Abstract
The organization of the developing male rodent brain is profoundly influenced by endogenous steroids, most notably estrogen. This process may be disrupted by estrogenic endocrine disrupting compounds (EDCs) resulting in altered sex behavior and the capacity to attract a mate in adulthood. To better understand the relative role each estrogen receptor (ER) subtype (ERα and ERβ) plays in mediating these effects, we exposed male Long Evans rats to estradiol benzoate (EB, 10 μg), vehicle, or agonists specific for ERβ (DPN, 1 mg/kg) or ERα (PPT, 1 mg/kg) daily for the first four days of life, and then assessed adult male reproductive behavior and attractiveness via a partner preference paradigm. DPN had a greater adverse impact than PPT on reproductive behavior, suggesting a functional role for ERβ in the organization of these male-specific behaviors. Therefore the impact of neonatal ERβ agonism was further investigated by repeating the experiment using vehicle, EB and additional DPN doses (0.5 mg/kg, 1 mg/kg, and 2 mg/kg bw). Exposure to DPN suppressed male reproductive behavior and attractiveness in a dose dependent manner. Finally, males were exposed to EB or an environmentally relevant dose of genistein (GEN, 10 mg/kg), a naturally occurring xenoestrogen, which has a higher relative binding affinity for ERβ than ERα. Sexual performance was impaired by GEN but not attractiveness. In addition to suppressing reproductive behavior and attractiveness, EB exposure significantly lowered the testis to body weight ratio, and circulating testosterone levels. DPN and GEN exposure only impaired behavior, suggesting that disrupted androgen secretion does not underlie the impairment.
Keywords: sex behavior, endocrine disruptor, genistein, estrogen receptor, partner preference, DPN, estrogen, masculinization, virility, soy
Introduction
To display sex-specific reproductive behavior in adulthood, male mammals must undergo both defeminization and masculinization during critical windows of development that span the pre- and postnatal periods. Masculinization is the emergence of male-specific behaviors and the underlying circuitry responsible for those behaviors, while defeminization is the loss of female-specific behaviors and the underlying circuitry necessary for those behaviors. Steroid hormones are required for both the organization and activation of male-specific traits (Baum, 1979; Beach et al., 1969; McEwen, 1983; Morris et al., 2004; Phoenix et al., 1959) and defeminization (Kudwa and Rissman, 2003). In the present study, we explored the hypothesis that exposure to estrogen receptor (ER) agonists, selective for one of the two nuclear estrogen receptor (ER) isoforms (ERα and ERβ), or the endocrine disrupting compound (EDC) genistein (Meyers et al., 2001), during the neonatal critical period could disrupt this organizational process, resulting in impaired reproductive behavior and reduced attractiveness to a potential mate.
Classically, in male rodents, estrogens, aromatized from testicular androgens, bind to the two nuclear ER subtypes, ERα and ERβ within the brain to induce male-specific neuroendocrine development (Kuiper et al., 1996; Merchenthaler et al., 2004; White et al., 1987). The specific mechanistic roles these two receptors play in the masculinization and defeminization process, however, remain largely unclear. Based primarily on data obtained from knockout (KO) mice, null for either ERα or ERβ, it has been hypothesized that ERα is primarily responsible for masculinization while ERβ is more important for defeminization (Kudwa et al., 2006). Accordingly, ERαKO mice of both sexes exhibit malformed gonads, infertility, and disrupted reproductive behavior (Lubahn et al., 1993; Rissman et al., 1997), while ERβKO mice develop subfunctional gonads (Krege et al., 1998), but are able to reproduce and display sex-typical reproductive behavior (Kudwa et al., 2005; Ogawa et al., 1999; Temple et al., 2003). Male ERβKO mice, however, display higher levels of lordosis than wild type conspecifics under certain experimental conditions, suggesting that ERβ is important for defeminization (Kudwa et al., 2005). To test this hypothesis, Kudwa and colleagues (Kudwa et al., 2006) injected female mice on postnatal days (PNDs) 1–3 with either a selective ERα agonist, 4,4',4'-(4-propyl-[1H]-pyrazole-1,3,5-triyl) tris-phenol (PPT) or an ERβ selective agonist diarylproprionitrile (DPN) and found that neonatal administration of DPN (but not PPT) significantly reduced lordosis behavior in females, a result which supports a defeminizing role for ERβ during neonatal life. By contrast, to date, few studies have explored how neonatal exposure to ER selective agonists during critical windows of development affect male reproductive behavior or neuroendocrine development. Thus, the present study sought to fill this data gap.
Both ER subtypes have been shown to be present in areas of the brain important for male reproductive behavior, such as the medial preoptic area (mPOA), throughout perinatal life and into adulthood (DonCarlos, 1996; Karolczak and Beyer, 1998; Merchenthaler et al., 2004; Shughrue et al., 1998) suggesting that either could be important for crucial aspects of masculinization and defeminization. Notably, ER expression in many hypothalamic regions is sexually dimorphic throughout development (Cao and Patisaul, 2011; Lemmen et al., 1999; Perez et al., 2003). In mice, sex differences in the number of ERβ immunoreactive cells within the mPOA, for example, have been shown to be present both at birth and in adulthood, with males having a greater number than females (Kudwa et al., 2004; Wolfe et al., 2005). This pattern has also been seen in adult rats (Zhang et al., 2002). We hypothesized that agonism of ERβ could disrupt male rat reproductive behavior, and the ability to attract a mate thereby demonstrating that it may play a role in masculinization, as well as defeminization.
To test this hypothesis, we first performed a pilot study, during which we neonatally exposed male rats to vehicle, estradiol benzoate (EB), or agonists selective for ERα (PPT), or ERβ (DPN) to determine which agonist had a more profound impact on the emergence of male sexual behavior and attractiveness. Results from this pilot study suggested that agonism of ERβ could reduce male sexual vigor. Thus, we conducted a subsequent experiment using additional doses of DPN, followed by a third study using the naturally occurring ERβ selective agonist, GEN to determine if the emergence of male sexual behavior is vulnerable to endocrine disruption. GEN is an isoflavone phytoestrogen produced by legumes, such as soy, and commonly found in soy-based food products and beverages, including soy-based infant formula (Dixon and Ferreira, 2002). Developmental GEN exposure has been linked to detrimental changes in female reproductive health (Jefferson et al., 2009; Jefferson et al., 2006, 2007; Kouki et al., 2003; Patisaul et al., 2006) and possibly male reproductive health and sexual differentiation (Atanassova et al., 2000; Bu and Lephart, 2007; Cederroth et al.; Naciff et al., 2005; Scallet et al., 2004) in rodents. GEN has a nine fold higher relative binding affinity for ERβ than ERα (Barkhem et al., 1998; Kuiper et al., 1998) suggesting it has the potential to interfere with ERβ dependent brain organization.
This series of experiments served to: 1) help elucidate the ER-dependent mechanisms underlying the masculinization of male sex behavior, 2) determine if neonatal exposure to an ERβ selective EDC would have an impact on male sexual behavior and, through partner preference testing, 3) examine how this would impact attractiveness.
Materials and Methods
Animals
Long Evans rats were maintained on a soy-free diet (5K96, Purina Test Diets, Richmond, IN) and a reversed (12:12) light cycle at 23°C and 50% relative humidity for the duration of the experiments. The light cycle for the male animals in Experiment 1 was changed to 13:11 prior to the last round of testing to increase sexual motivation. The animals used in Experiments 1, 2 and 4 were bred in-house, and the dams for Experiment 3 were ordered timed-pregnant from Charles River Laboratories. All pups were weaned on post natal day (PND) 21 into same sex littermate pairs until two months of age, at which time they were singly housed. Animal care and testing procedures followed the relevant guidelines stated in the Animal Welfare Act and U.S. Department of Health and Human Service “Guide for the Care and Use of Laboratory Animals.” The protocol was approved by the North Carolina State University Animal Care and use Committee and supervised by animal care personnel and veterinary staff. All animals were tested as young adults, with onset of testing occurring the week of PND 70, but the specific age range varied somewhat between experiments. This was not considered to be a potential confound because male rat reproductive behavior remains relatively consistent until approximately 10 months of age, at which point it begins to decline (Clark, 1995).
Experiment 1 (Pilot). Impact of ERα agonist PPT and ERβ agonist DPN on reproductive behavior, physiology, and attractiveness
On the day of birth, all animals were cross-fostered, as we have done previously (Adewale et al., 2009; Patisaul et al., 2006), to minimize litter effects (as described in (Raubertas et al., 1999) (n = 9 dams). Each litter contained a mixture of animals (n = 12 maximum), only two of which were related to each other. All pups (the females were ultimately used for other projects) were subcutaneously (sc) injected with either a sesame oil based vehicle (OIL), 10 μg EB (Sigma St. Louis IL), 1 mg/kg bw DPN (Tocris Cookson, Ellisville, MO), 1 mg/kg bw PPT (Tocris Biosciences), or a mixture of PPT and DPN (1 mg/kg bw PPT + 1 mg/kg bw DPN (PPT/DPN)) from the day of birth (defined as PND 0) through PND 3. This exposure window was selected because it is well established that this is a critical period of sexual differentiation in the male rat brain (Gore, 2008; McCarthy, 2008; Simerly, 2002). All compounds were first dissolved in 100% ethanol and then sesame oil (Sigma, St. Louis, MO) at a ratio of 10% as we have done previously (Patisaul et al., 2007; Patisaul et al., 2009b). These concentrations of DPN and PPT reflect those successfully used in prior experiments (Donner and Handa, 2009; Handa et al., 2008; Lund et al., 2006; Patisaul et al., 2009a). Male sexual performance was evaluated within PNDs 78 – 142 with hormone replaced ovariectomized (OVX) females. These males (n = 10 OIL; 4 EB; 10 DPN; 8 PPT; 10 PPT/DPN) were then used as mate choices in a partner preference paradigm during PNDs 155–168 for intact, sexually naïve females (n = 10; age PND 108–123). All males were weighed on PND 182, castrated under isoflurane anesthesia, and returned to the colony for future work beyond the scope of this project. At the time of surgery, the testes were weighed upon removal and blood was collected via tail clip then spun at 4°C at 13,000 rpm for 10 minutes to separate the plasma fraction. The plasma was extracted using a transfer pipet and stored at −80°C until analysis. Plasma testosterone levels were subsequently measured by radioimmunoassay using Siemens Coat-A-Count total testosterone kit with an analytical sensitivity of 4 ng/dl as described previously (Patisaul et al., 2009a).
Experiment 2. Dose dependent impact of DPN on reproductive behavior and attractiveness
Males were administered DPN at three different doses (0.5, 1, or 2 mg/kg bw) to establish a dose response on male reproductive behavior and attractiveness. On the day of birth males were cross-fostered as in Experiment 1 (n = 8 dams). All pups were then administered vehicle (OIL, n = 9), EB (10 μg, n = 4), 0.5 mg/kg bw DPN (n = 12), 1 mg/kg bw DPN (n = 7), or 2 mg/kg bw DPN (n = 12) prepared as in Experiment 1, from PND 0 through 3 by sc injection. This dose range is narrow but was selected because these three concentrations of DPN bracket the range of doses successfully used in prior experiments (Donner and Handa, 2009; Handa et al., 2008; Lund et al., 2006; Patisaul and Bateman, 2008; Weiser et al., 2009) and that used in Experiment 1. DPN has a 70-fold higher binding affinity for ERβ than ERα, and the highest dose used was not found to activate ERα dependent gene expression (data not shown). Males were sex tested within PNDs 73 – 113 with hormone replaced OVX females and then used as mate choices in partner preference over PNDs 113 – 123 for intact, sexually naïve females age PND 179 –187 (n = 12). The males exposed to the low dose (0.5 mg/kg bw DPN) were not tested for attractiveness because they showed intromission latencies and counts equivalent to those of the controls.
Experiment 3. Impact of GEN and EB on reproductive behavior, physiology, and attractiveness
On the day of birth, male animals were again cross-fostered to minimize litter effects (n = 15 dams). All pups were sc injected with either a sesame oil based vehicle (OIL), EB (10 μg, Sigma St. Louis, MO), or GEN (10 mg/kg bw, Indofine Chemical Company Hillsborough NJ) from PND 0 through 3. The dose of GEN used for this experiment is approximately equivalent to the total amount of isoflavone phytoestrogens consumed daily by infants reared exclusively on soy infant formula (Setchell et al., 1998). EB was used as a positive control (Levine and Mullins, 1964). Males (n = 15 GEN; 12 EB; 16 OIL) were sex tested between PNDs 71 – 85 with age-matched, hormone replaced OVX females and then used as mate choices for age-matched, gonadally intact, sexually naïve females (n = 11) during PNDs 92 – 156 to assess partner preference. Partner preference testing was performed twice. All animals were novel to one another in both rounds. All males were then weighed on PND 155, and sacrificed by CO2 asphyxiation followed by rapid decapitation on PND 170 and 171 to collect trunk blood and testes for evaluation. Plasma was extracted and analyzed for circulating testosterone levels as described for Experiment 1, and the testes were weighed upon removal using an electronic balance.
Experiment 4. Replicate of Experiment 3, with no cross-fostering
A second group of animals was used for Experiment 4, which was designed to replicate Experiment 3, but in this case the animals were bred in-house and the pups were not cross-fostered because they were not all born on the same day (n = 10 dams) and a recent study reported that cross-fostering may confound EDC effects (Cox et al., 2010). Thus it became important to determine if the behavioral changes observed after GEN exposure were also evident in animals that had not been cross-fostered. All compounds were prepared and administered as described in Experiment 3. Males (n = 11 GEN; 11 EB; 11 OIL) were sex tested within PND 69–95 with OVX females. These males were then used as mate choices in partner preference during PND 127–173 for age matched, intact, sexually naïve females (n = 12). Partner preference testing was performed twice. All animals were novel to one another in both rounds.
Assessment of reproductive behavior
After the onset of puberty, males in each experiment were tested for reproductive behavior once each week for a total of three (Experiment 3), four (Experiment 2 and 4), or five (Experiment 1) sessions dependent upon vehicle exposed (OIL) male performance. Because inexperienced males often perform poorly at the onset of testing, sexual performance was not statistically compared between exposure groups until ≥ 25% of vehicle exposed males had intromissions within the session. A fourth round was added to Experiments 2 and 4, while a fifth round was added to Experiment 1 because the vehicle exposed males did not meet this performance criteria in the first four rounds.
In each session, males were placed in a clean, novel cage and introduced to a sexually receptive, hormone replaced, OVX female, a procedure similar to what we and others have done previously (Becker et al., 2005; Hardy and DeBold, 1971; Patisaul et al., 2002). Testing duration was 15 minutes for each round in Experiments 1, 2, 3, and the first two rounds of 4. Duration was increased to 30 minutes for the final rounds of Experiment 4 to extend the observation period and better characterize sexual performance. All testing began within two hours of dark onset, under red-light illumination. All stimulus females were deemed receptive via a lordosis response to male contact. Behavior was recorded using a Sony camcorder set on night vision and then hand-scored by an investigator blind to the treatment groups using the behavioral analysis software package Stopwatch (courtesy of David A. Brown, Center for Behavioral Neuroscience, Emory University). Measures included latency to first intromission and total number of intromissions made during the trial because they were robustly displayed in all groups and most reliably captured the sexual motivation of the male subjects. Other measures, most notably number of mounts, and latency to first mount, were not included because they were not consistently performed by all males, particularly after they were sexual experienced.
Assessment of partner preference
After all rounds of sex testing were completed, the same test males were used as choices for intact, sexually naïve, proestrus (determined by vaginal cytology as described in (Becker et al., 2005)) females in a series of partner preference tests. All animals were habituated to the arena prior to testing. The plexiglass testing arena was a total length of 198.12 cm, 30.48 cm deep and 30.48 cm wide and divided into three chambers roughly equal in size with small compartments (17.78 × 30.48 × 30.48 cm) on either side. Each small compartment contained a single male enclosed behind a wire mesh composed of ¾ inch hardware cloth. A control male was placed on one side and an exposed male on the other. Positions were randomized to control for side preference. Females could spend time alone in the central chamber, or interact with one of the males in either of the end chambers though the mesh. Testing began within 2 hours of dark onset, under red-light illumination, with each session lasting 20 min. Preference was determined by quantifying the amount of time spent in each chamber next to the stimulus male. In Experiments 3 and 4, the males were presented as mate choices to the females for a first round (3a, 4a) and then again for a second round (3b, 4b) to demonstrate reproducibility. In all cases, all males were novel to the selecting female. Behavior was video recorded and scored by an observer blind to the exposure groups as described above.
Statistical analysis
For the sex behavior tests, differences in number of intromissions and intromission latency were compared by two-way ANOVA with round and exposure as factors. Significant effects were then followed up by one-way ANOVA, first with exposure as a factor for each week, then with week as a factor within each exposure group. Significant effects in the one-way ANOVAs were followed up with Fisher's Least Significant Differences post hoc tests. For all measures, analyses were two tailed and the level of significance was set at P ≤ 0.05.
For the partner preference data, preference was established by comparing the total time spent in the chamber containing the unexposed control (OIL) male to the time spent with the exposed male using a Fisher's Studentized t-test with the level of significance set at P ≤ 0.05. Because some of the males did not intromit during the sex testing, when analyzing the partner preference results, all data were first analyzed to determine if there was an effect of sexual experience, and no effect was found in any of the experiments (t-test; data not shown). The data were also analyzed (t-test) for a side preference. A side preference was identified in the second round of Experiment 3, thus that experiment was ultimately repeated (Experiment 4). The SYSTAT software package was used to perform all statistical analyses, and graphs were generated in SigmaPlot.
Results
Experiment 1 (Pilot): Impact of ERα agonist PPT and ERβ agonist DPN on reproductive behavior, physiology, and attractiveness
Reproductive behavior
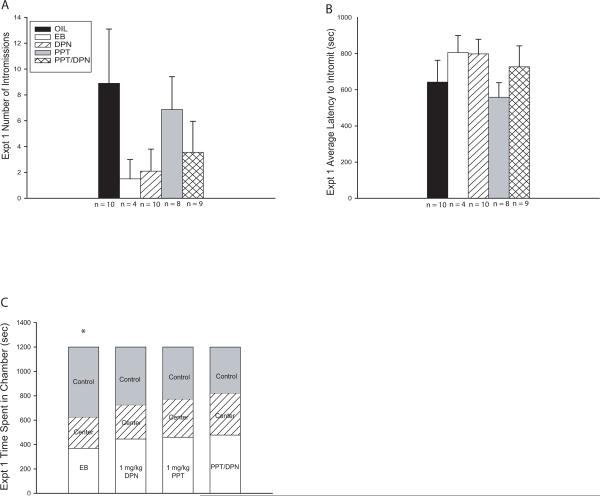
Only round 5 met the behavioral threshold for analysis and although a pattern did arise, there was no main effect of exposure group on either intromission number (F(4,36) = 1.125, P = 0.360) or intromission latency (F(4, 36) = 0.817, P = 0.523; Fig. 1A–B). Analysis of performance among only the intromitting males also failed to reveal a main effect of exposure group for either intromission number (F(4,10) = 0.508, P = 0.731) or intromission latency (F(4, 10) = 0.335, P = 0.849). In both analyses, However, a behavioral pattern emerged with the EB and DPN exposed males performing poorly, but not to a significant degree, compared to the other groups. These results constituted the rationale for the subsequent studies.
Fig. 1.
Experiment 1 sexual behavior and partner preference results. (A – C) Only behavior from the fifth round of testing was analyzed and no significant differences between groups emerged for any endpoint, but the outcome suggested an emasculating effect of neonatal EB or DPN exposure. (A) EB and DPN exposed males had fewer intromissions compared to vehicle exposed (OIL) males. (B) EB and DPN exposed males had longer intromission latencies compared to vehicle exposed males. (C) Females selected against the EB exposed males by spending significantly more time in the chamber containing the vehicle exposed (control) males. Partner preference was unaffected in the other groups (Mean ± SEM; * P ≤ 0.05)
Partner Preference
Females selected against EB exposed males compared to vehicle exposed control males (P ≤ 0.008; Fig. 1C). In contrast, females showed no clear preference between DPN and vehicle (P = 0.679), PPT and vehicle (P = 0.772), or PPT/DPN and vehicle (P = 0.160) exposed males (Fig. 1C).
Testosterone and Testis/Body Weight
There was a main effect of exposure group on plasma testosterone levels (F(4, 38) = 3.212, P ≤ 0.03). EB exposed males had significantly lower testosterone levels compared to that of the vehicle exposed control (OIL) group (P = 0.02), however, none of the other exposure groups had testosterone levels that were significantly different from the controls (Table 1).
Table 1.
Experiment 1. Plasma testosterone levels and testes to body weight ratios were significantly lower in the EB exposed males compared to the vehicle exposed (OIL) males. Adult body weight did not significantly differ between groups.
| Experiment 1 | n | Body Wt (kg) | Testes/body Wt Ratio (kg/g) | Total Testosterone (ng/dl) |
|---|---|---|---|---|
| OIL | 10 | 730.5 ± 17.87 | 2.61 ± 0.09 | 16.76 ± 3.35 |
| EB | 4 | 710.5 ± 35.84 | 1.3 ± 0.41* | 2.21 ± 0.72* |
| DPN | 10 | 785.9 ± 15.48 | 2.33 ± 0.13 | 10.16 ± 1.35 |
| PPT | 8 | 737.5 ± 14.2 | 2.54 ± 0.1 | 21.64 ± 5.12 |
| PPT/DPN | 9 | 743.78 ± 19.11 | 2.48 ± 0.1 | 17.71 ± 4.22 |
P ≤ 0.001
Of the four EB exposed males, two had retained testes. This was not seen in any of the other exposure groups. EB exposed males were the lightest and the DPN males were the heaviest (Table 1), but there was only a weak trend for a main effect of exposure on body weight (P = 0.103). The testis to body weight ratio, however, was significantly affected by exposure (F(4, 41) = 8.857, P ≤ 0.001; Table 1). The ratio of the EB exposed males was significantly smaller than that of the vehicle exposed (OIL) males (P ≤ 0.001), but the other groups did not differ from the controls.
Collectively the results from this pilot study indicated that neonatal exposure to EB results in significantly lower adult testosterone levels, improper testis development, significantly lower attractiveness, and, potentially, impaired male reproductive behavior. None of these were recapitulated in the group receiving both DPN and PPT. The ERβ agonist, DPN, appeared more effective than PPT in compromising both male sexual behavior and attractiveness. Therefore we chose to further explore the impact of ERβ selective agonism by testing additional doses of DPN.
Experiment 2: Dose dependent impact of DPN on reproductive behavior and attractiveness
Reproductive behavior
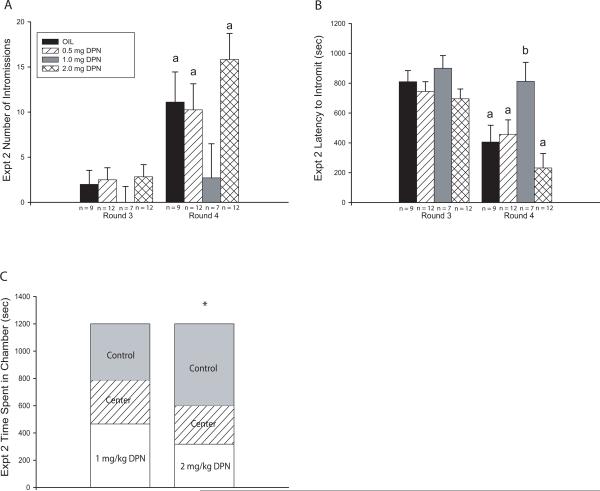
Only rounds 3 and 4 met the behavioral threshold for analysis and two-way ANOVA revealed a significant effect of testing round (F(1,72) = 19.319, P ≤ 0.001) as well as exposure (F(3,72) = 2.874, P ≤ 0.05) on the number of intromissions but no significant interaction (Fig. 2A). The vehicle exposed control males (OIL) made significantly more intromissions in round 4 than round 3 (F(1,16) = 4.630, P ≤ 0.05). The 0.5 mg DPN exposed males (F(1,22) = 4.590, P ≤ 0.05) and the 2 mg DPN exposed males (F(1,22) = 14.288, P ≤ 0.001) also significantly improved over time, however the 1 mg DPN exposed males did not (F(1,12) = 2.548, P = 0.136).
Fig. 2.
Experiment 2 sexual behavior and partner preference results. (A) The number of intromissions did not differ between groups in either round but the 1 mg/kg DPN exposed group was the only group that did not display increased numbers of intromissions in Round 4 compared to Round 3. (B) Intromission latency did not initially differ between groups, but significantly dropped over time in all groups except the 1 mg/kg DPN exposed group resulting in significantly longer latencies within this group compared to the vehicle (OIL) exposed controls. (C) Females significantly preferred vehicle exposed (Control) males over 2 mg/kg DPN exposed males but showed no preference between 1 mg/kg DPN exposed males and control males. (Mean ± SEM; * P ≤ 0.05; a = significant difference between rounds, b = significant difference from OIL controls)
Intromission latency was similarly affected (Fig. 2B). Two-way ANOVA revealed a significant effect of testing round (F(1,72) = 22.942, P ≤ 0.001) as well as exposure (F(3,72) = 5.772, P ≤ 0.002). Latency to intromit decreased in the vehicle exposed males between rounds 3 and 4 (F(1,16) = 9.634, P ≤ 0.008). A similar decrease was observed in the 0.5 mg DPN (F(1,22) = 4.053, P ≤ 0.05) and 2 mg DPN (F(1,22) = 16.012, P ≤ 0.001) exposed groups, whereas the 1 mg DPN exposed (F(1,12) = 3.243, P = 0.136) males failed to improve (Fig. 2B). Within round 4, a significant difference in intromission latency between exposure groups emerged (F(3,36) = 4.847, P ≤ 0.006). The 1 mg DPN exposed males had a significantly longer latency than the vehicle (P ≤ 0.02), the 0.5 mg (P ≤ 0.03), and 2 mg DPN exposed males (P ≤ 0.001).
Partner preference
The males exposed to the low dose (0.5 mg/kg bw DPN) were not tested for attractiveness because they showed intromission latencies and counts equivalent to those of the vehicle exposed males. Females significantly preferred the vehicle exposed males over the 2 mg DPN exposed males (P ≤ 0.02; Fig. 2C) while showing no preference between the 1 mg DPN exposed and the vehicle exposed males (P = 0.452; Fig. 2C).
Experiment 3. Impact of GEN and EB on reproductive behavior, physiology, and attractiveness
Reproductive behavior
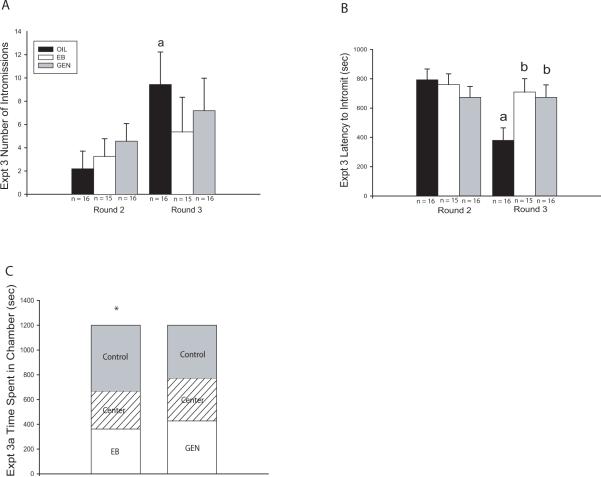
Behavior in the control group did not meet the threshold for statistical analysis until round 2. Therefore, only rounds 2 and 3 were statistically analyzed (Fig. 3A–B). Two-way ANOVA revealed a significant effect of testing round, but not exposure group, on the number of intromissions (F(1,88) = 4.683, P ≤ 0.03) with the number of intromissions increasing over time. Only within the vehicle exposed group did the number of intromissions significantly increase from round 2 to round 3 (F(1,30) = 8.168, P ≤ 0.008).
Fig. 3.
Experiment 3 sexual behavior and partner preference results. (A) The number of intromissions did not significantly differ between groups in either round. The vehicle exposed group was the only group that had significantly more intromissions over time. (B) Intromission latency was similar across exposure groups in Round 2 but by Round 3, the EB and GEN exposed males had significantly longer latencies compared to controls, with only the controls having shorter latencies in Round 3 compared to Round 2. (C) Females significantly preferred vehicle exposed (Control) males over EB exposed males but showed no preference between GEN exposed and control males. (Mean ± SEM; * P ≤ 0.05; a = significant difference between rounds, b = significant difference from OIL controls)
Intromission latency was similarly affected (Fig. 3B). Two way ANOVA revealed a significant effect of testing round (F(1,88) = 5.558, P ≤ 0.02) as well as a significant interaction between round and exposure (F(1,88) =3.990, P ≤ 0.02) but no main effect of exposure (P = 0.19). Intromission latency was roughly equivalent within each exposure group in round 2, but significant differences emerged in round 3 (F(2,43) = 4.356, P ≤ 0.02). Intromission latency was significantly shorter in the vehicle exposed males compared to the EB (P ≤ 0.01) and GEN (P ≤ 0.02) exposed males (Fig. 3B). Significantly decreased intromission latency between rounds 2 and 3 was only observed in the vehicle exposed males (F(1,30) = 17.466, P ≤ 0.001).
Partner preference
Partner preference was performed twice (2 rounds) with all animals being novel to one another in both rounds. In the first round of testing, females spent significantly more time in the chamber containing the vehicle exposed (OIL) male than the EB exposed male (P ≤ 0.006; Fig. 3C). In contrast, females did not show a preference for vehicle exposed males compared to the GEN exposed males (P = 0.67). Results from the second round of testing were confounded by a distinct left side preference and were therefore not analyzed further (data not shown). Instead, the experiment was repeated with a new group of animals to validate the initial findings (Experiment 4).
Testosterone and Testis/Body Weight
Exposure significantly affected plasma testosterone levels (F(2, 39) = 17.320, P ≤ 0.001; Table 2). Testosterone levels were significantly lower in the EB exposed males compared to that of the vehicle (OIL) and GEN exposed males (P ≤ 0.001). In contrast, the plasma testosterone levels in the vehicle exposed males and the GEN exposed males did not significantly differ from each other (P = 0.578; Table 2).
Table 2.
Experiment 3. Plasma testosterone levels and testes to body weight ratios were significantly lower in the EB exposed males compared to the vehicle exposed (OIL) males. Adult body weight did not significantly differ between groups.
| Experiment 3 | n | Body Wt(kg) | Testes/body Wt Ratio (kg/g) | Total Testosterone (ng/dl) |
|---|---|---|---|---|
| OIL | 15 | 647.13 ± 12.59 | 2.99 ± 0.09 | 89.09 ± 7.98 |
| EB | 11 | 649.82 ± 14.99 | 1.12 ± 0.10* | 8.88 ± 5.33* |
| GEN | 13 | 623.92 ± 15.89 | 3.06 ± 0.10 | 81.27 ± 14.34 |
P ≤ 0.001
During testes collection, it was discovered that 82% (9 out of 11) of the EB exposed males had retained testes. In all cases, both testes were retained. This was not seen in the other exposure groups. Body weight did not significantly differ (P = 0.392) between groups. Testis to body weight ratio, however, was significantly affected by exposure (F(2, 39) = 127.984, P ≤ 0.001) with the EB exposed males having a smaller ratio than either the vehicle (P ≤ 0.001) or GEN exposed (P ≤ 0.001) males (Table 2). There was no significant difference between the ratios of the vehicle and the GEN exposed males (P = 0.584).
Experiment 4. Replicate of Experiment 3, with no crossfostering
Reproductive behavior
Rounds 3 and 4 met the behavioral threshold for analysis and two-way ANOVA revealed a significant effect of exposure group, but not testing round or an interaction between the two factors, on intromission number (F(2,60) = 5.312, P ≤ 0.008). Significant differences in the number of intromissions made between the groups emerged in round 3 (F(2,30) = 3.414, P ≤ 0.05), with the vehicle exposed males intromitting significantly more than the EB (P ≤ 0.02) or the GEN exposed males (P ≤ 0.05). Within round 4, a similar performance pattern was observed, but was not found to be statistically significant (P = 0.09; Fig. 4A).
Fig. 4.
Experiment 4 sexual behavior and partner preference results. (A) Number of intromissions was significantly lower in the EB and GEN exposed groups compared to the OIL control males in both rounds of testing. Numbers did not significantly increase over time in any group. (B) Latency to intromit was significantly longer in the EB exposed groups compared to the OIL group in Round 3 but this difference was lost in Round 4. (C, D) Partner preference results differed between rounds. In both cases, females significantly preferred control males over EB exposed males. However, females displayed a significant preference for GEN exposed males compared to OIL control males in the first round of partner preference testing (Experiment 4a) but not the second (Experiment 4b). (Mean ± SEM; * P ≤ 0.05; b = significant difference from OIL controls)
Intromission latency was similarly affected (Fig. 4B). Two-way ANOVA only revealed a significant effect of exposure (F(2,60) = 5.161, P ≤ 0.01). A significant difference in intromission latency between groups arose in round 3 (F(2,30) = 4.231, P ≤ 0.02) with the vehicle exposed males having a significantly shorter latency than the EB (P ≤ 0.007) exposed males. The vehicle exposed males also had a shorter latency than the GEN exposed males (Fig. 4B), however it did not reach statistical significance (P = 0.08). There were no significant differences between groups in round 4 (P = 0.25).
Partner preference
Partner preference was performed twice (two rounds) with all animals being novel to one another in both rounds. The females significantly preferred the vehicle (control) males over the EB exposed males in both the first (Fig. 4C; P ≤ 0.01) and second rounds (Fig. 4D; P ≤ 0.01). In contrast, the females preferred GEN exposed males over vehicle exposed males in the first round (Expt. 4a) (P ≤ 0.001), but in the second round (Expt. 4b) they again showed no preference (P = 0.276; Fig. 4C–D).
Discussion
Here we clearly showed that neonatal exposure to EB negatively impacted male reproductive performance and the ability to attract a mate in adulthood, observations which are consistent with prior studies (Diamond et al., 1973; Zadina et al., 1979). Moreover, we also found that selective agonists for ERβ (DPN and GEN) had an emasculating effect on these behaviors, suggesting that ERβ activation during the neonatal critical period can potentially interfere with the sex specific organization of the neuroendocrine pathways which mediate male reproductive behavior. Reduced testis weight and significantly lower circulating testosterone levels were only observed in the EB exposed group, indicating that the disruption of male reproductive behavior caused by the ERβ agonists was not the result of abnormal testicular development or circulating androgen levels during adulthood. Instead, these observations signify that disruption occurred elsewhere in the neuroendocrine system. Collectively, the partner preference results reveal that compromised reproductive behavior is not always accompanied by a loss of attractiveness, indicating that females may not always be capable of detecting the subtle loss of virility.
Despite the narrow dose range, the effect of DPN on male reproductive behavior was dose specific, but not linear. This non-monotonic effect may not be uncommon for some neuroendocrine endpoints (Kendig et al., 2010), including male reproductive behavior (Jones et al., 2010). This phenomenon has recently emerged as an area of intense interest within the endocrine disruption field because some weakly estrogenic compounds, such as Bisphenol A (BPA), have produced non-linear effects at low doses in a variety of model systems (Vandenberg et al., 2009). Unfortunately, the molecular and biochemical mechanisms associated with this type of dose response curve are poorly understood, but the prevailing hypothesis is that it likely reflects the intersection of multiple mechanisms. For the present study, one possibility is that ERα and ERβ are modulating each other's activity and that this relationship differs at high and low doses of estrogen (or ER-selective agonists). It is now widely recognized that the relationship between ERα and ERβ is dynamic and complex. For example, ERβ activation can antagonize ERα-dependent transcription (Matthews and Gustafsson, 2003; Matthews et al., 2006; Rissman, 2008) but the two ER subtypes can also have synergistic or sequential effects (Rissman, 2008). It is possible that, at the 1 mg/kg dose, agonism of ERβ by DPN dampened the activity of ERα, thereby resulting in reduced virility but that other activities occurred at the 0.5 and 2 mg/kg doses. Further studies using additional doses of DPN and PPT, as well as antagonists for ERα and ERβ would be required to further elucidate these interactions. Moreover, prior work has established that although postnatal administration of testosterone to neonatal castrates can rescue male sexual behavior, exogenous administration to gonadally intact neonates results in compromised male sexual performance (Henley et al., 2010; Pollak and Sachs, 1975; Zadina et al., 1979). Thus there appears to be an “optimal range” in which steroid hormones induce masculinization, and doses above or below that range can result in partial demasculinization. This phenomenon is intrinsically non-monotonic.
The significantly lower circulating testosterone levels observed in the EB exposed animals is likely a substantive contributing factor for compromised reproductive performance and attractiveness in this group (Baum, 2009), but not for the 1 mg/kg DPN group or the GEN groups, as androgen levels in those groups did not significantly differ from controls. Unfortunately, plasma concentrations were not measured in the 2 mg/kg DPN exposed animals but presumably their levels were within the normal range because virility was not found to be compromised. The emasculating effects of EB observed here are consistent with prior studies examining the impact of postnatal estrogen exposure on male reproductive physiology and behavior (Diamond et al., 1973; Zadina et al., 1979) but also a recent study employing the postnatal administration of an aromatizable androgen (Henley et al., 2010). Male sexual performance was significantly compromised and circulating testosterone levels were reduced compared to unexposed control males. Moreover, partner preference was altered such that exposed males spent more time with another male conspecific, when given a choice between a male and an estrous female, than control males. This result was interpreted to indicate partial demasculinization and the development of a bi-sexual social preference, an effect which could reduce reproductive fitness because the hyper-androgenized male would presumably be just as likely to approach a male or a female.
The results from the present study suggest that postnatal agonism of ERβ also induces a partial demasculization. A role for ERβ in the masculinization process is further supported by the discovery that androgen metabolites, most notably 5α-androstane-3β, 17β-diol (3β-Adiol), interact with ERβ in the brain and other organs (Handa et al., 2009; Kuiper et al., 1998; Lund et al., 2006; Weiser et al., 2009). During development, 3β-Adiol is secreted from immature testes and can also be synthesized de novo in cells that express 5α-reductase and 17β-hydroxysteroid dehydrogenase type 7 (Weihua et al., 2002). It has previously been shown that 3β-Adiol activates estrogen response element (ERE) dependent ERβ-induced transcription equivalent to that of 17β-estradiol in neural tissue (Pak et al., 2005). Thus it has been proposed that 3β-Adiol is the native ligand for ERβ in perinatal life (Fan et al., 2010). Moreover, expression of ERβ within the mPOA, a region long recognized to be important for both appetitive and consummatory aspects of male sexual behavior (Balthazart et al., 1998; Hull and Dominguez, 2007; McCarthy et al., 2009; Phillips-Farfan and Fernandez-Guasti, 2009), is robust within the neonatal period (Cao and Patisaul, 2011) suggesting that emerging sexual dimorphisms within this region may be particularly sensitive to ERβ agonists during this time. For example, synaptic spine density in the mPOA is positively correlated with male mounting behavior (McCarthy et al., 2009) and the density of dendritic spines in the mPOA is higher in males than females as early as PND 0 (Amateau and McCarthy, 2002). Neonatal agonism of ERβ may have altered the masculinization of this pathway, leading to fewer mPOA dendritic spines and therefore compromised male sexual performance.
Paradoxically, females selected against the males neonatally exposed to the high dose (2 mg/kg) of DPN in the partner preference test even though the reproductive behavior of these males did not appear to be compromised. Conversely, females failed to select against the males exposed to the intermediate (1 mg/kg) dose of DPN or GEN, even though both of these groups had lower virility. In one round of Experiment 4, females actually displayed a preference for the GEN exposed males so collectively the data indicate that, if anything, neonatal GEN exposure could slightly enhance attractiveness, rather than reduce it, despite compromised sexual vigor. In all cases, females consistently favored the control males over the EB exposed males, thus it is certain that the females were being selective. It is not clear upon what pheromonal or other cues the females based their preference, but it is possible that females need to physically interact with the males to make a more accurate assessment of virility.
The results observed following neonatal GEN exposure on male reproductive physiology and behaviors are consistent with prior studies. For example, a recent study also failed to find any impact of perinatal GEN exposure, across a wide dose range, on testis weight or blood androgen levels in a variety of species (Cederroth et al., 2010a). Effects on male reproductive parameters may be more subtle, however. A recent study characterized the impact of lifetime (gestation to adulthood) exposure to a soy-rich diet containing GEN on a range of reproductive parameters in male mice and found a 25% decrease in epididymal sperm count as well as a 20% reduction in litter size. Moreover, expression of numerous germ cell specific genes involved in sperm glycolysis and mobility were also significantly lower in the soy-exposed males (Cederroth et al., 2010b). This is important to note in light of the partner preference results because if males exposed to GEN are reproductively impaired, but females continue to select them as a mate, then fitness could be decreased and this may ultimately negatively affect a population.
Only two other published studies have examined the effects of exposure to an environmentally relevant dose of endocrine disrupting chemicals on partner preference (Crews et al., 2007; Markman et al., 2008). Collectively, the results from these two prior studies indicate that EDC exposure might induce multigenerational effects, and a transglobal population decline in a given species. Crews and colleagues (2007) found that exposure to vinclozolin, an anti-androgenic fungicide, decreased male attractiveness, and that this effect persisted across generations such that females with no exposure history and females three generations removed from the exposure, selected against males with an exposure history. By contrast, males failed to select against exposed females. Further assessment revealed a transgenerational impairment in male fertility (Anway et al., 2005) suggesting that vinclozolin exposure induces epigenetic changes within the male germ line that impact both fertility and attractiveness in subsequent generations of males (Anway and Skinner, 2008). Markman and colleagues (2008) exposed juvenile male song birds to different combinations of estrogenic EDCs, all present in the natural feeding areas of the study population, in a manner consistent with the natural exposure route. A mixture of estradiol, dioctylphthalate, BPA and dibutylphthalate, appeared to act synergistically, producing a hypermasculinized song system, and hence a more attractive male, based on female selection of song quality. Exposure to these EDCs, however, also led to these males being immunocompromised. Therefore, song quality was no longer a reliable indicator of overall fitness which, over time, could contribute to population decline.
Finally, no significant effect of PPT was observed in Experiment 1. It should be noted that, overall, male reproductive performance was poor in Experiments 1 and 4 which contributed to the high degree of variability. This project began and ended during the winter months and, although the environment in our vivarium is tightly controlled, it is possible that there was an effect of season on reproductive performance. To compensate, the experimental paradigm was amended in several ways as the studies progressed. For example, additional rounds of testing were included, the light cycle was extended, and the observational period was lengthened. It is also possible that the dose of PPT was insufficient to induce a response. Because male mounting behavior is compromised by perinatal androgen (Henley et al., 2010) or estrogen (Zadina et al., 1979) administration, it was hypothesized that PPT administration could impair virility. Further work will be needed to adequately test this possibility.
It is important to note that maternal behavior is well recognized to influence the subsequent male reproductive behavior of their offspring, thus EDCs or experimental manipulations that impact maternal care can be potential confounds (Cox et al.; Del Cerro et al., 2010; Rhees et al., 2001). The dams in this study were not dosed directly, as we targeted the developmental window of PNDs 0–3. Therefore if dams were exposed, it would only be minimally via ingestion during the licking and grooming of pups. Prior studies have established that dietary genistein does not significantly impact licking and grooming or nest building in rats (Ball et al., 2010) and either fails to alter (Flynn et al., 2000), or enhances nursing behavior (Ball et al., 2010). Handling stress is a concern when cross-fostering (Caldji et al., 2003; Cox et al., 2010). Thus, to account for that, the GEN exposure was conducted twice, once with cross-fostering and once without. The results were similar in both cases, suggesting that cross-fostering was not a confound and did not significantly modify the effects of GEN.
Collectively, the data indicate that ERβ is involved in the masculinization of neuroendocrine pathways that regulate sex specific behavior, and imply that environmental exposures during critical stages of neuroendocrine development can evoke long term effects on complex behaviors. Our results show that neonatal agonism of ERβ, either with a synthetic agonist or a naturally occurring EDC, can impair reproductive behavior in male rats. This effect, however, does not always accompany decreased capacity to attract a mate. Furthermore, DPN produced a non-monotonic effect on male virility. Because GEN and other EDCs are increasingly ubiquitous components of our environment, a critical next step would be to explore the dose-specific effects of more ERβ agonists like GEN and determine if these effects persist in subsequent generations, potentially through epigenetic mechanisms.
Acknowledgements
The authors gratefully acknowledge Joseph Grappé for constructing the partner preference arenas, Linda Hester and Raeford McKinley for assistance with animal husbandry and support, and Karina Todd, John Vandenbergh, and Heather Bateman Adewale for critically reading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal Bisphenol-A Exposure Alters Rat Reproductive Development and Ovarian Morphology Without Impairing Activation of Gonadotropin Releasing Hormone Neurons. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online. 2008;16:23–25. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: Evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141:3898–3907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- Ball ER, Caniglia MK, Wilcox JL, Overton KA, Burr MJ, Wolfe BD, Sanders BJ, Wisniewski AB, Wrenn CC. Effects of genistein in the maternal diet on reproductive development and spatial learning in male rats. Horm Behav. 2010;57:313–322. doi: 10.1016/j.yhbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson JA, Nilsson S. Differential responces of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Molecular Pharmacology. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentiation of coital behavior in mammals: A comparative analysis. Neuroscience & Biobehavioral Reviews. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav. 2009;55:579–588. doi: 10.1016/j.yhbeh.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA, Noble RG, Orndoff RK. Effects of perinatal androgen treatment on responses of male rats to gonadal hormones in adulthood. J Comp Physiol Psychol. 1969;68:490–497. doi: 10.1037/h0027658. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bu L, Lephart ED. AVPV neurons containing estrogen receptor-beta in adult male rats are influenced by soy isoflavones. BMC Neurosci. 2007;8:13. doi: 10.1186/1471-2202-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. The Journal of comparative neurology. 2011 doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Auger J, Zimmermann C, Eustache F, Nef S. Soy, phytooestrogens and male reproductive function: a review. Int J Androl. 2010a;33:304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P, Doerge DR, Pralong FP, Vassalli JD, Nef S. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Mol Cell Endocrinol. doi: 10.1016/j.mce.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P, Doerge DR, Pralong FP, Vassalli JD, Nef S. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Mol. Cell. Endocrinol. 2010b doi: 10.1016/j.mce.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Clark JT. Sexual function in altered physiological states: comparison of effects of hypertension, diabetes, hyperprolactinemia, and others to “normal” aging in male rats. Neurosci Biobehav Rev. 1995;19:279–302. doi: 10.1016/0149-7634(94)00058-9. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Hormones and Behavior. 58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cerro MC, Perez-Laso C, Ortega E, Martin JL, Gomez F, Perez-Izquierdo MA, Segovia S. Maternal care counteracts behavioral effects of prenatal environmental stress in female rats. Behavioural brain research. 2010;208:593–602. doi: 10.1016/j.bbr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Diamond M, Llacuna A, Wong CL. Sex behavior after neonatal progesterone, testosterone, estrogen or antiandrogens. Horm Behav. 1973;4:73–88. [Google Scholar]
- Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205–211. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL. Developmental profile and regulation of estrogen receptor (ER) mRNA expression in the preoptic area of prenatal rats. Brain Res Dev Brain Res. 1996;94:224–233. doi: 10.1016/0165-3806(96)00067-3. [DOI] [PubMed] [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Xu H, Warner M, Gustafsson JA. ERbeta in CNS: new roles in development and function. Prog Brain Res. 2010;181:233–250. doi: 10.1016/S0079-6123(08)81013-8. [DOI] [PubMed] [Google Scholar]
- Flynn KM, Ferguson SA, Delclos KB, Newbold RR. Multigenerational exposure to dietary genistein has no severe effects on nursing behavior in rats. Neurotoxicology. 2000;21:997–1001. [PubMed] [Google Scholar]
- Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D, DeBold J. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 1971;2:287–297. [Google Scholar]
- Henley CL, Nunez AA, Clemens LG. Exogenous androgen during development alters adult partner preference and mating behavior in gonadally intact male rats. Horm Behav. 2010;57:488–495. doi: 10.1016/j.yhbeh.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod. 2009;80:425–431. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Studies of the effects of neonatal exposure to genistein on the developing female reproductive system. J AOAC Int. 2006;89:1189–1196. [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol. 2007;23:308–316. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Jones BA, Shimell JJ, Watson NV. Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Karolczak M, Beyer C. Developmental sex differences in estrogen receptor-beta mRNA expression in the mouse hypothalamus preoptic region. Neuroendocrinology. 1998;68:229–234. doi: 10.1159/000054370. [DOI] [PubMed] [Google Scholar]
- Kendig EL, Le HH, Belcher SM. Defining hormesis: evaluation of a complex concentration response phenomenon. Int J Toxicol. 2010;29:235–246. doi: 10.1177/1091581810363012. [DOI] [PubMed] [Google Scholar]
- Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, Yamanouchi K. Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm Behav. 2003;44:140–145. doi: 10.1016/s0018-506x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: Defeminization of male brain and behavior. Proc Natl Acad Sci U S A. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Gustafsson JA, Rissman EF. Estrogen receptor beta modulates estradiol induction of progestin receptor immunoreactivity in male, but not in female, mouse medial preoptic area. Endocrinology. 2004;145:4500–4506. doi: 10.1210/en.2003-1708. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Broekhof JL, Kuiper GG, Gustafsson JA, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech Dev. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- Levine S, Mullins R., Jr. Estrogen Administered Neonatally Affects Adult Sexual Behavior in Male and Female Rats. Science. 1964;144:185–187. doi: 10.1126/science.144.3615.185. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman S, Leitner S, Catchpole C, Barnsley S, Muller CT, Pascoe D, Buchanan KL. Pollutants increase song complexity and the volume of the brain area HVC in a songbird. PLoS One. 2008;3:e1674. doi: 10.1371/journal.pone.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol. 2006;20:534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Gonadal steroid influences on brain development and sexual differentiation. Int Rev Physiol. 1983;27:99–145. [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Hess KA, Overmann GJ, Torontali SM, Carr GJ, Tiesman JP, Foertsch LM, Richardson BD, Martinez JE, Daston GP. Gene expression changes induced in the testis by transplacental exposure to high and low doses of 17{alpha}-ethynyl estradiol, genistein, or bisphenol A. Toxicol Sci. 2005;86:396–416. doi: 10.1093/toxsci/kfi198. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ER agonist increases adult anxiety and aggression in gonadally intact male rats. Hormones and Behavior. 2008;53:580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav. 2009a;55:319–328. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Melby M, Whitten PL, Young LJ. Genistein affects ER□- but not ER□-dependent gene expression in the hypothalamus. Endocrinology. 2002;143:2189–2197. doi: 10.1210/endo.143.6.8843. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009b;30:350–357. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Phillips-Farfan BV, Fernandez-Guasti A. Endocrine, neural and pharmacological aspects of sexual satiety in male rats. Neurosci Biobehav Rev. 2009;33:442–455. doi: 10.1016/j.neubiorev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pollak EI, Sachs BD. Masculine sexual behavior and morphology: paradoxical effects of perinatal androgen treatment in male and female rats. Behav Biol. 1975;13:401–411. doi: 10.1016/s0091-6773(75)90949-9. [DOI] [PubMed] [Google Scholar]
- Raubertas RF, Davis BA, Bowen WH, Pearson SK, Watson GE. Litter effects on caries in rats and implications for experimental design. Caries Res. 1999;33:164–169. doi: 10.1159/000016511. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Lephart ED, Eliason D. Effects of maternal separation during early postnatal development on male sexual behavior and female reproductive function. Behavioural Brain Research. 2001;123:1–10. doi: 10.1016/s0166-4328(00)00381-8. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Divine RL, Newbold RR, Delclos KB. Increased volume of the calbindin D28k-labeled sexually dimorphic hypothalamus in genistein and nonylphenol-treated male rats. Toxicol Sci. 2004;82:570–576. doi: 10.1093/toxsci/kfh297. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Temple JL, Scordalakes EM, Bodo C, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta gene disrupts pubertal male sexual behavior. Horm Behav. 2003;44:427–434. doi: 10.1016/j.yhbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Dunlap JL, Gerall AA. Modifications induced by neonatal steroids in reproductive organs and behavior of male rats. J Comp Physiol Psychol. 1979;93:314–322. doi: 10.1037/h0077564. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]