Abstract
Background and objectives
The frequency, aetiologies, and outcomes of normal chest radiographs (CXRs) among HIV-seropositive patients with suspected pulmonary tuberculosis (TB) have been infrequently described.
Methods
Consecutive HIV-seropositive adults hospitalized for cough of ≥ 2 weeks duration at Mulago Hospital (Kampala, Uganda), between September 2007 and July 2008, were enrolled. Baseline CXRs were obtained on admission. Patients with sputum smears that were negative for acid-fast bacilli (AFB) were referred for bronchoscopy with bronchoalveolar lavage (BAL). BAL fluid was examined for mycobacteria, Pneumocystis jirovecii, and other fungi. Patients were followed for two months after enrolment.
Results
Of the 334 patients, 54 (16%) had normal CXRs. These patients were younger (median age 30 vs. 34 years, P=0.002), had lower counts of CD4+ T lymphocytes (median 13 vs. 57 cells/μL, P<0.001), and were less likely to be smear positive for AFB (17% vs. 39%, P=0.002) than those with abnormal CXRs. Pulmonary TB was the most frequent diagnosis (44%) among those with normal CXRs, followed by unknown diagnoses, pulmonary aspergillosis, and pulmonary cryptococcosis. The frequency of normal CXRs was 12% among pulmonary TB patients. There was a trend towards increased two-month mortality among patients with normal CXRs compared to those with abnormal CXRs (40% vs. 29%, P=0.15).
Conclusions
Normal CXR findings were common among HIV-seropositive patients with suspected TB, especially those who were young, those with low CD4+ T cell counts, and those with sputum smears that were negative for AFB. Mortality was high among those with normal CXRs. Normal CXR findings should not preclude further diagnostic evaluation in this population.
Keywords: clinical epidemiology, critical care medicine, immunodeficiency, radiology and other imaging, tuberculosis
INTRODUCTION
Chest radiographs (CXR) play an important role in the diagnosis of pulmonary disease in HIV-seropositive patients, although radiographic patterns may be substantially different from those observed in HIV-seronegative patients.1, 2 Patients with HIV infection and pulmonary tuberculosis (TB) may have atypical CXR findings when CD4+ T lymphocyte counts are low, 3–5 and CXRs may even appear normal in 7–14% of HIV-seropositive patients with pulmonary TB.6, 7 Normal CXR findings have also been reported in patients with HIV infection and other opportunistic respiratory diseases, such as pulmonary Kaposi’s sarcoma (KS), 8 Pneumocystis jirovecii pneumonia (PCP),9 and other fungal pneumonias.10, 11 In such patients, normal CXRs may delay diagnosis and the initiation of appropriate treatment, resulting in increased mortality among affected patients.12 Nevertheless, few studies have investigated the epidemiology of normal CXR findings among HIV-seropositive patients with suspected TB. Therefore, data from a prospective cohort study of HIV-associated pneumonia in Uganda was used to assess the frequency, aetiologies, and outcomes of respiratory complaints in this population.
METHODS
Participants
Consecutive adult patients who were admitted to the Medical Emergency Ward at Mulago Hospital in Kampala, Uganda between September 2007 and July 2008, were screened for eligibility, if they were suspected of having pulmonary TB, as defined by cough of at least two weeks duration.13 Patients were excluded if they had had a cough for longer than six months or if they were already receiving anti-tuberculosis treatment at the time of initial evaluation.
Ethical issues
The Makerere University Faculty of Medicine Research and Ethics Committee (#2006-017), the Mulago Hospital Institutional Review Board (#2006-017), the Committee on Human Research at the University of California, San Francisco (#H8660-27882), and the Uganda National Council for Science and Technology (#HS 259) approved the study protocol.
Data collection
After informed consent was obtained, research officers collected clinical and demographic information from study participants, using a standardized questionnaire, and blood was collected for measurement of the CD4+ T lymphocyte count. Each patient submitted two sputum samples (one spot and one early morning), which were examined by direct light microscopy and concentrated fluorescence microscopy according to standard protocols at the Uganda National TB Reference Laboratory.14 Processed sputum specimens were inoculated on two Lowenstein-Jensen slants for mycobacterial culture. Cultures were read weekly and were considered positive if there was adequate growth (≥ 1 colony forming unit) within eight weeks. Patients with negative smears were referred for bronchoscopy with BAL. A pulmonologist conducted a thorough bronchoscopic inspection of all visible airways for lesions consistent with Kaposi’s sarcoma. BAL fluid was examined for Mycobacterium tuberculosis [acid-fast bacilli (AFB) smear and Lowenstein-Jensen culture] at the reference laboratory, and for Pneumocystis jirovecii (modified Giemsa stain), and other fungi (potassium hydroxide and India ink stains, and culture on Sabouraud’s agar) in the Department of Microbiology at Mulago Hospital.
Vital status was assessed for all patients, either by telephone or in-person, two months after enrolment in the study. Patients who returned in person were administered a clinical questionnaire and underwent physical examination. After clinical and microbiological data were finalized, at least two pulmonary physicians assigned final diagnoses according to explicit pre-defined criteria (online supporting information, pages S1–S5).
Chest radiography
All patients underwent baseline postero-anterior (PA) or, in very sick patients, antero-posterior (AP) CXR, as a routine diagnostic procedure within 24 h of admission. Films were taken at a penetration of 60–80 kVp and a contrast of 5–8 mAS at a focal distance of 150 cm. If a patient had had a CXR within seven days prior to admission, it was not repeated. A medical officer photographed CXRs using a 4.1 mega-pixel digital camera. Two Ugandan board-certified radiologists, who were blinded to the clinical presentations and final diagnoses of the patients, reviewed and scored photographs of CXRs using a standardized interpretation form. Any differences in interpretation were resolved by consensus, including the interpretation of a third radiologist if necessary.
Statistical analysis
Bivariate analyses were performed to compare demographic and clinical characteristics and outcomes in patients with or without normal CXRs, using chi-squared or Fisher’s exact tests for dichotomous variables and the Mann-Whitney rank-sum test for continuous variables that were not normally distributed. Clinical predictors included age, gender, smoking history, a previous diagnosis of TB, use of antibiotics for respiratory complaints that preceded hospitalization, prophylaxis for PCP, use of antiretroviral therapy (ART), current CD4+ T cell count, and sputum AFB smear status. Subsequently, multivariate logistic regression analysis with backward selection was performed to assess whether predictor variables were associated with normal CXRs, and whether normal CXRs were associated with increased mortality. Variables were included based on face validity or empirical association with the outcome, if there was no possibility that they could be mediators. Because of the relatively small sample size, an inclusive cutoff was chosen for the empirical level of significance (P ≤ 0.2) for retention of variables. Predictor variables were also excluded if they did not alter by 10% or more the log odds of any predictor variable that was associated with the outcome at P ≤ 0.2. Missing continuous values were imputed as the median for that characteristic, and it was assumed that when patients reported prior treatments as unknown those treatments did not occur. Final models were assessed for goodness of fit using the Hosmer-Lemeshow test, and for omitted covariates and model misspecification using the link test. Statistical analyses were performed using STATA 11.0 (Stata Corporation, College Station, Texas, USA), with the level of significance specified as P < 0.05.
RESULTS
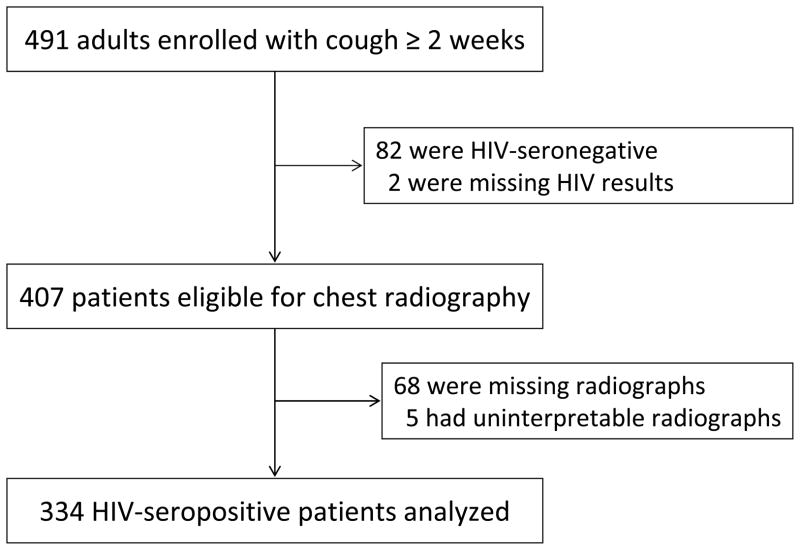
Of 491 consecutive patients enrolled, a total of 407 were HIV-seropositive. CXRs were available for 334 of these patients (Fig. 1). CXR findings were normal in 54 (16%) of these 334 HIV-seropositive patients.
Figure 1.
Flow diagram showing number of patients enrolled, number who were eligible, and number for whom data was analyzed.
Comparison of patients with normal and abnormal chest radiography
Patients with normal CXRs were younger (median age 30 vs. 34 years, P=0.002) and were more likely to be females (72% vs. 51%, P=0.004) compared to those with abnormal CXRs. Having a normal CXR was associated with a lower likelihood of past or current smoking (15% vs. 28%, P=0.045), and a higher likelihood of normal physical findings (35% vs. 22%, P=0.041). Patients with normal CXRs had lower median CD4+ T lymphocyte counts (13 vs. 57 cells/μL, P<0.001), and were less likely to be sputum smear-positive for AFB than those with abnormal CXRs (17% vs. 39%, P=0.002). The frequencies of other characteristics, symptoms, and signs were similar in the two groups (Table 1).
Table 1.
Characteristics of patients with normal or abnormal chest radiographs
| Characteristic | Chest radiograph interpretation | P value | |
|---|---|---|---|
| Normal (n = 54) | Abnormal (n = 280) | ||
| Age, years, median (IQR) | 30 (25–35) | 34 (28–40) | 0.002 |
| Female gender, n (%) | 39 (72) | 143 (51) | 0.004 |
| Sputum production, n (%) | 47 (87) | 239 (85) | 0.75 |
| Haemoptysis, n (%) | 9 (19)* | 75 (31)** | 0.09 |
| Dyspnoea, n (%) | 26 (48) | 152 (54) | 0.41 |
| Past or current tobacco use, n (%) | 8 (15) | 78 (28) | 0.045 |
| Previous history of TB, n (%) | 0 (0)† | 12 (11)†† | 0.36 |
| Use of antibiotics prior to admission, n (%) | 36 (67) | 186 (66) | 0.97 |
| Prophylaxis for PCP on admission, n (%) | 35 (65) | 151 (54) | 0.14 |
| Use of ART on admission, n (%) | 9 (17) | 40 (14) | 0.65 |
| Respiratory rate, breaths/min, median (IQR) | 26 (20–32) | 26 (20–32) | 0.84 |
| SpO2, %, median (IQR) | 97 (94–99) | 96 (92–98) | 0.037 |
| Normal lung examination, n (%) | 19 (35) | 62 (22) | 0.041 |
| CD4+ T cells/uL, median (IQR) | 13 (5–91)‡ | 57(20–153)‡‡ | <0.001 |
| AFB-positive sputum smear, n (%) | 9 (17)§ | 109 (39)§§ | 0.002 |
| Two-month mortality, n (%) | 19 (40)¶ | 73 (29)¶¶ | 0.15 |
n =47,
n =239,
n =14,
n =110,
n =52,
n =274,
n =53,
n =276,
n =48,
n =251
AFB, acid-fast bacilli; ART, antiretroviral therapy; IQR, interquartile range; PCP, Pneumocystis jirovecii pneumonia; SpO2, Oxyhemoglobin saturation measured by transcutaneous pulse oximetry; TB, tuberculosis.
In the multivariate analysis, having a normal CXR was not associated with the use of antibiotics, prophylaxis for PCP, or use of ART prior to admission. After adjusting for age, gender, smoking history, CD4+ T cell count, and sputum smear AFB status, three predictors (younger age, lower CD4+ T lymphocyte counts, and negative sputum smear AFB status) differed significantly between the two groups (Table 2).
Table 2.
Unadjusted and adjusted analyses for associations of clinical characteristics with normal chest radiography
| Characteristic | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age, per 10-year increase | 0.61 (0.43–0.87) | 0.007 | 0.67 (0.46–0.97) | 0.034 |
| Female gender | 2.49 (1.31–4.72) | 0.005 | 1.67 (0.77–3.63) | 0.19 |
| Past or current tobacco use | 0.45 (0.22–0.90) | 0.024 | 0.57 (0.21–1.55) | 0.27 |
| Use of antibiotics prior to admission | 1.01 (0.54–1.87) | 0.97 | – | – |
| Prophylaxis for PCP on admission | 1.57 (0.86–2.88) | 0.14 | – | – |
| Use of ART on admission | 1.20 (0.54–2.64) | 0.65 | – | – |
| CD4+ T cell count ≤50 cells/uL | 2.66 (1.42–5.00) | 0.002 | 3.67 (1.86–7.23) | <0.001 |
| AFB-positive sputum smear | 0.31 (0.15–0.67) | 0.003 | 0.22 (0.10–0.50) | <0.001 |
AFB, acid-fast bacilli; ART, antiretroviral therapy; PCP, Pneumocystis jirovecii pneumonia; TB, tuberculosis.
Diagnoses among HIV-seropositive patients with normal chest radiography
Pulmonary TB was the most common diagnosis among 54 patients with normal CXRs (n=24, 44%), followed by unknown diagnoses (n=19, 35%), pulmonary aspergillosis (n=3, 6%), and pulmonary cryptococcosis (n=2, 4%). Four (7%) of the 54 patients had two simultaneous respiratory infections; two had pulmonary TB and pulmonary cryptococcosis, and two had pulmonary TB and pulmonary aspergillosis.
The frequency of normal CXRs among culture-positive pulmonary TB patients was 9% (14/148) compared with 20% (10/50, P=0.048) among culture-negative pulmonary TB patients. Twenty-nine percent of patients (19/66) for whom the final diagnosis was unknown, 14% (2/14) of those with pulmonary cryptococcosis, 60% (3/5) of those with pulmonary aspergillosis, 0% (0/8) of those with pulmonary KS, and 0% (0/5)of patients with PCP had normal CXRs.
TB patients with normal CXRs had a longer median hospital stay (6 days) than those with abnormal CXRs (5 days, P=0.021), with the greatest difference being for those with culture-negative TB (9.5 days vs. 6 days, P=0.05). In addition, TB patients with normal CXRs were less likely to be discharged on TB treatment than those with abnormal CXRs(59% vs. 82%, P=0.014).
Two-month follow-up and mortality
Information was available on 299 of the 334 HIV-seropositive patients (90%), two months after discharge from hospital, including 48 of 54 patients (89%) with normal CXRs. Mortality among these 48 patients was 40%, compared with 29% among 251 patients with abnormal CXRs (difference 10%, 95% CI −4% to +25%, P=0.15). There were no significant differences in mortality between those with normal and abnormal CXRs within the different diagnostic categories, although mortality was generally higher among those with normal radiographs compared with those with abnormal radiographs (Table 3). Even after adjustment for potential confounders, there was no significant association between having a normal CXR and two-month mortality (data not shown).
Table 3.
Two-month mortality by chest radiography result and final diagnostic category
| Final diagnosis, n (%) | Two-month mortality* | P value | |
|---|---|---|---|
| Normal CXR (n =48) | Abnormal CXR (n =251) | ||
| Culture-positive pulmonary TB | 6/13 (46) | 36/114 (32) | 0.29 |
| Culture-negative pulmonary TB | 0/10 (0) | 0/40 (0) | -- |
| Acute bronchitis | 0/10 (0) | 0/36 (0) | -- |
| Pulmonary cryptococcosis | 0/2 (0) | 1/11 (9) | 1.0 |
| Pulmonary aspergillosis | 3/3 (100) | 0/2 (0) | 0.10 |
| Pulmonary Kaposi’s sarcoma | -- | 6/8 (75) | -- |
| Other causes of pneumonia | 1/2 (50) | 7/8 (75) | 1.0 |
| Unknown | 12/14 (86) | 27/38 (71) | 0.47 |
Vital status at two months was missing for 35 patients (6 with normal and 29 with abnormal baseline CXRs. The sum of patients in all categories may exceed the total number of patients because some patients had more than one final diagnosis. TB, tuberculosis
DISCUSSION
This is the first report on the frequency, disease aetiologies, and outcomes of normal CXRs among HIV-seropositive patients who were hospitalized with cough of at least two weeks duration. CXRs were normal in 16% of this population. Younger patients were more likely to have normal CXRs, as were those with lower CD4+ T lymphocyte counts and those with sputum smears that were negative for AFB. There was a moderate difference in mortality between patients with normal CXRs and patients without normal CXRs, although this analysis was under-powered and did not reach statistical significance.
Many studies have reported that radiographic findings may be normal in HIV-seronegative patients with culture-positive TB.6, 7, 15–19 The frequency of normal CXRs has consistently been found to be even higher among HIV-seropositive patients (7–14%)6, 7, 18 than among HIV-seronegative patients (1–3%).15, 17 Similar to the findings from previous studies, 9% of patients with culture-positive pulmonary TB had normal CXRs, whereas normal CXRs were more common among patients with culture-negative TB. Although CXR has been reported to be a good screening tool for increasing the sensitivity of TB diagnosis in sub-Saharan Africa, 20, 21 it is insufficiently sensitive to exclude pulmonary TB in symptomatic HIV-seropositive patients, as previously reported.22, 23
Similar to previous reports, HIV-seropositive patients with pulmonary aspergillosis and pulmonary cryptococcosis were also found to present with normal CXRs.10, 24 While no patient in the present study with PCP or pulmonary KS had a normal CXR, these diagnoses were infrequent (five patients with PCP, eight patients with pulmonary KS). Patients with PCP or pulmonary KS are well known to present with normal CXRs, even when their disease is clinically symptomatic.8, 9, 25–27
In the present study, CXRs were more frequently normal in patients with CD4+ T cell counts ≤50 cells/μL than in those with higher CD4+ T cell counts. It is well-known that HIV-seropositive patients with advanced CD4+ T cell depletion show atypical CXR findings when co-infected with TB.4, 6, 7, 28 In one study, patients with fewer than 200 CD4+ T cells/μL were as likely to have a normal CXR (21%) as they were to have post-primary patterns (23%).6 Unfortunately, little is known about the relationship between opportunistic non-TB pulmonary infections and normal CXR findings among HIV-seropositive patients stratified by CD4+ T cell count.
Patients with sputum smears that were negative for AFB showed a higher frequency of normal CXRs than those with sputum smears that were positive for AFB. This finding was consistent with three studies on normal CXR findings among HIV seropositive, culture-positive pulmonary TB patients, 12, 19, 29 one of which reported that those with normal CXRs had a significantly lower frequency of AFB-positive sputum smears than those with abnormal CXRs.29 Normal CXR findings among HIV-seropositive pulmonary TB patients with AFB-negative sputum smears may be associated with lower CD4+ T cell counts.12 In the present study, patients with AFB-negative sputum smears alone showed a higher frequency of normal CXRs, after adjusting for other predictors, including CD4+ T cell count.
In the present study, mortality tended to be higher among HIV-seropositive patients with normal CXRs than among those with abnormal CXRs, although the small sample size meant that the study was insufficiently powered to confirm this association. High mortality among patients who did not undergo bronchoscopy (e.g. patients with AFB-positive smears) may be associated with undiagnosed or co-prevalent pulmonary conditions, although the study protocol did not allow us to test this hypothesis. However, there was evidence of diagnostic and treatment delay in the form of prolonged hospitalization and under-treatment of patients with pulmonary TB. The high mortality among patients with unknown diagnoses may reflect the case definitions, in which culture-negative patients who died without improvement after receiving antibiotics or anti-TB medications, were categorized as “unknown diagnoses.” Similarly, none of the patients with culture-negative TB died, because the case definitions were based on a favourable clinical response to treatment.
There were some limitations to this study. First, bacterial culture was not performed to establish possible aetiologies. However, bacterial culture is not specific for bacterial pneumonia, which is less likely among patients with cough lasting more than two weeks. Second, postmortem examinations were not performed to establish definitive causes of death among the study patients. Third, CD4+ T cell counts during acute illness may underestimate the underlying true baseline value.
In summary, CXR findings were frequently normal among HIV-seropositive inpatients with suspected TB, especially when they were younger, or had lower CD4+ T cell counts, or AFB-negative sputum smear results. Two-month mortality among patients with normal CXRs may be higher than that of patients with abnormal CXRs. Therefore, a normal CXR in a symptomatic patient with unexplained cough should not preclude further diagnostic evaluation, such as chest computerized tomography (CT), culture for Mycobacterium tuberculosis or fungi, or empirical treatment directed at the most epidemiologically likely pathogens.
Supplementary Material
Acknowledgments
The authors thank the patients, staff, and administration of Mulago Hospital, as well as the staff and administration of the Infectious Disease Research Collaboration, without whom this study would not have been possible. We also thank two members of the Mulago Hospital Department of Radiology, Professor Michael Kawooya of Makerere University and Dr. Richard Okello of the Uganda Ministry of Health, for their assistance in interpreting the chest radiographs, and Cecily Miller at the University of California, San Francisco, for assistance with final proof-reading and copy-editing.
The National Institutes of Health provided financial support for this study through the following award mechanisms: K23AI080147 (JLD), K23HL094141 (AC), KL2 RR024130 (JLD, AC), K24HL087713 (LH), and R01HL090335 (LH).
References
- 1.Post FA, Wood R, Pillay GP. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+ T-lymphocyte count. Tuber Lung Dis. 1995;76:518–21. doi: 10.1016/0962-8479(95)90527-8. [DOI] [PubMed] [Google Scholar]
- 2.Shah NS, Anh MH, Thuy TT, et al. Population-based chest x-ray screening for pulmonary tuberculosis in people living with HIV/AIDS, An Giang, Vietnam. Int J Tuberc Lung Dis. 2008;12:404–10. [PubMed] [Google Scholar]
- 3.Geng E, Kreiswirth B, Burzynski J, et al. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA. 2005;293:2740–5. doi: 10.1001/jama.293.22.2740. [DOI] [PubMed] [Google Scholar]
- 4.Perlman DC, el-Sadr WM, Nelson ET, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. Clin Infect Dis. 1997;25:242–6. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 5.Jones BE, Young SM, Antoniskis D, et al. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SD, Frager D, Suster B, et al. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance) Radiology. 1994;193:115–19. doi: 10.1148/radiology.193.1.7916467. [DOI] [PubMed] [Google Scholar]
- 7.Long R, Maycher B, Scalcini M, et al. The chest roentgenogram in pulmonary tuberculosis patients seropositive for human immunodeficiency virus type 1. Chest. 1991;99:123–7. doi: 10.1378/chest.99.1.123. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell DM, McCarty M, Fleming J, et al. Bronchopulmonary Kaposi’s sarcoma in patients with AIDS. Thorax. 1992;47:726–9. doi: 10.1136/thx.47.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Stansell J, Osmond D, et al. Performance of an algorithm to detect Pneumocystis carinii pneumonia in symptomatic HIV-infected persons. Pulmonary Complications of HIV Infection Study Group. Chest. 1999;115:1025–32. doi: 10.1378/chest.115.4.1025. [DOI] [PubMed] [Google Scholar]
- 10.Keating JJ, Rogers T, Petrou M, et al. Management of pulmonary aspergillosis in AIDS: an emerging clinical problem. J Clin Pathol. 1994;47:805–9. doi: 10.1136/jcp.47.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman EP, Miller RF, Severn A, et al. Cryptococcal pneumonia in patients with the acquired immunodeficiency syndrome. Clin Radiol. 1995;50:756–60. doi: 10.1016/s0009-9260(05)83214-3. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri F, Girardi E, Pellicelli AM, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30:68–74. doi: 10.1007/s15010-002-2062-9. [DOI] [PubMed] [Google Scholar]
- 13.Hopewell PC, Pai M, Maher D, et al. International standards for tuberculosis care. Lancet Infect Dis. 2006;6:710–25. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- 14.Kent PT, Kubica GP. Public Health Mycobacteriology: A Guide for the Level III Laboratory. Centers for Disease Control; Atlanta: 1985. [Google Scholar]
- 15.Miller WT, MacGregor RR. Tuberculosis: frequency of unusual radiographic findings. AJR Am J Roentgenol. 1978;130:867–75. doi: 10.2214/ajr.130.5.867. [DOI] [PubMed] [Google Scholar]
- 16.Woodring JH, Vandiviere HM, Fried AM, et al. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol. 1986;146:497–506. doi: 10.2214/ajr.146.3.497. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PF, Verdegem TD, Vachon LA, et al. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94:316–20. doi: 10.1378/chest.94.2.316. [DOI] [PubMed] [Google Scholar]
- 18.FitzGerald JM, Grzybowski S, Allen EA. The impact of human immunodeficiency virus infection on tuberculosis and its control. Chest. 1991;100:191–200. doi: 10.1378/chest.100.1.191. [DOI] [PubMed] [Google Scholar]
- 19.Marciniuk DD, McNab BD, Martin WT, et al. Detection of pulmonary tuberculosis in patients with a normal chest radiograph. Chest. 1999;115:445–52. doi: 10.1378/chest.115.2.445. [DOI] [PubMed] [Google Scholar]
- 20.Day JH, Charalambous S, Fielding KL, et al. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis. 2006;10:523–9. [PubMed] [Google Scholar]
- 21.Kivihya-Ndugga LE, van Cleeff MR, Ng’ang’a LW, et al. Sex-specific performance of routine TB diagnostic tests. Int J Tuberc Lung Dis. 2005;9:294–300. [PubMed] [Google Scholar]
- 22.Koppaka R, Bock N. How reliable is chest radiography? In: Frieden T, editor. Toman’s Tuberculosis. World Health Organization; Geneva: 2004. p. 59. [Google Scholar]
- 23.Davis JL, Worodria W, Kisembo H, et al. Clinical and radiographic factors do not accurately diagnose smear-negative tuberculosis in HIV-infected inpatients in Uganda: a cross-sectional study. PLoS One. 2010;5:e9859. doi: 10.1371/journal.pone.0009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balloul E, Couderc LJ, Molina JM, et al. Pulmonary cryptococcosis during HIV infection. 15 cases. Rev Mal Respir. 1997;14:365–70. [PubMed] [Google Scholar]
- 25.Brechot JM, Dore MF, Capron F, et al. Pneumocystis carinii pneumonia in AIDS patients. Aspects of clinical evolution in 37 cases. Rev Pneumol Clin. 1989;45:114–17. [PubMed] [Google Scholar]
- 26.Opravil M, Marincek B, Fuchs WA, et al. Shortcomings of chest radiography in detecting Pneumocystis carinii pneumonia. J Acquir Immune Defic Syndr. 1994;7:39–45. [PubMed] [Google Scholar]
- 27.Richards PJ, Riddell L, Reznek RH, et al. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689–93. doi: 10.1016/s0009-9260(96)80239-x. [DOI] [PubMed] [Google Scholar]
- 28.Goodman PC. Pulmonary tuberculosis in patients with acquired immunodeficiency syndrome. J Thorac Imaging. 1990;5:38–45. doi: 10.1097/00005382-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Pepper T, Joseph P, Mwenya C, et al. Normal chest radiography in pulmonary tuberculosis: implications for obtaining respiratory specimen cultures. Int J Tuberc Lung Dis. 2008;12:397–403. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.