Abstract
Objective
Assess the association between depressive symptoms (not meeting the criteria for major depression) and gait dysfunction in older adults.
Design
Cross-sectional study.
Setting
Einstein Aging Study, a community-based longitudinal aging study.
Participants
Six hundred ten nondemented and nondepressed community-residing adults age 70 and older.
Measurements
Depressive symptoms measured using the 15-item Geriatric Depression Scale. To obtain a comprehensive assessment of gait, eight individual quantitative gait parameters were assessed: velocity (cm/s), stride length (cm), cadence (steps/min), swing phase (seconds), stance phase (seconds), double support phase (seconds), stride length variability (SD of stride length), and swing time variability (SD of swing time). Multiple linear regression analysis was applied to study the association of depressive symptoms with gait, adjusting for potential confounders including demographic variables, medical illnesses, and clinical gait abnormalities.
Results
Increased level of depressive symptoms was associated with worse velocity, stride, and swing time variability. The relationship of the remaining five gait variables with depressive symptoms was not significant in the fully adjusted models.
Conclusions
Higher levels of depressive symptoms are associated with worse performance in specific quantitative gait variables in community-residing older adults.
Keywords: depressive symptoms, epidemiology, elderly, gait
It is estimated that about 14% of the U.S. population of age 65 and older suffer from depressive symptoms that do not meet criteria for major depressive disorder (MDD), which is approximately 2.5 times higher than the prevalence of MDD.1 In addition to the associated risk of converting to MDD,2 depressive symptoms in older adults have been reported to be associated with increased risk of disability,3 decreased quality of life, and result in increased societal costs due to higher health care needs.4
Gait performance is recommended as a clinical marker of health and function in older adults,5,6 and predicts multiple adverse health outcomes including disability, falls, dementia, and death in our and other studies.5,7,8 Like depressive symptoms, the prevalence of gait disorders is high in older populations.6 Although slowing of gait in clinical depression is well established,9–12 little is known about the nature of gait dysfunction in elderly without MDD. It is unclear from studies that have included both depressed and nondepressed elderly4,13 whether the observed associations between gait and depressive symptoms are explained by the presence of subjects with MDD in these samples. Thus, these studies do not establish whether gait dysfunction occurs in the earliest or mildest stages of depressive symptoms. On the contrary, previous studies of gait in adults with depressive symptoms but who did not meet criteria for MDD have been mostly limited to older adults in hospital or nursing homes9,10,12 or younger adults.9–12 Older individuals with depressive symptoms have been reported to have slow gait velocity.13 However, gait is a complex motor behavior with many other measurable facets besides velocity. For instance, gait variability was a stronger predictor of falls than velocity and was the only predictor of injurious falls in our cohort.7
A more comprehensive examination of gait performance in older adults may not only increase our understanding of brain substrates and pathologic processes that are common to gait and depressive symptoms but may also provide insights into new interventions. To address limitations of previous studies, we conducted a cross-sectional study of the relationship between depressive symptoms and gait function, in an ambulatory community-residing sample of 610 older adults (age 70 and older) who were free of dementia and MDD.
METHODS
Sample
Subjects were participants in the Einstein Aging Study.14 The primary aim of the Einstein Aging study is to identify risk factors for cognitive decline. Study design and methods have been previously reported.6 In brief, potential subjects (age 70 and older) identified from population lists of Bronx County were contacted by a letter that explained the purpose and nature of the study and then by telephone. The telephone interview included verbal consent, medical history questionnaire, and cognitive screening tests.7 Exclusion criteria included severe audiovisual loss, bed bound because of illness, and institutionalization. After the interview, an age-stratified sample of subjects who matched on a computerized randomization procedure was invited for further evaluation at our clinical research center at the Albert Einstein College of Medicine. Informed consent was obtained at enrollment according to protocols approved by the local institutional review board. Subjects returned for yearly follow-up assessments.
Mobility Assessment
Quantitative Gait Assessment
Subjects received quantitative gait assessments at baseline and at each annual follow-up visit using a computerized walkway (180.0 × 35.5 × 0.25 inches) with embedded pressure sensors (GAITRite; CIR Systems. Havertown, PA). Research assistants asked subjects to walk on the instrumented mat at their normal pace for two trials in a quiet well-lit hallway wearing comfortable footwear and without any attached monitoring devices. White lines on the floor marked start and stop points, which included three feet from the walkway edge for initial acceleration and terminal deceleration. On the basis of footfalls recorded on the walkway, the software automatically computes gait parameters as the mean of two trials. The following eight gait variables that have been reported to have associations with cognitive function and dementia as well as falls in our previous studies were selected for analysis:7,15 velocity (cm/s), stride length (cm), cadence (steps/min), swing phase (seconds), stance phase (seconds), double support phase (seconds), stride length variability (standard deviation; SD of stride length), and swing time variability (SD of swing time). The GAITRite system is widely used in clinical research settings, and excellent reliability has been reported in our and other studies.7,16
Clinical Gait Assessment
Study clinicians clinically evaluated gait at each visit as part of the standard neurologic examination, blinded to the quantitative assessment. Gait was determined to be normal or abnormal due to neurologic (such as hemiparesis or Parkinson disease) or nonneurologic (such as arthritis or joint deformities) causes after visual inspection of walking patterns. More detailed descriptions and Web links to videos of individual gait subtypes are available in our previous publications.6,14 The clinical gait assessment has established test–retest reliability and predictive validity for outcomes such as dementia and falls.6,14
Depressive Symptoms
Participants were screened for depressive symptoms with the 15-item Geriatric Depression Scale (GDS).17,18 The GDS has been reported to have Cronbach’s α coefficient of 0.94 and a split-half reliability coefficient of 0.9419 in elderly populations. The GDS has been utilized to describe the relationship of depressive symptoms with other health outcomes in older adults including mortality20 as well as risk of developing major depression21 and falls.22
Study Sample
Between June 2004 and February 2010, 723 Einstein Aging Study participants received detailed clinical and quantitative motoric assessments as part of a gait and mobility substudy.6,7 Reasons for not obtaining assessments included clinician or tester unavailability for conduction motoric assessments, subject illness, or subject refusal. Subjects who were not included were similar in terms of age, sex, and educational status at enrollment.
Of the 723 subjects, 41 with prevalent dementia and 70 with MDD (defined as self-reported history of MDD and previously validated GDS cutscore of 10 or higher to capture undiagnosed cases of MDD23) were excluded. Two additional subjects with missing GDS scores were excluded. Thus, 610 older adults were eligible for this analysis. The subjects who were excluded had significantly different GDS scores but were not significantly different in age, sex, race, and education compared to eligible subjects.
Covariates
Study subjects were interviewed using structured questionnaires at baseline and annual follow-up study visits about sociodemographic variables, medical illnesses, medications, and activities of daily living. Details were verified with family members and caregivers when available. History of diabetes mellitus, heart failure, hypertension, angina pectoris, myocardial infarction, strokes, Parkinson disease, chronic obstructive lung disease, and arthritis reported by subjects at baseline gait visit or at any previous Einstein Aging Study visit was used to calculate a summary illness index (range: 0–9) as previously reported.24 Medical records and primary care providers were also consulted to verify or obtain additional information.6 Any use of antidepressant, antipsychotic, antianxiety, or antimanic drugs by subjects was summarized as “psychotropic use.” Research assistants recorded blood pressure, height, and weight using standard protocols. Mean arterial pressure was calculated as the sum of diastolic blood pressure and one-third pulse pressure (systolic–diastolic blood pressure). General cognitive status was assessed with the Blessed-Information-Memory-Concentration test.25 Participation in 10 common physical leisure activities (tennis, golf, swimming, bicycling, dancing, brisk walking, yoga, aerobics, weight training, and bowling) was assessed using our validated and reliable Physical Activity Scale (PAS).26 For each activity, subjects received two points for participating two or more days per week; one point for weekly participation; and zero points for participating occasionally or never. We summed individual activity points for each individual activity to generate a summary PAS score.26
Data Analysis
Multiple linear regression analysis was used to investigate the association between depressive symptoms and each of the eight gait parameters (variability measures were log transformed to account for skewed distributions), adjusting for age, sex, race, and education. Associations are reported as parameter estimates with 95% confidence intervals (CIs). The Benjamini and Hochberg’s approach27 was used to control the false discovery rate and p-values for individual gait variables that remained significant (Table 1) were indicated.
Table 1.
Association between depressive symptoms measured by the GDS and individual gait variables
| Linear Regression Model 1‡ | Linear Regression Model 2§ | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variable |
GDS Coefficient |
Lower CI | Upper CI |
P value | GDS Coefficient |
Lower CI | Upper CI |
P value |
| Velocity, cm/s | −2.98 | (−3.85 to | −2.10) | <0.001† | −1.17 | (−2.05 to | −0.30) | 0.01† |
| Stride Length, cm | −2.39 | (−3.14 to | −1.64) | <0.001† | −0.95 | (−1.69 to | −0.21) | 0.01† |
| Cadence, steps/min | −1.17 | (−1.66 to | −0.68) | <0.001† | −0.45 | (−0.99 to | 0.09) | 0.1 |
| Stance Time, seconds | 0.01 | (0.01 to | 0.02) | <0.001† | 0.004 | (−0.003 to | 0.01) | 0.29 |
| Swing Time, seconds | 0.003 | (0.001 to | 0.005) | 0.01† | 0.002 | (−0.001 to | 0.004) | 0.17 |
| Double Support Time, seconds | 0.01 | (0.01 to | 0.02) | <0.001† | 0.003 | (−0.004 to | 0.01) | 0.36 |
| Stride Length Variability, log SD | 0.02 | (−0.01 to | 0.04) | 0.15 | 0.01 | (−0.02 to | 0.03) | 0.51 |
| Swing Time Variability, log SD | 0.06 | (0.04 to | 0.09) | <0.001† | 0.03 | (0.002 to | 0.06) | 0.03 |
Indicates significant P-value (<0.05) after applying correction for multiple comparisons (see Methods for description).
Linear regression model adjusted for baseline gds, age, sex, race, and education; p-value for variables are derived from t-statistic and has 603 residual degrees of freedom
Linear regression model additionally adjusted for medical illness index, presence of clinical gait abnormalities, Blessed-Information-Memory-Concentration test score, mean arterial pressure, body mass index, physical activity scale score, and psychotropic use; 489 residual degrees of freedom
In additional analyses (Model 2), adjustments for the following potential confounders were applied: medical illness index, presence of clinical gait abnormalities, PAS score, Blessed test score, mean arterial pressure, body mass index (BMI), and psychotropic medication use. Missing values appeared random and showed no evidence of being related to gait using multivariate logistic regression. Thus, the main analysis for Model 2 is based on the 503 subjects without missing covariate data. Regression diagnostics for all models were examined analytically and graphically and were adequately met.
All statistical analyses were performed in STATA 10.1 (StataCorp LP, College Station, TX, 2009).
RESULTS
Baseline characteristics of study subjects are presented in Table 2. The sample was predominantly female (61%), well-educated (mean [SD]: 14.0 [3.4] years), mostly Caucasian (67%), with mean age 80.3 (SD: 5.5) years and a mean GDS score of 2.0 (SD: 1.9).
Table 2.
Baseline Characteristics
| Mean (N=610) |
SD | |
|---|---|---|
| Characteristic | ||
| Age | 80.3 | 5.5 |
| Education, years | 14 | 3.4 |
| N | % | |
| Female | 374 | 61.3% |
| Race | ||
| White | 406 | 66.6% |
| Black | 171 | 28.0% |
| Other | 33 | 5.4% |
| Mean | SD | |
| Geriatric Depression Scale score (range 0–15) | 2.0 | 1.9 |
| Blessed-Information-Memory-Concentration test score (range 0–32) | 2.0 | 1.9 |
| Height, cm (n=550) | 164.7 | 10.3 |
| Weight, kg (n=547) | 74.3 | 16.6 |
| Body Mass Index, kg/m2 (n=547) | 27.3 | 5.4 |
| Mean arterial pressure, mmHg (n=536) | 97.6 | 10.2 |
| Illness index (range 0–9) | 1.2 | 1.0 |
| Physical Activity Scale (range 0–20) | 1.6 | 2.2 |
| N | % | |
| Clinical gait abnormality (n=563) | 223 | 39.6% |
| Psychotropic use | 22 | 3.6% |
| Mean | SD | |
| Gait parameters | ||
| Velocity, cm/s | 93.1 | 23.4 |
| Cadence, steps/min | 101.3 | 12.3 |
| Stride Length, cm | 109.7 | 20.9 |
| Stance Time, seconds | 0.8 | 0.2 |
| Double Support Time, seconds | 0.3 | 0.1 |
| Swing Time, seconds | 0.4 | 0.1 |
| Swing Time Variability, SD | 0.0 | 0.0 |
| Stride Length Variability, SD | 4.4 | 2.5 |
Results of linear regression analysis of the association between depressive symptoms and individual gait parameters are presented in Table 1. In the basic model adjusting for age, sex, race, and years of education, higher GDS scores was associated with worse performance on all gait measures except stride length variability. A one-point increase in the GDS corresponded to decreases of 2.98 cm/s in velocity, 2.39 cm in stride length, and 1.17 steps/min in cadence; and increases of 0.01 seconds in stance time, 0.003 seconds in swing time, 0.01 seconds in double support time, and 0.06 in the log transformed swing time SD (seconds).
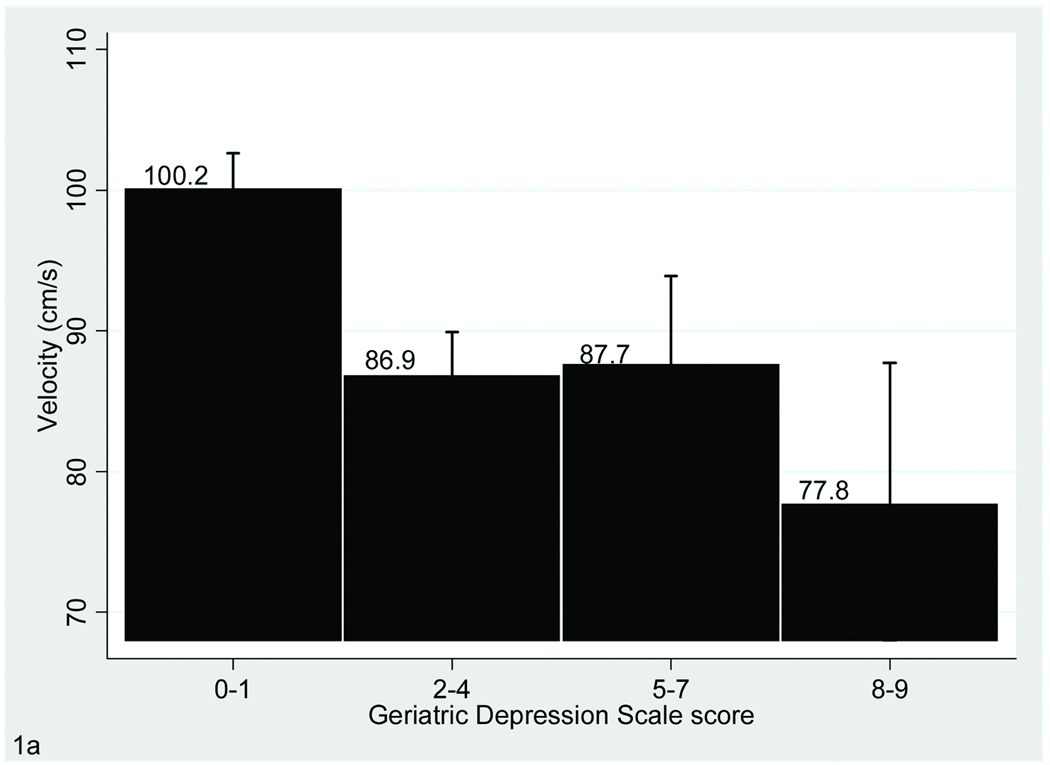
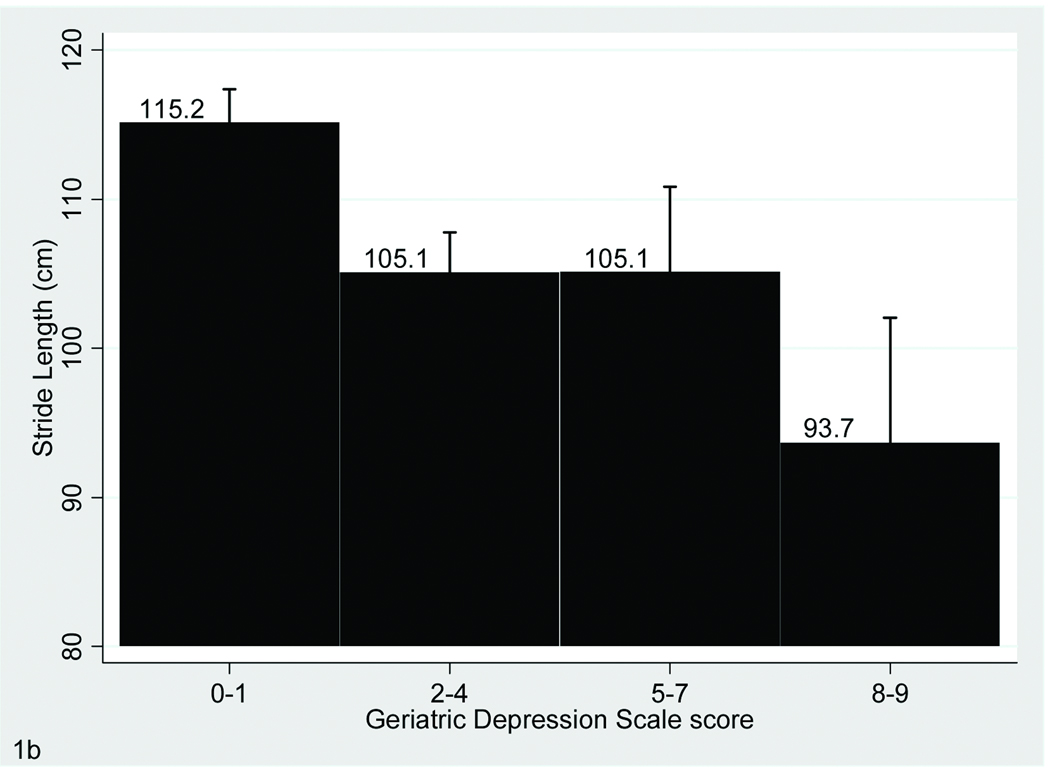
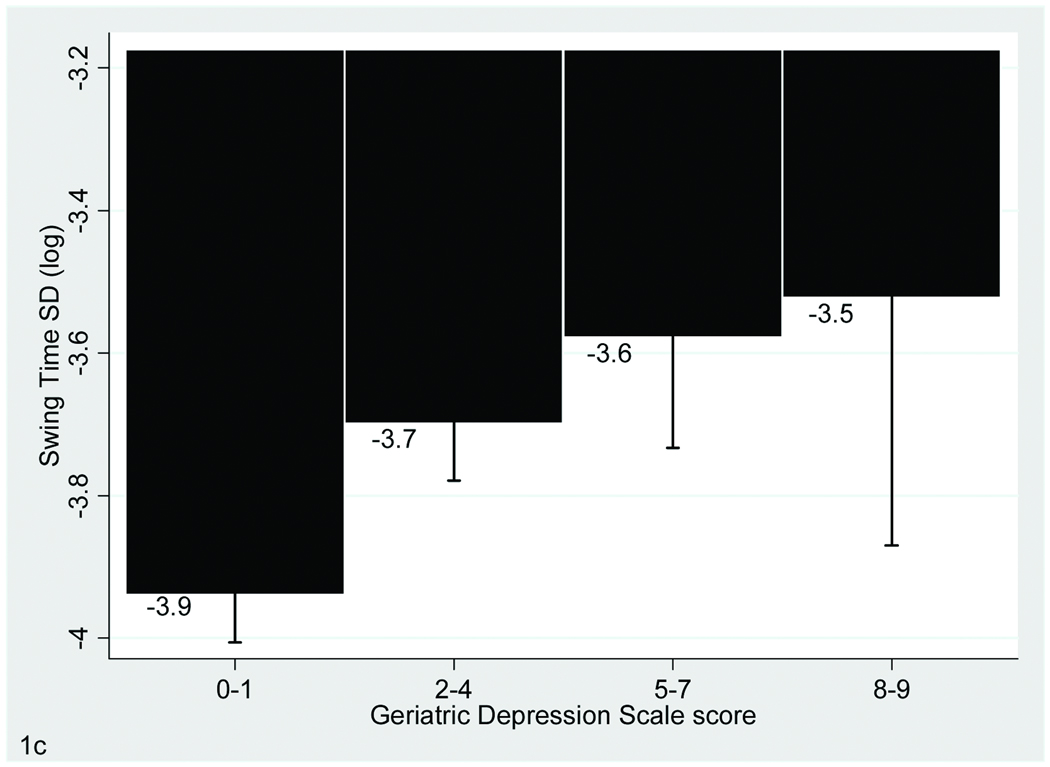
Upon further adjustment for additional covariates including in Model 2 the relationship with depressive symptoms remained significant for the velocity, stride length, and swing time variability variables only (Table 1 and Figure 1).
FIGURE 1. Association of Gait With Depressive Symptoms.
The bar graphs display the relationship between Geriatric Depression Scale scores and performance on gait velocity (A), stride length (A) and swing time variability (B), which are the three gait parameters that were significantly associated with depressive symptoms in the fully adjusted model. Bars show mean values for the gait variables and error bars indicate standard error of the mean.
CONCLUSIONS
The findings of this study show a significant association between depressive symptoms (in the absence of MDD) and quantitative gait dysfunction in a large, well characterized cohort of community-dwelling nondemented older adults. Increasing numbers of depressive symptoms in this sample was associated with worse performance on seven out of the selected eight gait measures in our study after adjusting for demographic variables. The association with depressive symptoms remained significant for velocity, stride length and swing time variability even after adjusting for several additional confounders such as vascular risk factors, overall disease burden, physical activity levels, and cognitive status. These results suggest that depressive symptoms have specific rather than global effects on gait performance in older adults.
To our knowledge, this is the first study to systematically examine the association of depressive symptoms with comprehensive gait assessments in community residing older adults. Our results are supported by previous studies that have examined the relationship between depressive symptoms and other aspects of mobility.4,12,13,28 Increasing levels of depressive symptoms, not meeting the criteria for MDD, were predictive of greater decline in standing balance, walking speed, and chair rise performance in a cohort of noninstituionalized older adults, age 71 and older.4 Low performance on tests of executive function, slower gait speed, and depressive symptoms has been identified to coexist in community-dwelling nondemented elderly.13 Differences in double support, stride length,10 gait velocity,10,12 arm swing, lateral body sway, posture of upper body, and vertical up and down movements of the upper body12 have been reported between patients with clinical depression and healthy elderly controls, with worse performance in the former group. These studies, however, have been mostly limited to older adults in hospital or nursing homes,10,12 patients with major depression,9–12 measures of mobility other than gait,4,13,22 gait velocity alone,4,13 or younger adults.9–12 Our study extends these findings to a broader assessment of gait performance in nondemented community-dwelling elderly without MDD.
Our study focused on older adults with depressive symptoms but not meeting criteria for MDD. Although MDD is the most frequently studied and well-characterized depressive syndrome,29 the incidence of clinically significant nonmajor forms of depression also steadily increases with age.1 We chose to exclude subjects with MDD, to address the nature of gait dysfunction in the earliest or mildest stages of depression. Moreover, it would be difficult to make inferences about the association between gait and depressive symptoms in the larger non-MDD population if subjects with MDD were included. When we repeated our analyses including subjects with clinical depression in the study sample, the association between depressive symptoms with velocity, stride length, and swing time remained significant. However, as may be expected, the strength of the association was increased. For instance, addition of clinically depressed subjects to the study sample strengthens the association of GDS scores with gait velocity (B −1.31, 95% CI −2.07 to −0.55) compared to the model that excluded depressed subjects (B −1.17, 95% CI −2.05 to −0.30).
There are various possible mechanisms by which depressive symptoms may be related to quantitative gait dysfunction. Vascular mechanisms may be an important link, as it has been reported that there are associations between late-onset depression and gait abnormalities with vascular diseases (such as hypertension, diabetes, or coronary artery disease), frontal–subcortical dysfunction, and white matter lesions.30–34 Slower velocity, shorter stride length, and longer double support time have been shown to be associated with subclinical strokes and high white matter ischemic disease on imaging studies.35 Subjects with extensive white matter lesions were three to five times more likely to have depressive symptoms as compared with individuals with mild or no white matter lesions in a population-based cohort study of adults age 60–90.36 Motor (i.e., gait), cognitive, and neuropsychiatric disorders such as depression, may concurrently develop due to disruption of neural circuits originating in the prefrontal cortex37 as the result of neurodegenerative disease, vascular disease, or other diseases.31 Neuroimaging data that could confirm this vascular hypothesis was not routinely obtained in all subjects in our cohort and needs to be done in follow-up studies.
Gait was a predictor of cognitive decline and incident dementia in our cohort.7 In addition, depression has been demonstrated to be a risk factor for Alzheimer disease.38 To account for cognitive impairment as a possible mechanism linking depressive symptoms and gait dysfunction we adjusted for the Blessed test,25 which correlates well with the pathology of Alzheimer disease.38 However, the significant association remained suggesting that cognitive dysfunction was not the only possible link between gait and depressive symptoms. Depressive symptoms are common in older adults with Mild Cognitive Impairment (MCI) syndrome.39 We have reported that gait dysfunction is common in MCI patients in our cohort.15 Despite excluding subjects with dementia and adjusting for the cognitive status using the Blessed test, the association of gait and depressive symptoms could in part be due to presence of subjects with MCI in this sample,40 and this possibility needs to be explored in future studies.41
Clinical gait abnormalities were included in our fully adjusted models to partially control for nonneurologic limitations of gait such as pain and arthritis as well as neurologic diseases. However, the significant relationships for the three gait variables remained in these additional analyses. We have reported a very low prevalence of neurologic gait subtypes such as parkinsonian, frontal, or hemiparetic that are also associated with mood disorders in the same cohort.6,42 Arthritis has been reported to be associated with depressive symptoms in adults43 and may be another link between depressive symptoms and gait.
The strengths of this study include the large sample size with systematic gait assessments7 and validated clinical and diagnostic procedures for motor and depressive symptoms and signs.7,19 Limitations of the study include the cross-sectional design, which limits inferences about temporality or causality. Although the presumption is that depressive symptoms lead to gait dysfunction, we cannot discount the possibility that gait dysfunction may limit mobility and social interactions increasing risk for developing depressive symptoms in older adults. In support of the reverse hypothesis, a recent study reported that lower walking capacity predicted increased risk of depressive symptoms in older men during an 8-year follow-up period.44 Our findings should be followed up in longitudinal observational studies or clinical trials. Our study excluded subjects with dementia or inability to ambulate at baseline. Therefore, our findings may not generalize to nursing home-based samples, though the observed associations might be stronger in less healthier populations. We limited our examination of gait to the eight variables that have been extensively studied in this cohort.7,15 It is likely that other gait variables may also show independent associations with depressive symptoms. However, most of these other gait variables are likely to be highly correlated with our selected gait variables. Although we examined some possible mechanisms such as vascular disease in our analyses, future studies should examine these observed relationships in more depth using more direct measures of pathology.
In conclusion, increasing depressive symptoms in community residing older adults are associated with quantitative gait dysfunction even in the absence of major depression or dementia. In the primary care setting, elderly patients with early signs of depressive symptoms should be evaluated for therapy or medical treatment and assessed for gait impairment. On the contrary, patients with gait abnormalities should be screened for depression and possible underlying pathologies such as vascular risk factors, which can be targeted for treatment.
Acknowledgments
The Einstein Aging Study is funded by the National Institute on Aging (AG03949, PI: R.B. Lipton, M.D.). Dr Verghese is funded by the National Institute on Aging (RO1 AG025119). Ms Tamar Brandler is supported by the CTSA Grant UL1 RR025750 and TL1 RR025748 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclosures to report.
REFERENCES
- 1.VanItallie TB. Subsyndromal depression in the elderly: underdiagnosed and undertreated. Metabolism. 2005;54(5) Suppl 1:39–44. doi: 10.1016/j.metabol.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Lyness JM, Heo M, Datto CJ, et al. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med. 2006;144(7):496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- 3.Barry LC, Allore HG, Bruce ML, et al. Longitudinal association between depressive symptoms and disability burden among older persons. J Gerontol a-Biol. 2009;64(12):1325–1332. doi: 10.1093/gerona/glp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 5.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol a-Biol. 2005;60(10):1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 6.Verghese J, LeValley A, Hall CB, et al. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54(2):255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verghese J, Wang C, Lipton RB, et al. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol a-Biol. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 9.Sloman L, Berridge M, Homatidis S, et al. Gait patterns of depressed patients and normal subjects. Am J Psychiatry. 1982;139(1):94–97. doi: 10.1176/ajp.139.1.94. [DOI] [PubMed] [Google Scholar]
- 10.Lemke MR, Wendorff T, Mieth B, et al. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J Psychiatr Res. 2000;34(4–5):277–283. doi: 10.1016/s0022-3956(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Peng CK, Goldberger AL, et al. Gait unsteadiness and fall risk in two affective disorders: a preliminary study. BMC Psychiatry. 2004;4:39. doi: 10.1186/1471-244X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalak J, Troje NF, Fischer J, et al. Embodiment of sadness and depression---gait patterns associated with dysphoric mood. Psychosom Med. 2009;2009(5):580–587. doi: 10.1097/PSY.0b013e3181a2515c. [DOI] [PubMed] [Google Scholar]
- 13.Hajjar I, Yang F, Sorond F, et al. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci. 2009;64(9):994–1001. doi: 10.1093/gerona/glp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verghese J, Lipton RB, Hall CB, et al. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 15.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1/2):165–173. [Google Scholar]
- 18.Mitchell AJ, Bird V, Rizzo M, et al. Which version of the geriatric depression scale is most useful in medical settings and nursing homes? diagnostic validity meta-analysis. Am J Geriatr Psychiatry. 2010;18(12):1066–1077. doi: 10.1097/jgp.0b013e3181f60f81. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 20.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 21.Low GD, Hubley AM. Screening for depression after cardiac events using the Beck Depression Inventory-II and the Geriatric Depression Scale. Soc Indic Res. 2007;82(3):527–543. [Google Scholar]
- 22.Turcu A, Toubin S, Mourey F, et al. Falls and depression in older people. Gerontology. 2004;50(5):303–308. doi: 10.1159/000079128. [DOI] [PubMed] [Google Scholar]
- 23.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psych. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Holtzer R, Ozelius L, Xue X, et al. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31(3):523–531. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 26.Verghese J, Cuiling W, Katz MJ, et al. Leisure activities and risk of vascular cognitive impairment in older adults. J Geriatr Psychiatry Neurol. 2009;22(2):110–118. doi: 10.1177/0891988709332938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate---a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 28.Barry LC, Murphy TE, Gill TM. Depressive symptoms and functional transitions over time in older persons. Am J Geriatr Psychiatry. doi: 10.1097/JGP.0b013e3181ff6669. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavretsky H, Kumar A. Clinically significant non-major depression: old concepts, new insights. Am J Geriatr Psychiatry. 2002;10(3):239–255. [PubMed] [Google Scholar]
- 30.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol a-Biol. 2004;59(8):818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 31.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry---an update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 32.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23(3):421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos GS. “The depression-executive dysfunction syndrome of late life”: a specific target for D3 agonists? Am J Geriatr Psychiatry. 2001;9(1):22–29. [PubMed] [Google Scholar]
- 34.Sneed JR, Culang-Reinlieb ME. The vascular depression hypothesis: an update. Am J Geriatr Psychiatry. doi: 10.1097/jgp.0b013e318202fc8a. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosano C, Brach J, Longstreth WT, et al. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26(1):52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 36.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57(11):1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 37.Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6(4):358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- 38.Grober E, Dickson D, Sliwinski MJ, et al. Memory and mental status correlates of modified Braak staging. Neurobiol Aging. 1999;20(6):573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg PB, Johnston D, Lyketsos CG. A clinical approach to mild cognitive impairment. Am J Psychiatry. 2006;163(11):1884–1890. doi: 10.1176/ajp.2006.163.11.1884. [DOI] [PubMed] [Google Scholar]
- 40.Buchman AS, Boyle PA, Leurgans SE, et al. Cognitive function is associated with the development of mobility impairments in community-dwelling elders. Am J Geriat Psychiat. doi: 10.1097/JGP.0b013e3181ef7a2e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomar JJ, Harvey PD, Bobes-Bascaran MT, et al. Development and cross-validation of the upsa short form for the performance-based functional assessment of patients with mild cognitive impairment and alzheimer disease. Am J Geriat Psychiat. doi: 10.1097/JGP.0b013e3182011846. In press. [DOI] [PubMed] [Google Scholar]
- 42.Verghese J, Ambrose AF, Lipton RB, et al. Neurological gait abnormalities and risk of falls in older adults. J Neurol. 2010;257(3):392–398. doi: 10.1007/s00415-009-5332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel-Nasser AM, El-Azim SA, Taal E, et al. Depression and depressive symptoms in rheumatoid arthritis patients: an analysis of their occurrence and determinants. Br J Rheumatol. 1998;37(4):391–397. doi: 10.1093/rheumatology/37.4.391. [DOI] [PubMed] [Google Scholar]
- 44.Smith TL, Masaki KH, Fong K, et al. Effect of walking distance on 8-year incident depressive symptoms in elderly men with and without chronic disease: the Honolulu-Asia aging study. J Am Geriatr Soc. 2010;58(8):1447–1452. doi: 10.1111/j.1532-5415.2010.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]