Abstract
Perfluorocarbon thin films and polymer brushes were formed on stainless steel 316L (SS316L) to control the surface properties of the metal oxide. Substrates modified with the films were characterized using diffuse reflectance infrared Fourier transform spectroscopy (DRIFT), contact angle analysis, atomic force microscopy (AFM), and cyclic voltammetry (CV). Perfluorooctadecanoic acid (PFOA) was used to form thin films by self-assembly on the surface of SS316L. Polypentafluorostyrene (PFS) polymer brushes were formed by surface initiated polymerization using SAMs of 16-phosphonohexadecanoic acid (COOH-PA) as the base. PFOA and PFS were effective in significantly reducing the surface energy and thus the interfacial wetting properties of SS316L. The SS316L control exhibited a surface energy of 38 mN/m compared to PFOA and PFS modifications, which had surface energies of 22 and 24 mN/m, respectively. PFOA thin films were more effective in reducing the surface energy of the SS316L compared to PFS polymer brushes. This is attributed to the ordered PFOA film presenting aligned CF3 terminal groups. However, PFS polymer brushes were more effective in providing corrosion protection. These low energy surfaces could be used to provide a hydrophobic barrier that inhibits corrosion of the SS316L metal oxide surface.
Keywords: stainless steel 316L, fluorine, thin films, surface initiated polymerization, low surface energy
INTRODUCTION
The surface of stainless steel 316L (SS316L) is readily corroded in the presence of water by the formation of a reactive metal oxide layer.1, 2 This is a significant issue because SS316L is used in many applications where structural integrity is crucial, such as marine pipelines and components of ships.1, 2 Corrosion is expedited not only by water, but when bacteria and marine organisms colonize the surface in a process called biofouling.3-6 Previous approaches to limit marine biofouling have consisted of using coatings, including paints, which actively release biocidal toxins such as cuprous oxide, tributyltin, and diuron.7-9 These coatings are usually not strongly bound at the surface, leading to delamination and exposing the metal oxide surface to corrosion and biofouling.10-12 Additionally, the effectiveness of the coating relies on release of toxic biocides, which have been shown to adversely affect the marine ecosystem.8-11 Here, steps have been taken towards an alternate approach to inhibit biofouling that limits the disruption of the marine ecosystem. Passive thin films which control the interfacial wetting properties of the metal oxide are formed. The films are strongly adhered and hydrophobic; forming low surface energy interfaces consisting of fluorinated carbon chains. The reduced surface wettability will limit corrosion and promote release of attached bacteria and marine organisms that cause biofouling.5, 13-15
In this study, organic thin films of perfluorooctadecanoic acid (PFOA) and polypentafluorostyrene (PFS) brushes were formed on the surface of SS316L. In a first approach, self-assembling molecules were examined as a way of forming thin films.16, 17 Perfluorooctadecanoic acid (PFOA) consists of a carboxylic acid head group with a fluorinated 18-carbon chain. Stable films of PFOA were formed on SS316L using a solution deposition method. The PFOA thin films formed were characterized using diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, atomic force microscopy (AFM), and contact angle analysis.
Surface initiated polymerization (SIP) of pentafluorostyrene (PFS) from 16-phosphonohexadecanoic acid (COOH-PA) monolayers was used to form polymer brushes. In this approach, the carboxylic acid tail group of a well-ordered COOH-PA thin film provides a network of densely packed initiator sites for polymerization to occur.18-20 Polymer brushes grown from these active sites impart high surface coverage and uniformity.19, 21 It has been found that the macromolecular polymer brushes can provide a more substantial coating than monomolecular thin films.21 This is because the polymer brush can mask defects in thin films which expose the metal oxide surface.19, 21 In this work, the 2,3,4,5,6-pentafluorostyrene monomer was successfully polymerized from a carboxylic acid terminated film in order to provide low surface energy polymer brush.19, 20 DRIFT was used to confirm each step in the SIP process and the wetting properties of the PFS brushes were assessed using contact angle analysis.
EXPERIMENTAL
Materials and Methods
Perfluorooctadecanoic acid (97%), pentafluorophenol (PFP, 99%), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC, 98+%), 16-phosphonohexadecanoic acid (COOH-PA, 97%) and 2,2α-azobis(2-amidinopropane)dihydrochloride (ABAP, 97%) were obtained from Sigma Aldrich and used as received. 2,3,4,5,6-pentafluorostyrene (98%, stab. with 250ppm 4-tert-butylcatechol) was purchased from Alfa Aesar and rinsed with 0.1M sodium hydroxide followed by distilled water before use. Tetrahydrofuran (THF) optima grade solvent was obtained from Fisher Scientific and was distilled over sodium and benzophenone before use. Stainless steel 316L (SS316L) foil of 0.5mm thickness was purchased from Goodfellow, Inc. and prepared as described below.
SS316L substrate preparation and cleaning
SS316L foil was sanded (150, 320, 400, 600 grit sandpaper) then cut to 1cm2 substrates. Samples for AFM analysis were additionally polished using a Buehler Metaserv polisher (400, 800, 1200 grit sandpapers followed by 1 m diamond suspension). The SS316L substrates were cleaned by rinse in acetone and sonication in methanol for 30 minutes then immersion in boiling methanol for 10 minutes. This cleaning procedure served to remove organic residue and metal dust from the surface. The clean SS316L substrates were placed in a 100°C oven to dry for 8-12 hours before further use.
Perfluorooctadecanoic acid (PFOA) film deposition
Thin films of perfluorooctadecanoic acid (PFOA) were formed on SS316L using a solution deposition method. A 0.5mM solution of PFOA in distilled THF was prepared. Cleaned SS316L substrates were heated in a 100°C oven for 60 minutes and then immersed in the PFOA solution for 5 minutes. Substrates were removed from solution and solvent was evaporated at room temperature before substrates were placed in an oven at 100°C for 30 minutes. This dip procedure was repeated before the substrates were placed in an oven at 100°C for 8-12 hours.
Polypentafluorostyrene (PFS) surface initated polymerization
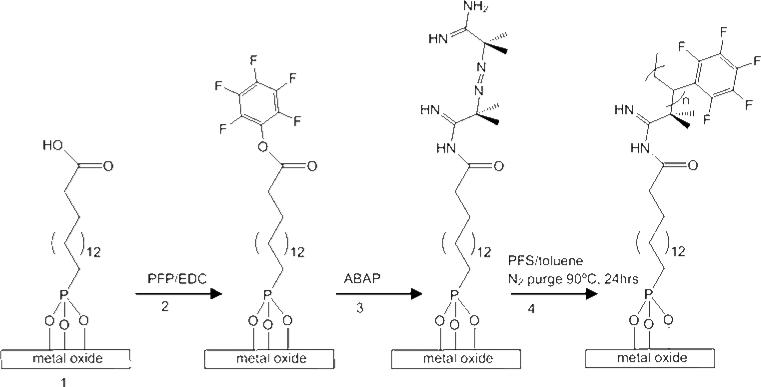
PFS brushes were formed by modifying the technique proposed by Roux, et al.20 First, films of 16-phosphonohexadecanoic acid (COOH-PA) were prepared on SS316L substrates using a solution deposition method (Scheme 1; 1). Substrates were immersed in a 0.5mM solution of COOH-PA in distilled THF for 24 hours. Substrates were then removed from solution and placed in an oven at 100°C for 8-12 hours. Next, the carboxylic acid film tail groups were used to attach pentafluorophenol (PFP) according to Scheme 1; 2. COOH-PA modified substrates were immersed in 2-propanol containing 0.2M PFP and 0.1M 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) for 3 hours. Excess solvent was removed by placing the substrates under vacuum (0.1 Torr). Finally, PFP modified substrates were used to attach the free radical initiator 2,2α-azobis(2-amidinopropane)dihydrochloride (ABAP) to the surface (Scheme 1; 3). Substrates with PFP were immersed in a solution (1:1 v/v MeOH: H2O) containing 0.1M ABAP for 1 hour. Excess solvent was removed by placing the substrates under vacuum (0.1 Torr) before PFS polymerization from the surface. 2,3,4,5,6-Pentafluorostyrene (PFS) was prepared by extracting in a separatory funnel 3 times with 30mL aliquots of 0.1 M sodium hydroxide followed by 3 times with 30mL aliquots of distilled water. The PFS was then diluted in a 1:1 v/v ratio with toluene. Substrates with covalently bound ABAP were placed in the PFS: toluene solution and heated to 90°C under N2 purge for 24 hours (Scheme 1; 4). Substrates were removed after 24 hours and placed in boiling toluene to remove visible residual polymer from the surface.
Scheme 1.
PFS surface initiated polymerization from SS316L metal oxide.
DRIFT spectroscopy
Diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy was used to confirm modification of SS316L substrates. A Nexus 470 Fourier Transform Infrared (FTIR) Spectrometer with the DRIFT attachment was used to analyze the functional group stretching regions of molecules attached to the surface. Each sample run consisted of 256 scans with 4cm-1 resolution.
Contact angle analysis
Contact angle analysis was used to evaluate the surface energy and wetting properties of PFOA thin films and PFS polymer brushes formed on SS316L. A Video Contact Angle (VCA) 2000 instrument was used to determine the advancing contact angle of deionized water (dH2O) and methylene iodide (CH2I2) test liquids with the surface. A syringe was used to deposit a 1 μL droplet of test liquid on a surface and the advancing contact angle (θ) the liquid made with the surface was measured.22, 23 Contact angles were acquired for each test liquid in three different spots on each of three samples. A one way analysis of variance (ANOVA) with a Bonferroni post-hoc test at the p <0.05 level of significance was used to compare the calculated surface energies for the modified and control substrates.
Atomic force microscopy (AFM)
PFOA thin film uniformity was qualitatively assessed using AFM to image control SS316L and PFOA modified substrates.24 A Molecular Imaging PicoSPM system was used in noncontact mode with silicon nitride cantilevers (Nanosensors Inc.). Image areas of 500nm2 were acquired in three different spots on each of three samples. The structure and uniformity of the SS316L surface before and after modification with PFOA thin films was evaluated by comparing the average root mean square (rms) roughness, which measures the standard deviation of all the height values in the imaged area.
Cyclic Voltammetry (CV)
Corrosion inhibition was assessed using CV to determine if PFOA thin films and PFS polymer brushes reduced oxidation of the surface compared to the control SS316L.19, 25 Measurements were taken using a VersaSTAT3 system (Princeton Applied Research, Inc.). Voltammograms were recorded with a standard three electrode system consisting of a saturated calomel reference electrode, a platinum wire auxiliary electrode, and SS316L with an exposed area of 1cm2 as the working electrode.19 Measurements were obtained in a quartz cell containing 0.1M NaOH with a potential scan ranging from -1 to +1V at 50mV/s.
RESULTS AND DISCUSSION
Perfluorooctadecanoic acid (PFOA) film formation was accomplished by immersion in a 0.5mM solution of PFOA in distilled THF for 5 minutes, solvent was evaporated and the substrates were heated as described above. This process was repeated. Film formation was confirmed by DRIFT spectroscopy. Peaks attributed to CF2 asymmetric stretches were present in the DRIFT spectrum at 1235, 1210, and 1153cm-1, confirming the presence of PFOA on the surface (Figure 1).26, 27
Figure 1.
CF2 asymmetric stretching region of the DRIFT spectra of perfluorooctadecanoic acid films formed on stainless steel 316L.
The binding mode of PFOA with the surface was also examined by comparing the DRIFT spectra of the PFOA surface film to that of the solid PFOA powder. The film is formed when carboxylic acid head groups of PFOA molecules adhere to the metal oxide layer of SS316L. The DRIFT spectrum of PFOA films revealed a strong peak attributed to νC-OO- at 1587cm-1 (Figure 2). This peak was not present in the spectrum of the PFOA solid powder and indicates the carboxylic acid is bound to the surface in a bidentate fashion.16, 28 The peaks of the PFOA film attributed to the carboxylic acid head group and fluorocarbon alkyl chain persisted through THF rinse (10 minutes) and sonication (15 minutes), indicating the film was strongly adhered to the SS316L surface.
Figure 2.
Carboxylic acid binding region of the DRIFT spectra of perfluorooctadecanoic acid (PFOA) solid powder (gray) and the PFOA surface film formed on stainless steel 316L (black).
The polypentafluorostyrene (PFS) brushes were formed by surface initated polymerization (Scheme 1) and each step was confirmed by DRIFT (Figure 3a-d).20 In the first step of the polymerization, thin films of 16-phosphonohexadecanoic acid (COOH-PA) were formed on the metal oxide surface of SS316L (Scheme 1; 1). The presence of these films and persistence through solvent rinse and sonication was confirmed by peaks in the DRIFT spectra assigned to the CH2 asymmetric and CH2 symmetric stretches of the alkyl chains at 2916 and 2848cm-1 (Figure 3a).29, 30 The binding mode of the COOH-PA films was also evaluated using DRIFT by comparing the phosphonic acid region of the COOH-PA film to the solid COOH-PA powder. The COOH-PA powder contained peaks in the DRIFT spectrum corresponding to P=O at 1260cm-1, P-O at 1077, 1007cm-1, and P-OH at 952cm-1. COOH-PA films displayed one broad peak in the DRIFT spectra assigned to P-O group stretches at 1082cm-1.30, 31 This data, coupled with the disappearance of peaks found in the spectrum of the COOH-PA solid powder attributed to the P=O and P-OH stretches at 1260cm-1 and 952cm-1, indicates that there was only one P-O species present.30 Therefore, it was determined that the phosphonic acid head group of the film was bound to the surface in a tridentate fashion. It was further confirmed that the carboxylic acid tail groups of COOH-PA were not bound to the surface through the evidence of peaks in the DRIFT spectra at 1690 and 1460cm-1, indicating “free” carboxylic acid.32, 33
Figure 3.
DRIFT spectra corresponding to the individual steps (a-d) in the polymerization of pentafluorostyrene (PFS) from the surface of stainless steel 316L (a) Surface modification with thin films of 16-phosphonohexadecanoic acid (COOH-PA) is confirmed by the methylene asymmetric and symmetric stretches at 2915, 2848cm-1 (b) Pentafluorophenol (PFP) linked to the terminal carboxylic acid of the COOH-PA film is confirmed by the peak at 2980cm-1 attributed to the benzene ring structure (c) 2,2α-azobis(2-amidinopropane)dihydrochloride (ABAP) displacement of PFP is confirmed by the amide and amine peaks present at 3310cm-1 and in the range 1700-1000cm-1 (d) PFS polymerization from the surface indicated by the peak at 2963cm-1 corresponding to the fluorine benzene ring structures that are pendant from the PFS polymer backbone.
The carboxylic acid tail groups were then used to immobilize pentafluorosytrene (PFP) using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (Scheme 1; 2). PFP attachment was verified by the peak in the DRIFT spectra at 2980cm-1 corresponding to the benzene ring structure (Figure 3b). The PFP is subsequently displaced by the free radical initiator 2,2α-azobis(2-amidinopropane)dihydrochloride (ABAP) (Scheme 1; 3). Attachment of ABAP was confirmed by a strong broad stretch at 3310cm-1 and multiple peaks in the 1700-1000cm-1 range of the DRIFT spectrum attributed to the secondary amide as well as primary, secondary, and tertiary amine groups present in ABAP (Figure 3c). Free radical polymerization of PFS from the surface (Scheme 1; 4) was confirmed using DRIFT. The peak in the spectrum at 2963cm-1 indicated the presence of the fluorine benzene ring structures that are pendant from the PFS polymer backbone (Figure 3d). The PFS brush formed was visually a polymer, generating a clear viscous residue on the surface of the SS316L foils and the sides of the reaction flask. The residue on the foils and from the flask matched and was confirmed to be PFS using DRIFT spectroscopy. Unbound PFS was removed from the surface of the SS316L foils by immersion in boiling toluene. PFS that remained on the surface was strongly bound as a clear polymer brush layer.
The wetting properties and surface energy of PFOA thin films and PFS brushes formed on SS316L were examined using contact angle analysis and the Young-Good-Girifalco-Fowkes equation (Equation 1).34-36
| (1) |
where θ is the advancing contact angle the test liquid makes with the surface. The values γLVP, γLVD, and γLV are the polar, dispersive, and total surface energy components of the test liquid. The values being determined experimentally, γSVP and γSVD, are the polar and dispersive surface energy components of the modified substrates. This approach requires the use of two liquids. Deionized water (dH2O) and methylene iodide (CH2I2) and were selected because of their known polar, dispersive, and total surface energy components (γLVP, γLVD, and γLV, respectively).36, 37 Furthermore, to obtain valid surface energy measurements, it is necessary to use a solvent pair consisting of a non-polar and a polar test liquid.38 In this study, CH2I2 served as a nonpolar test liquid (γLVP = 0 and γLVD = 50.8mN/m) and H2O served as a polar test liquid (γLVP = 51.0 and γLVD = 21.8mN/m).37 The contact angle (θ) of each test liquid was measured and inserted into Equation 1. Contact angles acquired using dH2O and CH2I2 (θH2O and θCH2I2) made it possible to solve for the polar (γSVP) and dispersive (γSVD) surface energy components of the modified substrates. These two values (γSVP and γSVD) were then summed to give the total surface energy (γSV) of the modified substrates. The average surface energies for the control SS316L, PFOA, and PFS modifications are displayed in Table 1, below.
Table 1.
Contact angles measured using water and methylene iodide (θH2O and θCH2I2) and experimentally derived values for the surface energy components of perfluorooctadecanoic acid (PFOA) thin films and pentafluorostyrene (PFS) polymer coatings formed on stainless steel 316L (SS316L) including the dispersive (γSVD), polar (γSVP), and total (γSV) average surface energy.
| Surface | θH2O (°) | θCH2I2 (°) | γSVD (mN/m) | γSVP (mN/m) | γSV (mN/m) |
|---|---|---|---|---|---|
| SS316L | 68 | 68 | 24 | 14 | 38 |
| PFOA | 126 | 72 | 21 | 9.0 × 10-1 | 22 |
| PFS | 112 | 68 | 24 | 8.4 × 10-5 | 24 |
From the contact angle data collected, it was shown that PFOA thin films and PFS polymer brushes formed on the surface of SS316L generated hydrophobic, non-wetting interfaces. In general, hydrophobic surfaces characteristically have θH2O values greater than or equal to 100°, while hydrophilic surfaces have lower θH2O values.5, 15 The θH2O value of control SS316L reported here (68°) was comparable with other studies examining hydrophilic metal oxide surfaces including titanium, nickel, and a stainless steel 304 alloy, which displayed θH2O values ranging from 42 to 81°.15, 39, 40 Compared to the control SS316L, measured values of θH2O were increased after modification with PFOA and PFS, indicating the modified surfaces were significantly more hydrophobic. Measured water contact angles (θH2O) of 126° for PFOA and 112° for PFS were comparable to other surfaces comprised of fluorinated alkyl chains.5, 41
Inserting the measured contact angles for each test liquid (Table 1) into Equation 1, allowed the surface energies of PFOA and PFS modifications to be calculated. It was found that both PFOA thin films and PFS polymer brushes lowered the surface energy of the control SS316L substrates. The control SS316L had a total surface energy of 38 mN/m compared to PFOA and PFS modifications which had surface energies of 22 and 24 mN/m, respectively. The value of 22 mN/m obtained for PFOA thin films was comparable to other studies done on films containing perfluorinated alkyl chains with terminal CF3 groups.4, 41 Meanwhile, PFS brushes had a similar surface energy to that reported for structurally similar fluoropolymers.5, 42 Both PFOA and PFS modifications were consistent with surface energy values reported for other hydrophobic surfaces including silicone (19.5 mN/m), perfluoroalkoxyalkane (16.29 mN/m), and polytetrafluoroethylene (21.35 mN/m).15 Comparatively, the surface energy of SS316L reported here (38 mN/m) was identical to a similar study examining polished and non-polished SS316L (γSV = 38.10 and 38.23 mN/m, respectively).15 Additional studies examining the surface energy of other hydrophilic metal oxide surfaces such as titanium, a stainless steel 304 alloy, and nickel alloys also reported similar values.5, 15
In order to assess the uniformity and structure of PFOA thin films, AFM imaging was used. The root-mean-squared (rms) roughness of 500nm2 image fields taken on control SS316L and substrates with adhered PFOA films were compared. Control SS316L exhibited an average rms of 9 ± 2 Å while PFOA modified surfaces had an average rms of 22 ± 7 Å. From the images acquired, it appears that the PFOA forms densely packed thin films (Figure 4a,b).
Figure 4.
AFM Topography images of 500×500nm for (a) control SS316L (b) SS316L with adhered PFOA surface film
The ability of the PFOA thin films and PFS polymer brushes to reduce corrosion was examined using cyclic voltammetry (CV). For all measurements, electrodes were immersed in a quartz cell containing 0.1M NaOH. SS316L substrates were immersed in solution so that a total of 1cm2 served as the working electrode.19 A saturated calomel reference electrode and platinum wire auxiliary electrode were used to complete the circuit. The current density of the substrates was recorded from -1 to +1V potential. Using CV, it was shown that both PFOA thin films and PFS brushes were capable of reducing the maximum current density compared to bare SS316L.19, 25 The maximum current density of bare SS316L (11.7 mA/cm2) was lowered by PFOA thin films (10.1 mA/cm2) and significantly reduced by PFS brushes (6.7 mA/cm2). Reduction of the maximum current density reflects the ability of the PFOA thin films and PFS polymer brushes to reduce the oxidation and, therefore, the corrosion of the SS316L surface.25 The reported value of the maximum current density for bare SS316L (11.7 mA/cm2) was similar (14.4 mA/cm2) to previous studies examining the corrosion behavior of stainless steel alloys.43 The application of thin films (PFOA) and polymer coatings (PFS) were successful in reducing the maximum current density value of SS316L. The ability of thin films to passivate the surface of SS316L shown here was consistent with other studies in which surface coatings were developed on SS316L and analyzed using CV resulting in current densities ranging from 1.5 to 20mA.44-46
CONCLUSION
Uniform, robust thin films of PFOA that persisted through solvent rinse and sonication were formed on the surface of SS316L and present an ordered interface of CF3 groups. This is significant because very hydrophobic films with carboxylic acid head groups have shown limited stability on metal oxides. PFS brushes were formed through surface initiated polymerization, which involves propagation from free radical initiators that are immobilized at the terminal groups of a surface film.20 The surface energy of SS316L foils was lowered using thin films and polymer brushes consisting of fluorinated carbon chains. The PFOA thin films were more effective in lowering the surface energy of the SS316L compared to PFS brushes. This can be attributed to the ordered thin film structure of the PFOA compared to the PFS brush.4 The PFOA thin films were shown to uniformly follow the contours of the SS316L foil surface using AFM analysis. Analysis of acquired voltammograms indicated that the PFOA thin films and PFS polymer brushes reduced the maximum current density compared to bare SS316L. This indicates that the oxidation, and therefore corrosion, of the SS316L surface can be reduced by using the PFOA and PFS modifications. The PFS modifications exhibit greater efficiency at reducing maximum current density due to the fact that they are a macromolecular polymer brush compared to the monomolecular PFOA thin film.19 Both PFOA and PFS surface modifications could be used in applications where corrosion prevention and low surface energy interfaces are required.4, 5, 14, 41
ACKNOWLEDGMENT
We thank NIH-NIAMS (R15 AR056864-0) for funding, Dr. Toby Chapman (University of Pittsburgh) for use of the contact angle meter, Mourad Frites (Duquesne University) for assistance with electrochemical characterization of surfaces using CV, and Mr. Mark Campbell (Agilent) for help in using the AFM.
Footnotes
BRIEFS Perfluorocarbon thin films and polymer brushes with low surface energies were developed to control the interfacial properties of a stainless steel 316L alloy.
REFERENCES
- 1.Yoon JH, Son KS, Kim HS, Mitton B, Latanision R, Yoo YR, Kim YS. Materials Science Forum. 2005;475-479:4207–4210. [Google Scholar]
- 2.Zheng L, Neville A. Corrosion. 2009;65(2):145–153. [Google Scholar]
- 3.Cassé F, Swain GW. International Biodeterioration and Biodegradation. 2006;57:179–185. [Google Scholar]
- 4.Lindner E. Biofouling. 1992;6:193–205. [Google Scholar]
- 5.Liu Y, Zhao Q. Biophys Chem. 2005;117(1):39–45. doi: 10.1016/j.bpc.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson WH, Caccavo F, Olesen B, Lewandowski Z. Appl Environ Microbiol. 1997;63(7):2502–6. doi: 10.1128/aem.63.7.2502-2506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Alba AR, Hernando MD, Piedra L, Chisti Y. Analytica Chimica Acta. 2002;456:303–312. [Google Scholar]
- 8.Koutsaftis A, Aoyama I. Sci Total Environ. 2007;387(1-3):166–74. doi: 10.1016/j.scitotenv.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Koutsaftis A, Aoyama I. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43(14):1581–5. doi: 10.1080/10934520802329794. [DOI] [PubMed] [Google Scholar]
- 10.Ranke J, Jastorff B. Environmental Science and Pollution Research. 2000;7(2):105–114. doi: 10.1065/espr199910.003. [DOI] [PubMed] [Google Scholar]
- 11.Thomas KV. Biofouling. 2001;17(1):73–86. [Google Scholar]
- 12.Krishnan S, Wang N, Ober CK, Finlay JA, Callow ME, Callow JA, Hexemer A, Sohn KE, Kramer EJ, Fischer DA. Biomacromolecules. 2006;7(5):1449–62. doi: 10.1021/bm0509826. [DOI] [PubMed] [Google Scholar]
- 13.Lindner Biofouling. 1992;6:193–205. [Google Scholar]
- 14.Liu H, Szunerits S, Xu W, Boukherroub R. ACS Applied Materials & Interfaces. 2009;1(6):1150–1153. doi: 10.1021/am900100q. [DOI] [PubMed] [Google Scholar]
- 15.Pereni CI, Zhao Q, Liu Y, Abel E,S. Colloids Surf B Biointerfaces. 2006;48(2):143–7. doi: 10.1016/j.colsurfb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Allara DL, Nuzzo RG. Langmuir. 1985;1(1):52–66. [Google Scholar]
- 17.Badia A, Lennox RB, Reven L. Acc. Chem. Res. 2000;33(7):475–481. doi: 10.1021/ar9702841. [DOI] [PubMed] [Google Scholar]
- 18.Chechick V, Crooks RM, Stirling CJM. Adv. Mater. 2002;12(16):1161–1171. [Google Scholar]
- 19.Quiñones R, Gawalt ES. Langmuir. 2008;24(19):10858–64. doi: 10.1021/la801906e. [DOI] [PubMed] [Google Scholar]
- 20.Roux S, Duwez A-S, Demoustier-Champagne S. Langmuir. 2003;19:306–313. [Google Scholar]
- 21.Schmelmer U, Jordan R, Geyer W, Eck W, Gölzhäuser A, Grunze M, Ulman A. Angew. Chem., Int. Ed. 2003;42(5):559–563. doi: 10.1002/anie.200390161. [DOI] [PubMed] [Google Scholar]
- 22.Bain CD, Whitesides GM. Science. 1987;40:62. doi: 10.1126/science.240.4848.62. [DOI] [PubMed] [Google Scholar]
- 23.Myers D. Surfaces, Interfaces, and Colloids: Principles and Applications. 2. Wiley-VCH; New York: 1999. p. 501. [Google Scholar]
- 24.Meyer E, Hug HJ, Bennewitz R. Scanning Probe Microscopy: The Lab on a Tip. Springer-Verlag; Berlin: 2004. [Google Scholar]
- 25.Ganesh V, Pal SK, Kumar S, Lakshminarayanan V. J Colloid Interface Sci. 2006;296(1):195–203. doi: 10.1016/j.jcis.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein RD, Moriarty J, Cushnie E, Colorado RJ, Lee TR, Patel M, Alesi WR, Jennings GK. J Phys Chem B. 2003;107:11626–11632. [Google Scholar]
- 27.Shaporenko A, Cyganik P, Buck M, Ulman A, Zharnikov M. Langmuir. 2005;21:8204–8213. doi: 10.1021/la050535b. [DOI] [PubMed] [Google Scholar]
- 28.Raman A, Gawalt ES. Langmuir. 2007;23(5):2284–2288. doi: 10.1021/la063089g. [DOI] [PubMed] [Google Scholar]
- 29.Gao W, Dickinson L, Grozinger C, Morin FG, Reven L. Langmuir. 1997;13(2):115–118. [Google Scholar]
- 30.Fiurasek P, Reven L. Langmuir. 2007;23(5):2857–2866. doi: 10.1021/la0629781. [DOI] [PubMed] [Google Scholar]
- 31.Pawsey S, Yach K, Reven L. Langmuir. 2002;18(13):5205–5212. [Google Scholar]
- 32.Gnauck M, Jaehne E, Blaettler T, Tosatti S, Textor M, Adler HJ. Langmuir. 2007;23(2):377–81. doi: 10.1021/la0606648. [DOI] [PubMed] [Google Scholar]
- 33.Faucheux A, Gouget-Laemmel AC, Villeneuve C. H. d., Boukherroub R, Ozanam F, Allongue P, Chazalviel J-N. Langmuir. 2006;22(1):153–162. doi: 10.1021/la052145v. [DOI] [PubMed] [Google Scholar]
- 34.Fowkes FM. The journal of industrial and engineering chemistry. 1964;56:40–52. [Google Scholar]
- 35.Girifalco LA, Good RJ. J Phys Chem. 1956;61:904–909. [Google Scholar]
- 36.Ozcan C, Hasirci N. Journal of Applied Polymer Science. 2007;108:438–446. [Google Scholar]
- 37.Birdi KS. Handbook of Surface and Colloid Chemistry. 3rd edition CRC Press; Boca Raton: 2009. [Google Scholar]
- 38.Dalal EN. Langmuir. 1987;3:1009–1015. [Google Scholar]
- 39.Quiñones R, Raman A, Gawalt ES. Thin Solid Films. 2008;516:8774–8781. [Google Scholar]
- 40.Raman A, Dubey M, Gouzman I, Gawalt ES. Langmuir. 2006;22(15):6469–6472. doi: 10.1021/la060636p. [DOI] [PubMed] [Google Scholar]
- 41.Tsibouklis J, Stone M, Thorpe AA, Graham P, Peters V, Heerlien R, Smith JR, Green KL, Nevell TG. Biomaterials. 1999;20:1229–1235. doi: 10.1016/s0142-9612(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 42.Gudipati CS, Finlay JA, Callow JA, Callow ME, Wooley KL. Langmuir. 2005;21(7):3044–53. doi: 10.1021/la048015o. [DOI] [PubMed] [Google Scholar]
- 43.Misirlioglu Z, Uneri S, Aksu ML. The Journal of Corrosion Science and Engineering. 2000;3(8):1–9. [Google Scholar]
- 44.Le DP, Yoo YH, Kim JG, Cho SM, Son YK. Corrosion Science. 2009;51:330–338. [Google Scholar]
- 45.Chaudhari S, Patil PP. Electrochimica Acta. 2010;55:6715–6723. [Google Scholar]
- 46.González MB, Saidman SB. Corrosion Science. 2011;53:276–282. [Google Scholar]