Abstract
Spontaneous intrusive recollections (SIRs) are known to follow emotional events in clinical and nonclinical populations. Previous work in our lab has found that women report more SIRs than men after exposure to emotional films, and that this effect is driven entirely by women in the luteal phase of the menstrual cycle. To replicate and extend this finding, participants viewed emotional films, provided saliva samples for sex hormone concentration analysis, and estimated SIR frequency following film viewing. Women in the luteal phase reported significantly more SIRs than did women in the follicular phase, and SIR frequency significantly correlated with salivary progesterone levels. The results are consistent with an emerging pattern in the literature suggesting that menstrual cycle position of female participants can potently influence findings in numerous cognitive domains. The potential implications of these results for disorders characterized by intrusions, such as post-traumatic stress disorder, are also discussed.
Keywords: intrusions, menstrual cycle, progesterone, estrogen, emotion
1. Introduction
Emotional events tend to be remembered better than are relatively neutral events (Christianson, 1992). This is true in the real world as well as in laboratory settings when the stimuli used are words, pictures, slideshows, or films (Rubin & Friendly, 1986; Heuer & Reisberg, 1990; Bradley, Greenwald, Petry & Lang, 1992; Cahill & McGaugh, 1995; Cahill et al., 1996; Kensinger & Corkin, 2003). Additionally, intrusive memories are known to occur in the aftermath of emotional events. Although intrusive memories are particularly common in patients with post-traumatic stress disorder and depression, non-clinical populations often report intrusions after traumatic or emotional events as well (Brown & Kulik, 1977; Wilkinson, 1983; Nolen-Hoeksema & Morrow, 1991; Brewin, Christodoulides & Hutchinson, 1996; Reynolds & Brewin, 1998; Reynolds & Brewin, 1999; Hall & Berntsen, 2008). A previous investigation in our lab (Ferree & Cahill, 2009) sought to determine whether the enhancement of memory for emotional stimuli is due in part to an increased tendency for people to experience post-event spontaneous intrusive recollections (SIRs) following exposure to emotional events or stimuli. SIRs would constitute unintentional covert rehearsal, which would in turn strengthen memory for those stimuli. In our previous study we found that emotional stimuli were associated with more SIRs than were neutral stimuli, and that there was a quantitative positive relationship between SIR frequency and subsequent memory strength for emotional stimuli (Ferree & Cahill, 2009). Our results were consistent with those of Hall and Berntsen (2008), who found that emotional arousal during the encoding of aversive pictures was associated with higher levels of both voluntary and involuntary (intrusions) recall.
Disorders characterized by intrusive thoughts and memories, such as depression and post-traumatic stress disorder (PTSD), are significantly more prevalent in women than men (Breslau, Davis, Andreski, Peterson & Schultz, 1997; Kendler, Thornton & Prescott, 2001). As such, we were not surprised to observe a sex difference in SIR frequency, such that women reported significantly more intrusions than did men (Ferree & Cahill, 2009). One key difference between the sexes is fluctuating levels of ovarian hormones throughout the menstrual cycle in female participants, and we examined the possibility that a woman’s reported frequency of SIRs might be affected by her circulating levels of estradiol and progesterone.
A good deal of evidence suggests that menstrual cycle position can influence many aspects of cognition including spatial and verbal performance (Halpern & Tan, 2001; Rosenberg & Park, 2002), as well as brain activity patterns during cognitive task performance (Maki & Resnick, 2001) and the neural circuitry underlying the response to stress and arousal (Goldstein et al., 2005). Menstrual cycle position is also thought to influence the stress hormone response, with higher levels of salivary cortisol in response to psychosocial stress during the luteal phase compared to the follicular phase (Kirschbaum, Kudeilka, Gaab, Schommer & Hellhammer, 1999). Some evidence suggests that whether a positive or negative correlation is observed between stress hormone levels and memory depends critically upon menstrual cycle position (Andreano, Arjomandi & Cahill, 2008).
Phase-dependent differences in stress hormone response may have clinical significance as well, as PTSD has long been associated with abnormalities in various aspects of the stress hormone response (Yehuda, 2002). Given the evidence relating stress hormones and PTSD, some authors have proposed that menstrual cycle influences on stress hormone reactivity may have an impact on PTSD symptoms (Saxe & Wolfe, 1999; Rasmusson & Friedman, 2002). As we regard the SIRs that we observe in the laboratory as a form of perseverative cognition that may be analogous to the intrusive symptoms that are a key symptom of PTSD, we hypothesized that we might observe menstrual phase-dependent differences in SIR frequency. In fact, in our previous study we found that women in the luteal phase of the cycle reported significantly more SIRs than did men or women in the follicular phase of the cycle (the latter two groups did not differ), suggesting that the sex difference we observed was driven entirely by the high level of SIR frequency reported by women in the luteal phase (Ferree & Cahill, 2009). However, the classification of female participants into follicular and luteal phases in the previous study was based on women’s self-reported position within the menstrual cycle, which may not be completely accurate (Maki & Resnick, 2001; Phillips & Sherwin, 1992b). Because we did not analyze levels of estradiol and progesterone in these participants, we could not verify the accuracy of their menstrual reports, nor could we begin to explore whether differences in estradiol, progesterone, or both, were responsible for the heightened SIR frequency in the luteal phase.
The current study was designed to further explore the menstrual cycle effect found in our previous study (Ferree & Cahill, 2009) and to extend these findings by incorporating analyses of salivary estradiol and progesterone levels. Salivary analysis offers the ability to verify a woman’s self-report of menstrual cycle position as well as to directly examine the potentially distinct contributions of estradiol and progesterone to the enhanced intrusion frequency in the luteal phase. We anticipated that, as in our previous study, women in the luteal phase would report more intrusions than would women in the follicular phase.
In the early follicular phase (days 1 through 5 from the start of menstruation), levels of estradiol and progesterone are very low. In the late follicular phase (days 6 through 13), estradiol levels rise until they peak the day before ovulation (which occurs on day 14) while progesterone levels remain low. Levels of progesterone are high throughout most of the luteal phase (peaking around day 22 and beginning to drop between days 24 and 28), while estradiol levels reach a second gradual peak that is significantly lower than the preovulatory peak but significantly higher than early follicular levels (Weis & Hausmann, 2009). Because we collapsed across the low estradiol early follicular and the high estradiol late follicular sub-phases into one follicular group, we expected that estradiol levels would not differ significantly between the follicular and luteal phases and that any observed menstrual phase effects would be due to the large expected differences in progesterone levels between the two groups. Consistent with the hypothesis that our observed effects are mediated primarily by progesterone, luteal levels of progesterone have been associated with increased amygdala activity, which is known to be involved in emotional tasks such as the one used in this study (van Wingen et al., 2007a; Andreano & Cahill, 2010).
2. Material and Methods
2.1. Participants
Fifty-two undergraduates at the University of California, Irvine between the ages of 18 and 23 participated in this study, which was approved by the university’s Institutional Review Board. All women were naturally cycling (not using hormonal birth control). Women reporting consistently irregular cycles less than 24 or more than 32 days in length were not included. Menstrual cycle position was determined by self report and salivary estradiol and progesterone levels were used to verify a woman’s position within the reported phase. Three participants (2 luteal, 1 follicular) were excluded based on estradiol or progesterone levels outside the range expected for their self-reported menstrual cycle position. Six participants were excluded due to SIR measures more than 2 standard deviations from the mean (3 follicular, 3 luteal). Additionally, three participants chose to withdraw from the experiment before its completion. The final analyses involved data from 40 women, with 19 in the follicular (days 1–13 since the start of menstruation) and 21 in the luteal phase (days 15–28) of the cycle.
2.2. Procedures
Each participant was shown a series of six emotional film clips depicting violence against humans or animals previously shown to reliably elicit emotional reactions (Ferree & Cahill, 2009). After each film clip, participants were asked to subjectively rate their emotional arousal in response to the film on a 1 to 10 scale, with 1 representing “completely unemotional” and 10 representing “extremely emotional.” Participants were told that they could discontinue participation at any time with no penalty if they felt uncomfortable while watching the films. In order to keep subjects unaware that their memory would be tested during the second session, participants were connected to non-functional galvanic skin response electrodes and a heart rate monitor and told that the experiment concerned physiological responses to the films. Three saliva samples were collected during the first experimental session at the following time points: prior to watching the films, as well as immediately and 10 minutes after the films ended.
Forty-eight hours later, participants returned for a second session during which they received a surprise memory test. Subjects were first asked to freely recall as many films as possible while not providing any specific details, and to order the films according to their subjective assessment as to which film they remembered best, second best, and so on for all films they recalled. Subjects were then asked to indicate in which order the recalled films were shown. Next, subjects were asked how many times each of the recalled films or scenes from the films had “spontaneously popped into their minds” since viewing them. Finally, subjects were asked to freely recall as many details as they could remember about each of the films with no time limit. At the end of the second experimental session, participants were debriefed about the study and compensated for their time with course credit or cash.
2.3. Scoring
Our definition of SIRs is in accordance with the intrusive memory literature, which describes intrusions as non-deliberate or involuntary spontaneously occurring recollections of events or stimuli (Holmes, Brewin & Hennessy, 2004; Holmes & Bourne, 2008). To reduce the likelihood that subjects would use their subjective rankings of memory strength when estimating SIR frequencies, subjects were asked to estimate SIR frequency in a pseudorandom order. For example, subjects were asked to first estimate SIR frequency for the third best remembered film, followed by the fifth best remembered film, the second best remembered film, the fourth best remembered film, the best remembered film, and then the sixth best remembered film. Six pseudorandom sequences were used, with equal numbers of participants assigned to each of the sequences. Because not all participants recalled all six films during the test, in order to fairly compare SIR frequency between subjects we used the measure mean SIRs per film to assess SIR frequency rather than total number of reported SIRs per subject.
In analyses of detail memory, only correctly recalled details were analyzed. Scoring of the freely recalled details was determined by a single judge (NKF). In the vast majority of cases, recalled items were readily identifiable as accurately pertaining to a specific film or clearly erroneous. In the rare cases in which accuracy of a response was questionable, a second judge unconnected to the study was consulted and a consensus between the two judges was established. Although the same author (NKF) scored the memory tests and entered the data, the phase information for each subject was not present on the memory tests and was therefore unlikely to influence the scoring of the memory test.
2.4. Salivary Hormones
All samples were collected with the “passive drool” method, in which participants collected saliva in polystyrene tubes, to avoid the contamination of estradiol levels caused by cotton salivette sampling (Shirtcliff, Granger, Schwartz & Curran, 2001). Participants were asked to take a drink of water upon arrival in the lab and the first saliva sample was collected 10 minutes afterward. All saliva samples were frozen after collection.
Saliva samples were thawed and centrifuged at 2080g for 15 minutes to remove particulate matter and separate mucous material from the samples. Cleared saliva was decanted into a second tube and centrifuged for 10 additional minutes before being decanted once more into a third tube.
Salivary estradiol and progesterone levels were assessed using Salimetrics enzyme-linked immunosorbent assay kits (State College, PA) and measured optically with a ThermoLabSystems Multiskan Ascent spectrophotometer (Waltham, MA). Salimetrics reports correlations between serum and salivary levels of r = 0.80 (p < .001) for estradiol and r = 0.87 (p < .001) for progesterone. The minimal concentration that can be distinguished from 0 is 5 pg/mL for progesterone, and 0.1 pg/mL for estradiol (Salimetrics package inserts). Multiple assays were done to determine salivary hormone levels, and within each assay there were roughly equal numbers of samples from subjects in the self-reported follicular and luteal phases. The manufacturers of the hormone assay kits suggest using an average of the levels observed in multiple samples due to the periodic secretion patterns of steroid hormones (Salimetrics kit inserts for Estradiol and Progesterone Enzyme Immunoassay Kits), and in accordance with this suggestion we measured hormone levels in all three samples provided by each participant and calculated average estradiol and progesterone levels to be used in all analyses.
2.5. Statistical Analysis
Arousal ratings, free recall memory, SIR measures, and hormone levels were compared between follicular and luteal groups using unpaired t-tests. The relationship between the number of reported SIRs and subjective memory strength was compared between follicular and luteal groups by a two way ANOVA (Phase and Subjective Rank) and Fisher’s PLSD post-hoc tests. The relationships between hormone levels and SIRs were characterized using regression analyses. Correlation coefficients (r) were computed for linear regression lines, and p values for significance of fit were computed using correlation z tests.
3. Results
3.1. Salivary Sex Hormone Levels
The mean levels of progesterone were reliably higher in the luteal phase than in the follicular phase (Table 1), unpaired t(38) = 3.41, p < .001. No difference in estradiol was predicted and the numerical difference between phases did not reach significance (Table 1), unpaired t(38) = −1.87, p > .05.
Table 1.
Mean salivary estradiol and progesterone levels, by phase.
| Follicular | Luteal | |
|---|---|---|
|
Estradiol (pg/mL) ± SEM |
5.01 ± 0.68 | 6.90 ± 0.74 |
|
Progesterone (pg/mL) ± SEM |
71.02 ± 11.41 | 143.83 ± 17.46*** |
Significance level determined by unpaired t test,
p = .001
Follicular, n = 19; Luteal, n = 21
3.2. Arousal Ratings
Mean arousal ratings did not differ between women in the two menstrual phases (Table 2), unpaired t(38) = −1.76, p > .05.
Table 2.
Mean Arousal, SIRs, and Memory, by phase.
| Follicular | Luteal | |
|---|---|---|
|
Arousal Rating ± SEM |
7.40 ± 0.35 | 8.09 ± 0.20 |
|
SIRs/Film ± SEM |
1.29 ± 0.14 | 1.87 ± 0.24* |
|
# Films Recalled ± SEM |
5.42 ± 0.18 | 5.38 ± 0.13 |
|
Details/Film ± SEM |
6.63 ± 0.55 | 6.87 ± 0.45 |
Significance level determined by unpaired t test,
p < .05
Follicular, n = 19; Luteal, n = 21
3.3. Memory Measures
The mean number of films recalled did not differ between women in the two menstrual phases (Table 2), unpaired t(38) = 0.19, p > .80. No difference was observed between the two phases in the number of correctly recalled details per film (Table 2), unpaired t(38) = −0.33, p > .70.
3.4. Spontaneous Intrusive Recollections
Women in the luteal phase reported significantly more SIRs per film than did women in the follicular phase (Table 2), unpaired t(38) = −2.06, p < .05].
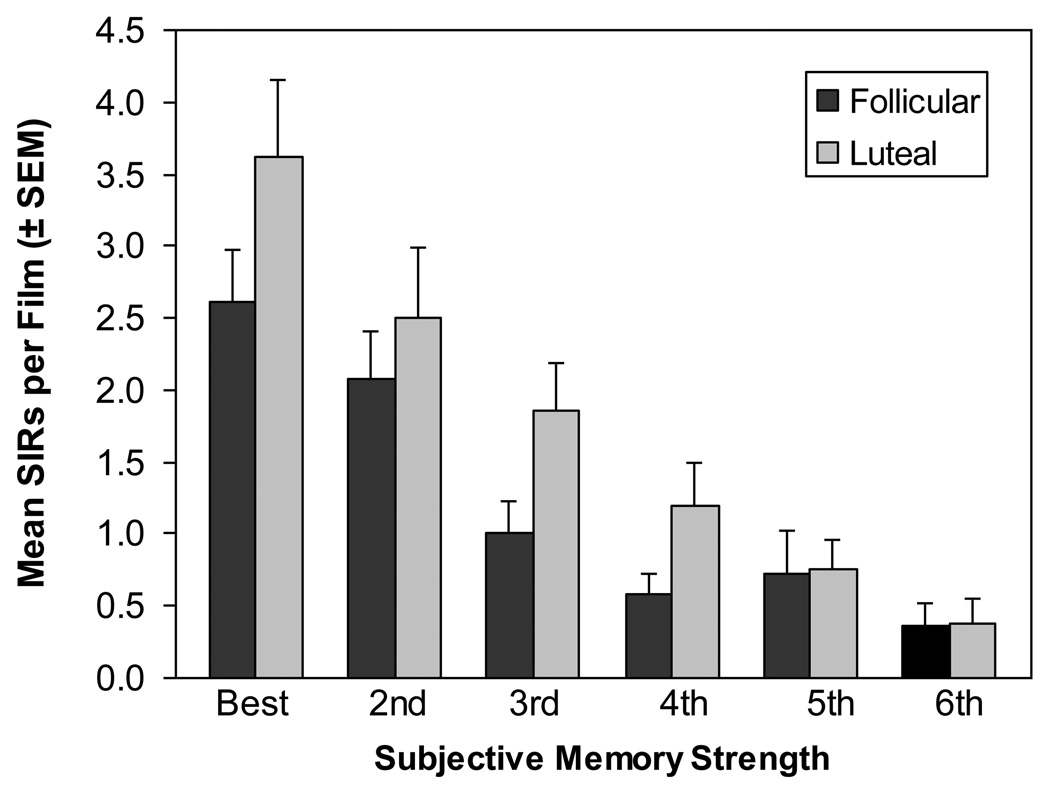
Participants in the luteal phase reported more SIRs than women in the follicular phase and more SIRs were reported for the films subjectively rated as better remembered (see Figure 1). A 2 (Phase) × 6 (Subjective Rank) ANOVA of the mean number of SIRs for the remembered films revealed main effects of Phase [F(1, 203) = 5.26, p < .05] and Subjective Rank [F(5, 203) = 16.48, p < .0001]. Fisher’s PLSD post-hoc tests revealed that the best remembered film was associated with more SIRs than was the second, third, fourth, fifth, or sixth best remembered films (all p < .01), that the second best remembered film was associated with more SIRs than was the third, fourth, fifth, or sixth best remembered films (all p < .01), and that the third best remembered film was associated with more SIRs than was the fifth or sixth best remembered films (all p < .05). The Phase × Subjective Rank interaction was not significant [F(5, 203) = 0.62, p > .65].
Figure 1.
Relationship between SIRs and subjective memory strength, by phase. Women in the luteal phase reported more SIRs than women in the follicular phase (p < .05), and more SIRs were associated with films subjectively rated as better remembered (p < .0001).
3.5. Relationships Between Hormone Levels, SIR Incidence, and Memory Measures
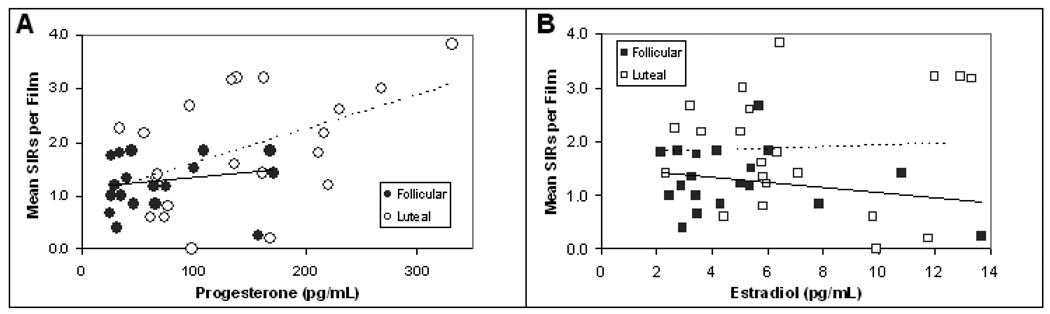
Regression analysis revealed a significant positive correlation between mean SIR frequency and salivary progesterone (r2 = 0.23, r = 0.48, p = .001). When considering women from the two phases separately, the relationship between SIRs and progesterone was significant in the luteal phase (Figure 2A; r2 = 0.22, r = 0.47, p < 0.05) but not in the follicular phase (Figure 2A; r2 = 0.03, r = 0.16, p > 0.5). No correlation was observed between mean SIR frequency and salivary estradiol (Figure 2B; r2 = 0.004, r = 0.07, p > .7). A stepwise multiple regression of models incorporating estradiol, progesterone, and arousal ratings indicated that the best predictor of SIRs was a model using only progesterone levels (R2= 0.23, RMSE = 0.82, AICc = 102.13). No relationships were observed between hormone levels and mean arousal ratings or the mean number of recalled details (all p > .60).
Figure 2.
(A) No correlation was observed between salivary progesterone levels and SIR frequency for women in the follicular phase (solid line; r = 0.16, p > 0.05), whereas a significant positive correlation was observed between salivary progesterone and SIR frequency for women in the luteal phase (dotted line; r = 0.47, p < 0.05). (B) No correlation was observed between salivary estradiol levels and SIR frequency for women in the follicular (solid line; r = −0.24, p > 0.05) or luteal phase (dotted line; r = 0.05, p > 0.05).
Additionally, significant positive relationships were observed between arousal ratings and SIR frequency (r2 = 0.06, r = 0.25, p < .001), arousal ratings and details (r2 = 0.02, r = 0.15, p < .05), and SIR frequency and details (r2 = 0.08, r = 0.28, p < .0001). A stepwise multiple regression of models incorporating SIRs, arousal ratings, estradiol, and progesterone revealed that the best predictor of correctly recalled details was a model using SIRs alone (R2= 0.08, RMSE = 2.22, AICc = 181.84).
4. Discussion
4.1. Implications and Discussion
The results of the current study support our hypothesis that a woman’s position within the menstrual cycle at the time of exposure to emotional films influences the subsequent frequency of spontaneous intrusive recollections for the films. Women in the luteal phase of the menstrual cycle at the time of film viewing reported significantly more intrusions than did women in the follicular phase, thus replicating the results of our previous study (Ferree & Cahill, 2009). Furthermore, hormone analyses revealed a significant positive correlation between salivary progesterone concentration and mean SIR frequency. In combination with the results of our previous study (Ferree & Cahill, 2009), these results suggest that menstrual phase does influence SIR frequency, and this effect appears to be mediated by the heightened levels of progesterone present during the luteal phase.
It seems unlikely that the reported SIR frequencies were simply a consequence of the demand characteristics of our study. First, participants were instructed to estimate SIR frequency only if SIRs occurred and each participant was assured that if she did not experience SIRs for any film she should indicate that she never had SIRs for that film by giving an estimate of zero. Second, because all participants were given the exact same set of instructions, one would expect the instructions to influence all participants equally and not preferentially influence women in one phase or the other. We have no particular reason to believe that women in the luteal phase should be more influenced by experimenter instructions than women in the follicular phase. In the analysis of SIR frequency by subjective memory strength, we attempted to minimize the likelihood that participants would use their own subjective rankings of strength when estimating SIR frequency. Participants were asked to estimate SIR frequency for the films in a pseudorandom order (e.g. third best, then best, then fourth best, then second best, then fifth best, and finally sixth best), such that it would have been very difficult to adjust their SIR estimates based on strength ranking. Additionally, there was a film viewing order reporting task between the subjective memory ranking and SIR estimation in order to further separate the tasks. Finally, participants gave SIR frequency estimates rather quickly and with apparently little effort, making it unlikely that they referred to previously reported memory strength rank order before responding. Because all participants were told that the experiment was designed to assess physiological responses to the films, they were unaware of the true nature of the study until debriefing at the end of the second session.
Our findings that women in the luteal phase report more SIRs than do women in the follicular phase but do not also remember more details may appear at first glance to contradict our previous results that SIR frequency correlates positively with details recalled. However, as discussed in our previous paper (Ferree & Cahill, 2009), we do not claim that SIR frequency is the only determinant of memory for details. As discussed in that paper, it seems likely that another factor, namely emotional arousal, leads to both increased SIRS and details, but that the additional rehearsal associated with SIRs contributes to the stronger memory observed under emotionally arousing conditions. In our previous paper we argued that emotional arousal and SIR frequency, when considered together, better predict the amount of recalled details than either factor considered alone, although the multiple regression analyses in the current study indicate that SIRs alone better predict detail memory than a model incorporating both SIRs and arousal ratings. It is likely that there are other factors that influence details that are not accounted for by our measures, and these may also help to explain why we did not observe heightened memory for details in the luteal compared to the follicular phase.
It is possible that the fact that women in the luteal phase have both higher levels of progesterone and report more SIRs than women in the follicular phase leads to the detection of an artificial correlation between progesterone and SIRs which in actuality only reflects a group difference. When the follicular and luteal groups are considered separately, progesterone levels significantly predict SIR frequency even when only subjects in the luteal phase are considered. This suggests that the correlation is not driven merely by the presence of two menstrual groups which, when considered together, creates an artificial correlation. Furthermore, the distinction between follicular and luteal phases may itself be thought of as an artificial bimodal division which is nonetheless helpful as a first pass to detect menstrual phase effects. Given that the hormone levels in the two phases overlap considerably, it seems to us that using continuous measures of estradiol and progesterone levels as they vary across the cycle is a more accurate representation of the influences of these hormones than the gross follicular versus luteal distinction. It should also be noted that, because progesterone levels do not fully account for the variance in intrusive memories, we cannot rule out the possibility that other factors not measured here may influence the reporting of SIR frequency besides hormone levels.
Our results appear to be a specific example of a more general phenomenon emerging from the literature, namely, the influence of sex hormones on cognition. It has been known for some time that menstrual cycle position can affect women’s performance on various cognitive tasks, especially those that tend to show large sex differences (Hampson, 1990; Halpern & Tan, 2001; Maki, Rich & Rosenbaum, 2002; Rosenberg & Park, 2002). Imaging studies also report differences, both between males and females, and between female participants in the different menstrual cycle phases, on patterns of brain activation during several cognitive tasks (Dietrich et al., 2001; Gizewski, Krause, Wanke, Forting & Senf, 2006). Comparisons between women in the early follicular, late follicular, and mid-luteal phases suggest that estradiol and progesterone both appear to play roles in these differential patterns of activation (Dietrich et al., 2001; Gizewski et al., 2006). Additionally, estradiol and progesterone levels have been found to correlate with performance on several cognitive tasks (Phillips & Sherwin, 1992b, Halpern & Tan, 2001; Maki, Rich & Rosenbaum, 2002). Studies of exogenous administration of estradiol reveal that such treatment can attenuate cognitive impairments observed in hysterectomized or gonadally suppressed women (Phillips & Sherwin, 1992a; Sherwin & Tulandi, 1996). Administration of either estradiol or progesterone to gonadally suppressed female subjects has been shown to restore normal patterns of brain activity during performance of some cognitive tasks (Berman et al., 1997).
Other imaging studies report menstrual phase influences on several brain circuits during performance of tasks with an emotional component. In an fMRI study comparing brain activity in late follicular and late luteal phases during performance of an emotional word task, patterns and levels of activity differed between the two phases (Protopopescu et al., 2005). Goldstein and colleagues found increased activation in a number of regions involved in the brain’s stress response in the early follicular compared to the late follicular phase when women were exposed to emotional pictures and argue that these results may reflect an ability of estrogen to attenuate the effects of arousal through cortical control of hypothalamic pituitary adrenal (HPA) axis circuitry in the latter portion of the follicular phase (Goldstein et al., 2005). A study by our lab designed to extend these results by comparing women in the midluteal phase to women in the early follicular phase found increased amygdala activation in the midluteal group (Andreano & Cahill, 2010). The finding that high levels of progesterone may be associated with heightened amygdala response to emotional tasks is consistent with the work of van Wingen and colleagues, who have observed increased amygdala activity and functional coupling of the amygdala with other brain regions after administration of progesterone resulting in levels similar to those observed in the luteal phase (van Wingen et al., 2007a). When women in the follicular phase were given higher doses of progesterone similar to levels seen during pregnancy, however, amygdala activity decreased (van Wingen et al., 2007b). These two results considered together suggest the possibility of an inverted-U dose-dependent relationship between progesterone administration and amygdala activity. van Wingen and colleagues attribute these effects of progesterone to its neuroactive metabolite allopregnanolone and suggests that allopregnanolone may influence mood, anxiety, and memory via its effects on amygdala activity, which may in turn affect processes in other brain regions (van Wingen et al., 2007a; van Wingen et al., 2007b).
It is also known that menstrual cycle position can affect the stress response. Kirschbaum and colleagues found that women in the luteal phase and men showed roughly equivalent cortisol responses to psychosocial stress which were greater than those observed in women in the follicular phase or those using oral contraceptives (1999). Women in the luteal phase show enhanced adrenocorticotropic hormone and cortisol responses to exercise stress relative to women in the follicular phase (Altemus, Roca, Galliven, Romanos & Deuster, 2001; Roca et al., 2003). These menstrual effects in stress reactivity occur in the absence of baseline HPA axis activity level differences between the phases in the studies mentioned above. The current findings are likely not to merely reflect a heightened stress reactivity in the luteal phase, however, because in a previous study using the same film stimuli (unpublished results), we found that the films did not cause a reliable cortisol response in women in either phase of the menstrual cycle.
The results of the current study then seem to add to the growing literature which suggests that menstrual cycle position may potently affect the results in numerous cognitive domains, and further indicate that ignoring the menstrual cycle position of female participants may lead to an oversimplification of the pattern of results that can be obtained by a finer-grained analysis. For example, when we combined women in both phases in our previous study (Ferree & Cahill, 2009), women reported more intrusions than men, but further examination showed that this effect was driven entirely by the heightened intrusion frequency observed in the luteal phase women. In other work in our lab (Andreano & Cahill, 2006), a comparison of men and women collapsed across the menstrual cycle suggested that there was a reliable enhancement of memory for a story after cold pressor stress in men but not women. However, further examination revealed that there was a significant positive correlation between cortisol and memory in women in the midluteal but not the early or late follicular phase (Andreano, Arjomandi & Cahill, 2008).
The current results also suggest that more work needs to be done to determine the influence of menstrual cycle position on intrusions, and potentially on the development of clinical disorders such as PTSD which are characterized by intrusive symptoms. If intrusive recollections can be thought of as a laboratory analog of the intrusive memories experienced after emotional events in real world settings, our results raise the possibility that a woman’s position within the menstrual cycle at the time of a trauma may influence her susceptibility to develop PTSD, or the severity of intrusive symptoms associated with PTSD. Although our subjects clearly did not develop PTSD after viewing our film stimuli, the films were sufficiently emotionally arousing to cause intrusions, and our results may therefore have some clinical significance. In fact, the trauma film paradigm has been used in many labs to study intrusive symptoms analogous to those observed in PTSD with the assumption that pathological intrusions such as those observed in PTSD can be thought of as an extreme on the continuum of intrusions occurring in everyday life (Holmes & Bourne, 2008). Based on their work showing that recurrent involuntary memories occur in everyday life following both positive and negative events, and that these recurrent memories appear to follow the basic principles of autobiographical memory and do not need to be explained by a “special mechanisms” view, Berntsen and Rubin (2008) encourage the extrapolation of results and theories from general autobiographical memory research to account for memory in disorders such as PTSD. However, it is important to be cautious about the potential clinical implications of our results because although the SIRs we observe in the lab are similar in that they involve visual or sensory elements, the intrusions experienced by patients with PTSD are often associated with extreme distress and an overwhelming feeling of reliving the events that is likely absent in our participants (Reynolds & Brewin, 1999). With these caveats in mind, the results of the current study, in combination with the results of our previous work (Ferree & Cahill, 2009), are the first to suggest that position within the menstrual cycle might influence SIR frequency following emotional stimuli.
While several authors have proposed that a woman’s position within the menstrual cycle at the time of a trauma may influence her likelihood of subsequently developing PTSD, no studies to date have directly investigated this possibility. In 1999, for example, Saxe and Wolfe suggested that, because noradrenergic and glucocorticoid responses—two systems implicated in PTSD—are affected by position in the menstrual cycle, women may be more vulnerable to developing PTSD if the precipitating trauma occurs during certain phases of the cycle (Saxe & Wolfe, 1999). Later, Rasmusson and Friedman suggested that studies of PTSD in women should take into account the influence of the menstrual cycle, given differences in the stress response across the cycle as discussed above (Rasmusson & Friedman, 2002). In a recent study of fear conditioning, Milad and colleagues found that women in the late follicular phase showed impaired extinction memory relative to men or women in the early follicular phase (Milad et al., 2006). It should be noted that, as they did not include participants in the luteal phase, it is impossible to determine the effects of high levels of progesterone on extinction memory. Because the authors noted that exposure-based therapy for PTSD relies on extinction processes and that failure to extinguish fear responses may influence the development of anxiety disorders, they suggest that these results may have clinical implications. The authors note that future studies should investigate the possibility that women in certain phases of the menstrual cycle at the time of a trauma may be more vulnerable to developing PTSD (Milad et al., 2006). Because women are more likely to develop PTSD than men after traumatic experiences (Breslau et al., 1997; Kendler, Thornton & Prescott, 2001), such an investigation would be interesting and possibly informative in understanding responses to trauma among women.
4.2. Limitations and Future Directions
Unlike other studies of intrusions, which have used rumination diaries (Holmes, Brewin & Hennessy, 2004), our participants were asked to give retrospective estimates of SIR frequency. We use this approach in our lab to avoid the potentially confounding effects of additional intentional rehearsal caused by recording intrusions in a diary, which could influence our memory measures. This was a stronger concern in our previous study (Ferree & Cahill, 2009), but as the current study is in part a replication of that study, we did not change our method of obtaining SIR frequency estimates. Rather than using the longer one week interval used in many of our previous studies of emotional memory, the retention interval used in the current study and the previous study was kept relatively short (48 hours) in order to increase accuracy of SIR estimation. Our participants did not appear to experience difficulty in estimating SIR frequency over the 48 hour retention interval. In several previous investigations of rumination and intrusion in both clinical and nonclinical populations, after-the-fact descriptions of the content of intrusive thought or memory have been successfully used (Brewin, Christodoulides & Hutchinson, 1996; Reynolds & Brewin, 1998; Steil & Ehlers, 2000; Berntsen & Rubin, 2008).
It should be noted that although we did not classify women into the follicular or luteal phase based strictly on estradiol or progesterone levels, we instead used the hormone levels to verify self-reported cycle position. Because hormone levels overlap between the two phases, it is difficult to classify women into phases based solely on hormone levels. In fact, several women were excluded because their hormone levels were significantly outside of the expected range for their self-reported phase. As expected, we did observe significantly higher progesterone levels in the luteal phase than in the follicular phase. Estradiol levels are low in the early follicular phase and high in the late follicular phase, but because we collapsed these two sub-phases into one overall follicular group, it was not unexpected that we would not observe differences in estradiol levels between the follicular phase and the luteal phase, which is itself characterized by moderate estradiol levels. Also, although progesterone levels in the follicular group were low, the observed levels were well above the lower limits of detectability of the assay.
Although the results of the present study may have clinical implications, this possibility will remain speculative until a systematic study is conducted to explore the potential influence of menstrual cycle position on the intrusive symptoms associated with PTSD. While depression and PTSD are associated with intrusive symptoms, it remains entirely speculative that the SIRs reported in the laboratory are analogous to the intrusions that characterize these disorders. It remains to be seen how the two phenomena are related, and as such we are currently attempting to extend the present basic science findings to the clinical conditions by studying trauma survivors. It should also be noted that our findings appear to be most closely related to intrusive symptoms, but PTSD is a complex condition which is also associated with significant hyperarousal and avoidance symptoms, so the relevance of our findings to PTSD as a whole is hard to predict. We strongly feel that, given the noted sex differences in PTSD development after a trauma, the examination of factors influencing a woman’s likelihood of developing the disorder is a rather important and yet still relatively unexplored field of study. The influence of hormonal fluctuations over the menstrual cycle on PTSD symptoms is therefore a potentially fruitful avenue of research and definitely worthy of further examination.
4.3. Conclusions
The current study’s findings replicate the findings of our previous work indicating that women in the luteal phase of the menstrual cycle experience more intrusions following exposure to emotional stimuli than do women in the follicular phase, and furthermore suggest that this effect is mediated by progesterone levels. Taken together, these results suggest that an examination of the effects of menstrual cycle position and hormone levels on intrusions, and possibly on clinical disorders characterized by intrusive symptoms such as PTSD, is an important goal for future research.
Acknowledgements
This study was supported by National Institute of Mental Health Grant RO1-57508 to L.C. We thank Dr. Joseph Andreano for his helpful consultation and input regarding the analysis and interpretation of this data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin response to stress in the midluteal phase of the menstrual cycle. The Journal of Clinical Endocrinology and Metabolism. 2001;86:2525–2530. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucorticoid release and memory consolidation in men and women. Psychological Science. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron emission tomography study in women. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen D, Rubin DC. The reappearance hypothesis revisited: recurrent involuntary memories after traumatic life events and in everyday life. Memory & Cognition. 2008;36:449–460. doi: 10.3758/mc.36.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Berntsen D, Rubin DC. The reappearance hypothesis revisited: recurrent involuntary memories after traumatic life events and in everyday life. Memory & Cognition. 2008;36:449–460. doi: 10.3758/mc.36.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Christodoulides J, Hutchinson G. Intrusive thoughts and memories in a nonclinical sample. Cognition & Emotion. 1996;10:107–112. [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;29:179–196. [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Consciousness and Cognition. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- Christianson S-A, editor. The Handbook of Emotion and Memory: Research and Theory. New Jersey: Erlbaum; 1992. [Google Scholar]
- Dietrich T, Krings T, Neulen J, Willmes K, Eberich S, Thron A, et al. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. Neuroimage. 2001;13:425–432. doi: 10.1006/nimg.2001.0703. [DOI] [PubMed] [Google Scholar]
- Ferree NK, Cahill L. Post-event spontaneous intrusive recollections and strength of memory for emotional events in men and women. Consciousness and Cognition. 2009;18:126–134. doi: 10.1016/j.concog.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizewski ER, Krause E, Wanke I, Forting M, Senf W. Gender-specific cerebral activation during cognitive tasks using functional MRI: Comparison of women in mid-luteal phase and men. Neuroradiology. 2006;48:14–20. doi: 10.1007/s00234-005-0004-9. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:9016–9039. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NM, Berntsen D. The effect of emotional stress on involuntary and voluntary conscious memories. Memory. 2008;16:48–57. doi: 10.1080/09658210701333271. [DOI] [PubMed] [Google Scholar]
- Halpern DF, Tan U. Stereotypes and steroids: using a psychobiosocial model to understand cognitive sex differences. Brain and Cognition. 2001;45:392–414. doi: 10.1006/brcg.2001.1287. [DOI] [PubMed] [Google Scholar]
- Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain and Cognition. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- Heuer F, Reisberg D. Vivid memories of emotional events: the accuracy of remembered minutiae. Memory & Cognition. 1990;18:496–506. doi: 10.3758/bf03198482. [DOI] [PubMed] [Google Scholar]
- Holmes EA, Bourne C. Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychologica. 2008;127:553–566. doi: 10.1016/j.actpsy.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Holmes EA, Brewin CR, Hennessy RG. Trauma films, information processing, and intrusive memory development. Journal of Experimental Psychology: General. 2004;133:3–22. doi: 10.1037/0096-3445.133.1.3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. American Journal of Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudeilka BM, Gaab J, Schommer N, Hellhammer D. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: a review of neuroimaging studies. Neuroimage. 2001;14:789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40:518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress disorder after a natural disaster: the 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory formation in surgically menopausal women. Psychoneuroendocrinology. 1992a;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Variations in memory function and steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992b;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Friedman MJ. Gender issues in the neurobiology of PTSD. In: Kimmerling R, Oimette P, Wolfe J, editors. Gender and PTSD. New York: Guilford Press; 2002. pp. 43–75. [Google Scholar]
- Reynolds M, Brewin CR. Intrusive cognitions, coping strategies, and emotional responses in depression, posttraumatic stress disorder and a non-clinical population. Behavior Research and Therapy. 1998;36:135–147. doi: 10.1016/s0005-7967(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Brewin CR. Intrusive memories in depression and posttraumatic stress disorder. Behavior Research and Therapy. 1999;37:201–215. doi: 10.1016/s0005-7967(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrernal axis in women with premenstrual syndrome and controls. Journal of Clinical Endocrinology and Metabolism. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27:835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Friendly M. Predicting which words get recalled: measures of free recall, availability, goodness, emotionality, and pronunciability for 925 nouns. Memory & Cognition. 1986;14:79–94. doi: 10.3758/bf03209231. [DOI] [PubMed] [Google Scholar]
- Saxe G, Wolfe J. Gender and posttraumatic stress disorder. In: Saigh PA, Bremner JD, editors. Posttraumatic Stress Disorder: A Comprehensive Text. Boston: Allyn & Bacon; 1999. pp. 160–179. [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. Journal of Clinical Endocrinology and Metabolism. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Steil R, Ehlers A. Dysfunctional meaning of posttraumatic intrusions in chronic PTSD. Behavior Research and Therapy. 2000;38:537–558. doi: 10.1016/s0005-7967(99)00069-8. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry. 2007a;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, et al. How progesterone impairs memory for biologically salient stimuli in healthy young women. Journal of Neuroscience. 2007b;27:11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Hausmann M. Sex hormones: Modulators of interhemispheric inhibition in the human brain. Neuroscientist. 2009;16:132–138. doi: 10.1177/1073858409341481. [DOI] [PubMed] [Google Scholar]
- Wilkinson CB. Aftermath of a disaster: the collapse of the Hyatt Regency Hotel skywalks. American Journal of Psychiatry. 1983;140:1134–1139. doi: 10.1176/ajp.140.9.1134. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. New England Journal of Medicine. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]