Abstract
Objectives
Bladder cancer expresses many potential therapeutic targets of biological agents including the vascular endothelial growth factor receptor (VEGFR). Pazopanib is a small molecule inhibitor of VEGFR-1, -2 -3, platelet derived growth factor receptor (PDGFR) and c-Kit. The current study investigates the efficacy of pazopanib, both alone and in combination with docetaxel, in bladder cancer cells.
Materials and Methods
Using human bladder cancer cells HTB3, HT1376, J82, RT4, CRL1749, T24, Sup and HTB9, the treatment effect of pazopanib and cytotoxic chemotherapy was assessed using a tetrazolium-based assay. The combinatorial effect of these agents on clonogenic growth was further examined. Western blotting was employed to assess changes in relevant downstream targets including phospho-AKT, phospho-FAK, total AKT and total FAK.
Results
Single-agent pazopanib had modest activity. However, synergy was seen with the combination of docetaxel and pazopanib in multiple these cells lines. J82 and T24 cells were selected for additional clonogenic testing due to their resistance to single-agent docetaxel chemotherapy. 1.25 nM of docetaxel had little effect on clonogenic formation; however, in combination with pazopanib, significant inhibition of colony formation was observed. This combination treatment additionally decreased phospho-AKT, an important mediator of cell survival in all cell lines, while phospho-FAK expression was variably affected.
Conclusions
The present study demonstrates synergistic efficacy of pazopanib with docetaxel in docetaxel-resistant bladder cancer cells. This work supports future evaluation of pazopanib with docetaxel for the treatment of bladder cancer with the potential of improved efficacy and toxicity.
Keywords: Urinary bladder neoplasms; Receptors, Vascular endothelial growth factor; Taxoids; Drug Therapy; Angiogenesis inhibitors
Introduction
Bladder cancer is a common cancer, with 70,980 new cases and 14,330 deaths estimated in the United States in 2009 [1]. Despite active chemotherapy regimens, the 5 year overall survival rate for patients with locally advanced or metastatic bladder cancer is only 10–15% [2]. The MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) regimen has been the cornerstone of treatment for advanced urothelial cancer for many years [3]. Although the regimen possesses significant activity, its limitations include a relatively short response duration and significant toxicities. Therefore, new approaches and agents are needed to improve both the activity and tolerability of systemic therapy in bladder cancer. Beyond the standard platinum-based regimens, taxanes have gained greater attention in recent years for the treatment for bladder cancer. In the front-line setting, the addition of paclitaxel to a platinum-gemcitabine doublet resulted in a response rate of 68% [4]. In a phase II trial, single-agent docetaxel showed activity in patients with advanced transitional-cell carcinoma (TCC) who progressed after cisplatin-based therapy [5]. Gitlitz et al [6] also conducted a phase II study of gemcitabine and docetaxel therapy in patients with advanced urothelial carcinoma. The results suggested that combination therapy including docetaxel was an effective treatment for patients with unresectable (Stage T4 or N1) metastatic or locally advanced TCC of the urothelial tract.
Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), is approved by the U.S. Food and Drug Administration for the treatment of advanced lung, breast and colon cancer [7]. The vascular endothelial growth factor receptor (VEGFR) pathway has a critical role in maintaining tumor microvasculature and has been successfully targeted in several cancer types. Studies showed that both VEGFR and the ligand VEGF are expressed in bladder cancer tissue and cells [8–10]. VEGFR expression is associated with invasive properties in cell culture studies and predicts an invasive phenotype in clinical samples [10].
Several other antiangiogenesis drugs, including sorafenib and sunitinib are currently used clinically to treat solid tumors. Pazopanib (GW786034) is a second-generation multitargeted tyrosine kinase inhibitor against VEGFR-1, -2, and -3, platelet-derived growth factor receptor-α, platelet-derived growth factor receptor-β, and c-kit [11]. Pazopanib was approved for use in advanced renal cell carcinoma (RCC) by the Food and Drug Administration in the United States in October of 2009. Previous pre-clinical testing has established the IC50 values of inhibition of VEGFR -1, -2, and -3 were 10, 30, and 47 nM, respectively [12]. Preclinical evaluation has revealed excellent antiangiogenic and antitumor activity. In a phase I study, pazopanib showed efficacy in treating several tumors including renal cell carcinoma, adenocarcinoma of the lung and melanoma [13]. However, to date, the activity of pazopanib in treating bladder cancer has not been reported.
The current study was designed to investigate the efficacy of pazopanib, both alone and in combination with docetaxel in bladder cancer cell lines in vitro.
Materials and Methods
Cell culture and reagents
Human bladder cancer cells HTB3, HT1376, J82, RT4, CRL1749, T24, Sup and HTB9 (American Type Culture Collection, Manassas, VA) were grown in OPTI-MEM (Gibco, Grand Island, NY) with 3.75% Fetal Bovine Serum (Gemini, Woodland, CA) and 100 u/ml streptomycin-penicillin sulfate (Life Technologies, Grand Island, NY). All cell lines were incubated at 37° C with 5% CO2. Pazopanib (from GlaxoSmithKline, GSK) was dissolved in DMSO and docetaxel in Ethanol (Sigma, Saint Louis, MO). Antibodies for the protein characterization were against total AKT, phospho-AKT (Ser473), total FAK, phospho-FAK (Tyr397) and β-actin (Cell signaling, Beverly, MA).
Cell viability assay
For all experiments, the cell viability was assessed using a tetrazolium-based assay (CellTiter 96 AQueous One Solution - Promega Corporation, Madison, WI). Approximately 3000 cells in 50 µl of media per well were plated in 96-well plates in triplicate. Twenty-four hours after plating, the cells were subjected to specific treatment regimens with the addition of 50 µl of treatment media to achieve the prescribed treatment concentrations. DMSO, in equal amounts to the treatment conditions, was added to the media in the control condition. Once the treatment was complete, 20 µl of the AQueous One Solution was added to each well, for a final volume of 120 µl. Colorimetric analysis using a 96-well plate reader (Vmax Kinetic Microplate Reader, Molecular Devices, Sunnyvale, CA) was performed between 1 and 4 hours (wavelength of 490 nm) after the addition of AQueous One Solution, depending on cell type and cell density. Cell viability assays were performed in triplicate. Synergy analysis was performed with the CalcuSyn software program (Biosoft, Ferguson, MO - version 2 - 2005) using non-constant ratio analysis and statistical method described by Chou and Talalay to calculate the combination index (CI) values [14]. The resulting CI is a quantitative measurement of the degree of interaction between difference drugs. A CI value of 1 signifies an additive effect, a CI more than 1 denotes antagonism and a CI of less than 1 indicates synergy.
Clonogenic assays
Clonogenic survival was defined as the ability of the cells to maintain their clonogenic capacity and to form colonies. J82 and T24 cells were selected for clonogenic assessment, representing highly and moderately docetaxel-resistant cell lines. Briefly, 5000 cells were seeded into 6-well dishes in 2 ml of medium. Twenty-four hours later, medium, docetaxel, pazopanib, or the combination of pazopanib plus docetaxel were each added to the cells, which were then maintained for 7–10 days in a CO2 incubator. The cells were fixed with 12.5% acetic acid in 30% methanol and then stained with Brilliant Blue R. Each experiment was performed in triplicate. Statistical analysis was prepared with the SPSS software program (13.0 for Windows, 2004 - Chicago, IL). The analysis of variance (ANOVA) was used to compare colony numbers and determine the statistical significance of these differences.
Protein characterization
For western blot assessment, the cells were plated in culture dishes. Twenty-four hours after seeding, the cells were treated with media alone, pazopanib 12.5µM, docetaxel 12.5nM, and pazopanib 12.5µM + docetaxel 12.5nM (concurrent) for 24 hours. After the treatment, the cells were harvested by scraping and washed with PBS. Cells were collected by centrifuge at 1100 rpm and pellets were resuspended in lysis solution (10 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.2 mM Sodium Orthovanadate, 0.5% NP-40, 0.3 mM PMSF, 10 ug/ml Aprotinin). Protein estimation was performed with Pierce protein dye, using a DU 800 Beckman Coulter spectrophotometer (Fullerton, CA). Fifty µg of protein was electrophoresed using NuPAGE 4–12% Bis-Tris Gel (Invitorgen, Carlsbad, CA). The protein was transferred to a nitrocellulose membrane (Invitorgen, Carlsbad, CA) using a wet method at 100 volts for 1 hour. The membrane was then blocked with 5% (w/v) milk and placed in a rotator for 1 hour at room temperature. The primary antibody was added in milk (2.5% w/v) and allowed to incubate overnight at 4° C after which it was washed with PBS/0.05% TWEEN-20 three times (15 minutes each) before the appropriate horseradish peroxidase-linked secondary antibody was added and incubated for an additional hour at room temperature. The membrane was again washed 3 × 15 minutes, before the addition of Pierce SuperSignal chemiluminescent substrate (Rockford, IL) and then immediately imaged on Chemi Doc (Bio-Rad, Hercules, CA).
Results
The effects of single-agent pazopanib or docetaxel on bladder cancer cell lines
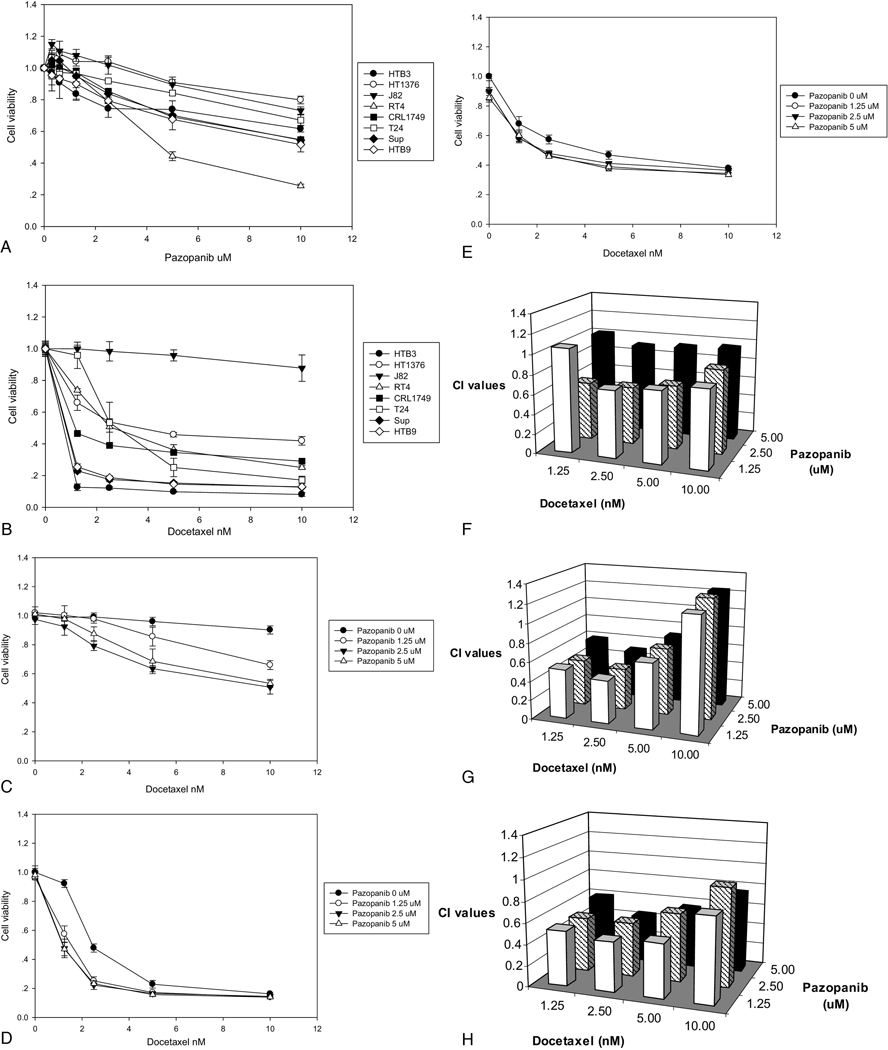
All the bladder cancer cells lines were exposed to pazopanib (0.3 µM, 0.6 µM, 1.25 µM, 2.5 µM, 5 µM, 10 µM; Figure 1A) or docetaxel (1.25 nM, 2.5 nM, 5 nM, 10 nM; Figure 1B), with cell viability determined by tetrazolium-based assay. The IC50 for pazopanib was more than 10 µM in all the cell lines except RT4. The IC50 values of J82, T24, HT1376, RT4, CRL1749, HTB9, Sup and HTB3 were 24.57µM, 52.45µM, 28.21µM, 5.14µM, 22.69 µM, 11.84 µM, 53.32 µM and 14.16 µM, respectively. Treatment with docetaxel was also performed in the same eight cell lines at the same passage number. The IC50 values of J82, T24, HT1376, RT4, CRL1749, HTB9, Sup and HTB3 were 68.67 nM, 4.18 nM, 3.78 nM, 3.05 nM, 0.79 nM, 0.07 nM, 0.02 nM, and < 0.01 nM respectively, with notable resistance observed in the J82 cells. We selected one very resistant (J82) and two moderately resistant cell lines (T24 and HT1376) for further investigation.
Figure 1.
The treatment effect of pazopanib and docetaxel on bladder cancer cell viability was assessed. Human bladder cancer cells HTB3, HT1376, J82, RT4, CRL1749, T24, SUP and HTB9 were seeded in triplicate with treatment started 24 hours after seeding and continued for 96 hours. Cell viability was assessed with a tetrazolium-based assay. The cells were treated with pazopanib (A) and docetaxel (B) with the concentrations indicated. Bars indicate the standard deviation. All cell lines studied, except RT4, had an inhibitor concentration 50 (IC50) of more than of 10 µM of pazopanib (A). Treatment with docetaxel demonstrated better single-agent activity compared with pazopanib, although J82 cells displayed strong resistance to docetaxel (B); The effect of combination treatment with pazopanib and docetaxel was also evaluated (C–E). Twenty-four hours after plating, the cells were treated by pazopanib and docetaxel concurrently for 96 hours. The cells were J82 (C), T24 (D) and HT1376 (E), with bars indicating the standard deviation. The combination index (CI) levels for concurrent treatment with pazopanib and docetaxel were calculated for J82 (F), T24 (G) and HT1376 (H) cells. CI values of less than 1 are consistent with synergy.
Pazopanib shows synergy when combined with docetaxel
The synergistic effects of pazopanib in combination with docetaxel were examined in J82, T24 and HT1376 cells. Pazopanib (0, 1.25 µM, 2.5 µM, and 5 µM) was tested concurrently with docetaxel (0, 1.25 nM, 2.5 nM, 5 nM, and 10 nM) for 96 hours. Among the three cell lines, synergy was seen with the combination of docetaxel with pazopanib, even at low concentrations of pazopanib (Figures 1C~E). In the T24 and HT1376 cells, it is noted that the vast majority of pazopanib’s benefit occurs at 1.25 µM, without clear additional activity at higher concentrations. The calculated CI values (Figures 1F~H) and IC50 (Table 1) quantified this pattern of synergy.
Table 1.
Inhibitory concentration 50% (IC50) for docetaxel
| docetaxel nM | ||||
|---|---|---|---|---|
| pazopanib 0 µM |
pazopanib 1.25 µM |
pazopanib 2.5 µM |
pazopanib 5 µM |
|
| J82 | 68.89 | 10.61 | 8.98 | 9.15 |
| HT1376 | 4.21 | 2.16 | 2.36 | 2.28 |
| T24 | 3.33 | 1.22 | 0.77 | 0.79 |
Suppression of colony formation
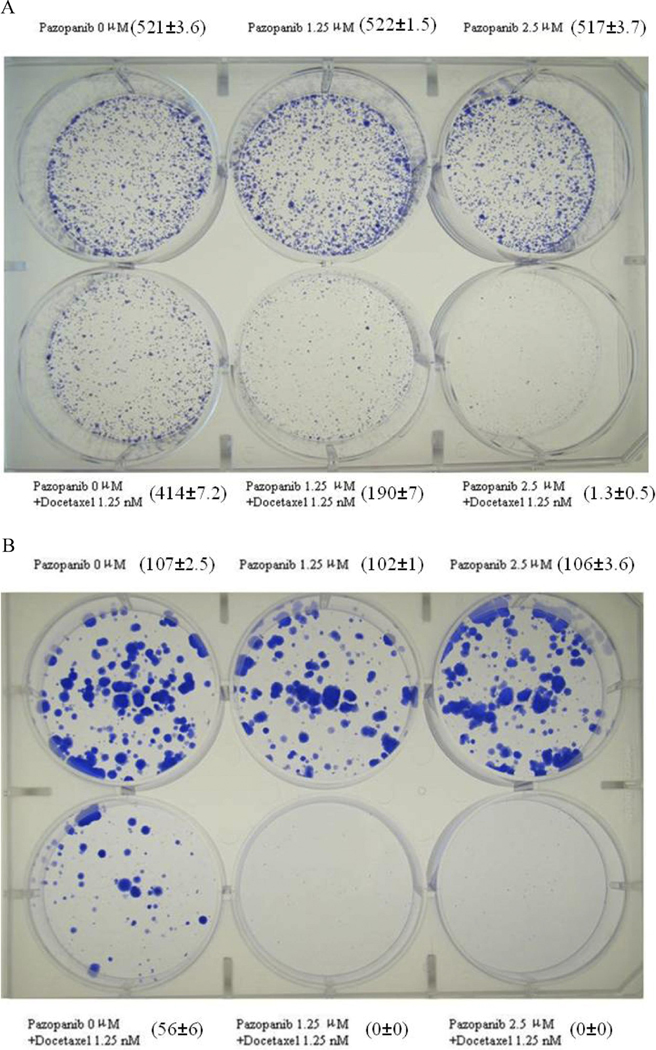
J82 and T24 cells were selected for additional clonogenic testing due to their high and moderate resistance (respectively) to single-agent docetaxel chemotherapy. The combination of pazopanib and docetaxel demonstrated improved suppression of clonogenic formation, compared to docetaxel alone (Figures 2A ~B). The experiments were repeated in triplicate and the colonies manually counted (numbers in brackets Figures 2A and 2B). There was no significant difference in the colonies numbers comparing treatment by medium alone to single-agent pazopanib at this concentration (Fig. 2A: p=0.212; Fig. 2B: p=0.083). However, a significant difference was shown with docetaxel treatment alone or the combination with pazopanib (Fig. 2A: p<0.001; Fig. 2B: p<0.001).
Figure 2.
The effect of pazopanib and docetaxel on colony formation in J82 (A) and T24 (B) cells was assessed. The treatment conditions are noted for each well. After 7–10 days of treatment, the cells were stained with Brilliant Blue R. The numbers adjacent to the corresponding wells indicate the mean number ± SD of the colonies per well in each treatment condition.
AKT and FAK phosphorylation and protein assessment
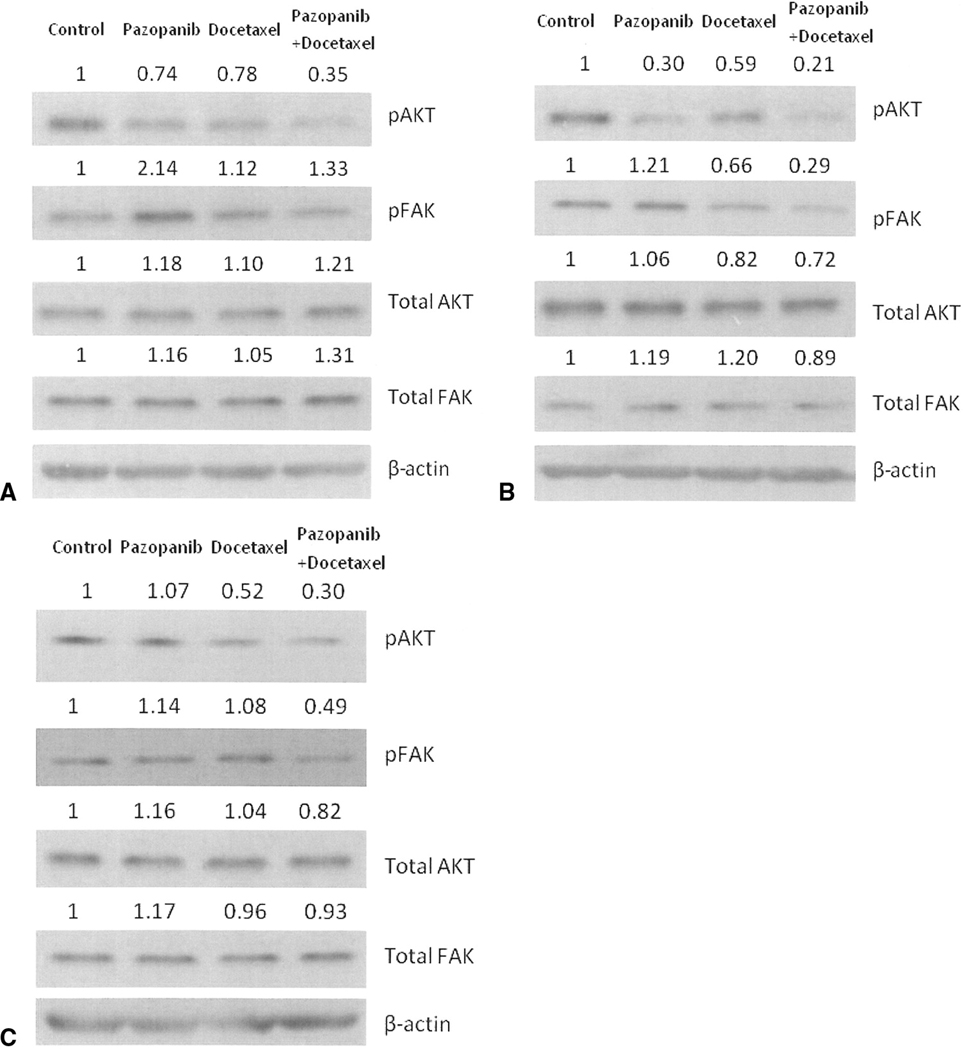
We further examined protein expression of phospho-AKT, phospho-FAK, total AKT and total FAK in cell lines of J82, T24 and HT1376. In the J82 and T24 lines, single-agent pazopanib decreased phospho-AKT expression, and the combination decreased the expression even further. In the HT1376 cells, phospho-AKT expression was not decreased with single-agent pazopanib, but was decreased by the combination of pazopanib and docetaxel compared to single-agent docetaxel. The combination treatment did decrease phospho-FAK expression in the T24 and HT1376 cell lines, but not in J82 cells, suggesting that this action is not independently necessary for the observed activity (Figures 3A~C). Total AKT and FAK did not show any great changes.
Figure 3.
Expressions of phospho-AKT, phospho-FAK, total AKT, total FAK were determined by Western blot analysis. The cells were J82 (A), T24 (B) and HT1376 (C) respectively. The relative expression ratios of the protein of interest and β-actin are indicated in the figure.
Discussion
These studies establish a pre-clinical rationale for the use of pazopanib in combination with cytotoxic chemotherapy for the treatment of bladder cancer. While pazopanib has modest single-agent activity in these in vitro experiments, synergistic activity was observed when pazopanib was combined with docetaxel. Clonogenic colony formation was also notably decreased with this combination.
Angiogenesis plays a critical role in the growth and metastatic spread of malignant disease [15]. VEGF is a key angiogenic factor with its effects mediated by VEGFR. Inhibition of VEGFR not only inhibits angiogenesis, but also tumor cell growth [16]. Podar et al [16] have reported that pazopanib inhibited in vitro myeloma cell growth, survival, and migration, with inhibition of VEGF-induced up-regulation of adhesion molecules on both endothelial and tumor cells. Pazopanib was recently approved for use in advanced RCC [17]. In phase III testing of patients with advanced RCC, pazopanib demonstrated a significant improvement in the median progression-free survival (9.2 versus 4.2 months), when compared to placebo, with an objective response rate of 30%. There are ongoing clinical trials examining its use as a single-agent in advanced urothelial cancer (ClinicalTrials.gov identifier: NCT01031875 and NCT00471536).
The mechanisms of chemotherapy insensitivity in advanced urothelial cancer are complex and not fully understood. Xia et al [10] utilized VEGF antisense oligodeoxynucleotide to inhibit activity of VEGF to investigate its role in chemotherapy sensitization. This work showed that VEGF inhibition augmented the effect of docetaxel in a murine xenograft model of bladder cancer with a significant inhibition of the proliferative index and microvascular density, with induction of apoptosis. In contrast to this antisense approach, pazopanib is in current clinical use with ongoing trials in urothelial cancers. Kamat et al [18] studied a combination therapy of docetaxel and AEE788, a dual epidermal growth factor receptor (EGFR) and VEGFR inhibitor. This combination was effective against ovarian cancer cells which were resistant to docetaxel. In the present study, pazopanib plus docetaxel showed synergy in docetaxel-resistant cells. The effect of these combinations was confirmed with clonogenic assays using J82 and T24 cells. Additionally, it must be emphasized that these experiments investigated the direct effect of pazopanib in combination with cytotoxicity chemotherapy in bladder cancer cells; however, the anti-angiogenic impact of pazopanib is not realized in the in vitro setting and further efficacy may be demonstrated in animal modeling where in vivo angiogenesis would also be impacted.
Signaling molecules implicated in the cellular actions of VEGF include phosphatidylinositol 3-kinase (PI3K), AKT, and endothelial nitric oxide synthase [19,20]. AKT regulates both growth and survival mechanisms by phosphorylation of a large number of substrates in cancer cells [21]. A great deal of study has been focused on blockade of the phosphoinositide 3-kinase/AKT survival signaling pathway in the quest for improved cancer therapeutics. Manipulation of AKT activity has been shown to modulate the response of tumor cells to chemotherapy [22]. Banerjee et al [23] confirmed docetaxel can induce growth inhibition and decrease the level of phospho-AKT in prostate cancer cells. Docetaxel can also decrease AKT phosphorylation in human umbilical vein endothelial cells [18]. Pazopanib can block VEGF-triggered activation of phospho-AKT[16]. The present study found that docetaxel decreased the expression of phospho-AKT and this effect was greatly potentiated with the addition of pazopanib in the two docetaxel-resistant cell lines. When combined with pazopanib, docetaxel further decreases phospho-AKT expression in T24 cells. Phospho-AKT is important for cell survival and these results support the importance of phospho-AKT inhibition in the anti-cancer efficacy of the combination of pazopanib and docetaxel in bladder cancer cells.
VEGF has also been shown to have multiple effects on the signaling components of the focal cell adhesion, such as FAK [24], which is expressed in several advanced cancer types [25]. FAK plays a key role in the dynamic reorganization of the cytoskeletal network and cell migration, with several pathways downstream of FAK able to contribute to cell survival [26]. The combination of pazopanib and docetaxel had a variable effect on phospho-FAK with notable inhibition noted in two cell lines. FAK’s role, if any, in the synergy of pazopanib and docetaxel in bladder cancer cells is not clear.
Conclusions
Despite multiple potential targets for biological agents, there is no “new generation” therapeutics currently in clinical use for the treatment of bladder cancer. The present study demonstrates synergistic efficacy of pazopanib with docetaxel in docetaxel-resistant bladder cancer cells. Pazopanib is a clinically relevant and accessible agent, with ongoing single-agent pazopanib human trials underway in advanced urothelial cancer patients. This work supports future evaluation of pazopanib with docetaxel for the treatment of bladder cancer with the potential of both improved efficacy and toxicity.
Acknowledgement
This work was supported in part by a Paul Calabresi K12 clinical scholar grant (TWF) awarded to the University of Colorado Denver (K12CA086913). The pazopanib used in these studies was supplied by GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602460–4602468. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, Yagoda A, Scher HI, Watson RC, Ahmed T, Weiselberg LR, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol. 1985;133:403–407. doi: 10.1016/s0022-5347(17)48996-8. [DOI] [PubMed] [Google Scholar]
- 4.Hussain M, Vaishampayan U, Du W, Redman B, Smith DC. Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol. 2001;19:2527–2533. doi: 10.1200/JCO.2001.19.9.2527. [DOI] [PubMed] [Google Scholar]
- 5.McCaffrey JA, Hilton S, Mazumdar M, Sadan S, Kelly WK, Scher HI, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15:1853–1857. doi: 10.1200/JCO.1997.15.5.1853. [DOI] [PubMed] [Google Scholar]
- 6.Gitlitz BJ, Baker C, Chapman Y, Allen HJ, Bosserman LD, Patel R, et al. A phase II study of gemcitabine and docetaxel therapy in patients with advanced urothelial carcinoma. Cancer. 2003;98:1863–1869. doi: 10.1002/cncr.11726. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- 8.Wu W, Shu X, Hovsepyan H, Mosteller RD, Broek D. VEGF receptor expression and signaling in human bladder tumors. Oncogene. 2003;22:3361–3370. doi: 10.1038/sj.onc.1206285. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann E, Eltze E, Bierer S, Köpke T, Görge T, Neumann J, Hertle L, et al. VEGF-C, VEGF-D and Flt-4 in transitional bladder cancer: relationships to clinicopathological parameters and long-term survival. Anticancer Res. 2007;27:3127–3133. [PubMed] [Google Scholar]
- 10.Xia G, Kumar SR, Hawes D, Cai J, Hassanieh L, Groshen S, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175:1245–1252. doi: 10.1016/S0022-5347(05)00736-6. [DOI] [PubMed] [Google Scholar]
- 11.Sonpavde G, Hutson TE. Pazopanib: a novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–119. doi: 10.1007/s11912-007-0007-2. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Dowlati A, Savage S, Fernando N, Lasalvia S, Whitehead B, et al. Safety, tolerability, and pharmacokinetics of oral administration of GW780634 in pts with solid tumors. J Clin Oncol. 2005;23 Suppl 16S:3012. [Google Scholar]
- 14.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 16.Podar K, Tonon G, Sattler M, Tai YT, Legouill S, Yasui H, et al. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc Natl Acad Sci U S A. 2006;103:19478–19483. doi: 10.1073/pnas.0609329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson TE, Figlin RA. Evolving role of novel targeted agents in renal cell carcinoma. Oncology (Williston Park) 2007;21:1175–1180. [PubMed] [Google Scholar]
- 18.Kamat AA, Kim TJ, Landen CN, Jr, Lu C, Han LY, Lin YG, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281–288. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 19.Murtagh J, Lu H, Schwartz EL. Taxotere-induced inhibition of human endothelial cell migration is a result of heat shock protein 90 degradation. Cancer Res. 2006;66:8192–8199. doi: 10.1158/0008-5472.CAN-06-0748. [DOI] [PubMed] [Google Scholar]
- 20.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 21.Toker A, Yoeli-Lerner M. AKT signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- 22.Brognard J, Clark AS, Ni Y, Dennis PA. AKT/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 23.Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, Cher M, Sarkar FH. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007;67:3818–3826. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 24.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- 25.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 26.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]