signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cartilage. 2001;9:518–526. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of. NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006;209:845–853. doi: 10.1002/jcp.20768. [DOI] [PubMed] [Google Scholar]
- 10.Kono T, Nishikori T, Kataoka H, et al. Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochem Funct. 2006;24:103–111. doi: 10.1002/cbf.1304. [DOI] [PubMed] [Google Scholar]
- 11.You J, Jacobs CR, Steinberg TH, Donahue HJ. P2Y purinoceptors are responsible for oscillatory fluid flow-induced intracellular calcium mobilization in osteoblastic cells. J Biol Chem. 2002;277:48724–48729. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs JJ, Hara MR, Davenport CL, et al. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399–407. doi: 10.1016/s8756-3282(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 14.Spurney RF, Flannery PJ, Garner SC, et al. Anabolic effects of a G protein-coupled receptor kinase inhibitor expressed in osteoblasts. J Clin Invest. 2002;109:1361–1371. doi: 10.1172/JCI14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990;30:109–116. doi: 10.1016/0922-3371(90)90079-c. [DOI] [PubMed] [Google Scholar]
- 16.Marais R, Marshall CJ. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 17.Fitzgerald JB, Jin M, Chai DH, et al. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283:6735–6743. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- 18.You J, Yellowley CE, Donahue HJ, et al. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122:387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- 19.You J, Reilly GC, Zhen X, et al. Osteopontin Gene Regulation by Oscillatory Fluid Flow via Intracellular Calcium Mobilization and Activation of Mitogen-activated Protein Kinase in MC3T3-E1 Osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 20.Tare RS, Howard D, Pound JC, et al. Tissue engineering strategies for cartilage generation--micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem Biophys Res Commun. 2005;333:609–621. doi: 10.1016/j.bbrc.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 21.Malafaya PB, Oliveira JT, Reis RL. The effect of insulin-loaded chitosan particle aggregated scaffolds in chondrogenic differentiation. Tissue Eng Part A. 2009 doi: 10.1089/ten.TEA.2008.0679. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1beta induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology. 2006;43:413–429. [PubMed] [Google Scholar]
- 23.Nicodemus GD, Bryant SJ. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage. 18:126–137. doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Ryan JA, Eisner EA, DuRaine G, et al. Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. J Tissue Eng Regen Med. 2009;3:107–116. doi: 10.1002/term.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knippenberg M, Helder MN, Doulabi BZ, et al. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780–1788. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 26.Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009;19:457–469. doi: 10.1111/j.1600-0838.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 27.Kudirka JC, Panupinthu N, Tesseyman MA, et al. P2Y nucleotide receptor signaling through MAPK/ERK is regulated by extracellular matrix: involvement of beta3 integrins. J Cell Physiol. 2007;213:54–64. doi: 10.1002/jcp.21087. [DOI] [PubMed] [Google Scholar]
- 28.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–446. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 29.Knight MM, McGlashan SR, Garcia M, et al. Articular chondrocytes express connexin 43 hemichannels and P2 receptors - a putative mechanoreceptor complex involving the primary cilium? J Anat. 2009;214:275–283. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal. 2008;20:1248–1255. doi: 10.1016/j.cellsig.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Kleibeuker W, Ledeboer A, Eijkelkamp N, et al. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur J Neurosci. 2007;25:1696–1704. doi: 10.1111/j.1460-9568.2007.05423.x. [DOI] [PubMed] [Google Scholar]
- 32.Hardy AR, Conley PB, Luo J, et al. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105:3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]