Abstract
Chronic renal failure patients receiving hemodialysis and continuous ambulatory peritoneal dialysis often encounter gastrointestinal troubles over their long treatment period. Helicobacter pylori infection has close association with development of peptic ulcer, gastric cancer and gastric lymphoma, and is thought to be one of the major risk factors for gastrointestinal troubles in dialysis patients. However, it is unclear whether H. pylori infection is directly associated with progression of renal dysfunction and prognosis of chronic renal failure patients. Recent consensus shows that the prevalence of H. pylori infection in chronic renal failure patients is significantly lower than in subjects with normal renal function. In the natural history of H. pylori infection in hemodialysis patients, the prevalence of infection decreases as dialysis periods progressed, in particular within the first four years after the start of treatment. However, the chance of natural eradication becomes rare for patients receiving dialysis treatment for a long time. Moreover, chronic renal failure patients with H. pylori infection have a higher incidence of gastroduodenal diseases, and therefore, are recommended to receive eradication therapies, especially for those receiving treatment for a long time and with higher risks of complication. Intensive endoscopic check-ups for the prevention of gastrointestinal events and the discovery of peptic ulcer and neoplastic diseases at an early phase may be required.
Keywords: Chronic renal failure, Continuous ambulatory peritoneal dialysis, Eradication therapy, Helicobacter pylori, Hemodialysis, Peptic ulcer
Chronic renal failure (CRF) patients receiving hemodialysis treatment consist of more than 1.1 million people in the world, and the size of this population is expanding at a rate of 7% per year according to the progress in medical and dialysis machine technique (1). Although heart failure, angina pectoris, hypertension, parathyroid-related disease, amyloidosis, renal anemia and infection are well known to occur by receiving hemodialysis and continuous ambulatory peritoneal dialysis (CAPD) for long periods (2), those patients also often suffer from gastrointestinal troubles including peptic ulcer, hemorrhage, abdominal symptoms, constipation, diarrhea, ileus, and perforation (3–12).
Helicobacter pylori is a spiral-shaped, microaerophilic Gram-negative flagellate bacterium that was isolated in 1983 from gastric biopsy specimens of patients with chronic gastritis (13). The gastric mucosa of approximately 50% of the world's population is infected with H. pylori, and the infection levels exceed 70% in some developing areas (14,15). Helicobacter pylori infection plays a crucial role in the development of gastrointestinal diseases, such as peptic ulcer, gastric hyperplastic polyps, gastric adenoma, gastric cancer, and gastric mucosa associated-lymphoid tissue lymphoma, both in individuals with normal renal function and in chronic renal failure patients receiving hemodialysis and CAPD (Tables 1 and 2) (4–9,16,18–32,34,36–45,47–60).
TABLE 1.
List of previous reports: association of Helicobacter pylori infection prevalence and patients with hemodislysis
| Year | Author | Period (months) | Patient (n) | Control (n) | Character of Control | PU | GIF |
|---|---|---|---|---|---|---|---|
| 1989 | Offerhaus GJA (16) | NA | 44% (50) | 45% (40) | Healthy | + | − |
| 1990 | Wee A (17)† | NA | 31% (322) | NA | NA | + | |
| 1991 | Davenport A (18) | 38 (1–76) | 34% (76) | 30% (247) | Healthy | NA | − |
| 1991 | Loffeld RJ (19) | 4 (2–16) | 43% (30) | 38% (344) | Healthy | NA | − |
| 1995 | Jasperson D (20) | NA | 23% (34)a | 37% (127) | Symptoms | + | + |
| 1995 | De Vecchi AF (21) | NA | 59% (29) | 72% (29) | Symptoms | + | − |
| 1996 | Luzza F (22) | 74 ± 63 | 73% (103) | 78% (103) | Symptoms | NA | − |
| 1996 | Seyrek N (23) | 23 ± 2 | 14% (91) | 23% (35) | Healthy | NA | − |
| 1996 | Krawczyk W (24) | 28 ± 12 | 62% (21) | 64% (22) | Dyspepsia | − | + |
| 1996 | Abu Farsakh NA (25) | 18 (1–108) | 57% (28)a | 73% (100) | NA | + | |
| 1997 | Hruby Z (26) | 52 ± 54 | 35% (25) | 44% (16) | Dyspepsia | NA | + |
| 1997 | Ozgur O (27) | 29 ± 29 | 60% (47) | 64% (100) | Symptoms | + | + |
| 1998 | Munoz de Bustillo E (28) | 40 ± 42 | 64% (52) | NA | + | + | + |
| 1999 | Yildiz A (29) | 33 ± 28 | 66% (47) | 73% (55) | Healthy | NA | − |
| 1999 | Fabirizi F (30) | NA | 56% (228) | 53% (158) | Healthy | NA | − |
| 1999 | Araki H (31)† | 91 ± 62 | 46% (63) | 66% (64) | Healthy | + | + |
| 1999 | Gut G (32) | 21 ~ 22 | 57% (44) | 55% (45) | Dyspepsia | − | + |
| 1999 | Boran M (33) | 52 ± 21 | 50% (50) | NA | NA | + | |
| 2000 | Huang JJ (34) | 28 (1–156) | 47% (70) | 60% (42) | Dyspepsia | + | + |
| 2001 | Wang YL (35) | 53 | 50% (80) | 60% (80) | Dyspepsia | + | + |
| 2002 | Nakajima F (36) | 47 ± 58 | 28% (51)a | 56% (25) | CRF (non-HD) | + | + |
| 2002 | Fabbian F (37) | 51 ± 58 | 13% (38) | NA | − | 4- | |
| 2002 | Tsukada K (38) | 76.5−57.4 | 30% (47)a | 56% (55) | Dyspepsia | + | + |
| 2003 | Sotoudehmanesh R (5) | 14 ± 29 | 59% (206) | NA | + | + | |
| 2003 | Marsenic O (39)‡ | NA | 9% (22) | NA | − | + | |
| 2004 | Lopez T (40) | 56 ± 61 | 42% (86) | NA | NA | − | |
| 2004 | Sezer S (4) | 67 ± 48 | 10% (163) | NA | − | + | |
| 2004 | Nakajima F (41) | 57 ± 62 | 37% (138) | 62% (138) | Healthy | NA | − |
| 2004 | Al-Mueilo SH (42) | NA | 63% (44) | 63% (60) | Dyspepsia | + | + |
| 2004 | Trimarchi H (43) | 19 ~ 20 | 28% (29) | NA | NA | − | |
| 2005 | Blusiewicz K (44) | NA | 63% (30) | 71% (31) | Dyspepsia | + | + |
| 2006 | Lentine (45) | 28 | 25% (97) | NA | NA | − | |
| 2007 | Khedmat H (9) | 47 ± 11 | 63% (73)b | 35% (305) | Dyspepsia | + | + |
| 2007 | Itatsu T (46) | NA | 40% (77) | NA | Symptoms | NA | + |
| 2008 | Gioe FP (47) | 42–85 | 53% (142) | 45% (132) | Symptoms | − | − |
| 2009 | Sugimoto M (48) | 59 ± 0.4 | 49% (539)a | 79% (400) | Symptoms | + | + |
P < 0.05 when significantly lower compared with control subjects;
P < 0.05 when significantly higher compared with control subjects.
Paper contains hemodialysis and CAPD patients
Paper contains hcmodialysis and CRF patients.
Treatment period was determined as median (range) or mean ± SE or SD according to reports. Gastrointestinal endoscopy (GIF)-positive (+) indicates that GIF was performed in the study, and peptic ulcer-positive (+) meant reports included peptic ulcer in both/either patient group and/or control group with normal renal function. CAPD, continuous ambulatory peritoneal dialysis; CRF, chronic renal failure; PU, peptic ulcer; NA, not available.
TABLE 2.
List of previous reports: association with Helicobacter pylori infection prevalence and patients with CAPD and Tx
| Treatment | Year | Author | Period (month) | Patient (n) | Control (n) | Character of Control | PU | GIF |
|---|---|---|---|---|---|---|---|---|
| CAPD | ||||||||
| 1995 | De Vecchi AF (21) | NA | 53% (38)a | 79% (38) | Symptoms | + | − | |
| 1997 | Antoniou S (49) | (1–108) | 64% (39) | NA | NA | − | ||
| 2001 | Aguilera A (50) | 18 ± 13 | 33% (48) | NA | − | − | ||
| 2005 | Lui SL (51) | 54 ± 42 | 26% (136) | NA | NA | − | ||
| 2008 | Altay M (52) | 21 ± 18 | 27% (64) | NA | NA | − | ||
| TX | ||||||||
| 1991 | Davenport A (18) | 45 (6–219) | 29% (202) | 30% (247) | Healthy | NA | − | |
| 1997 | Hruby Z (26) | 41 ± 35 | 62% (29) | 44% (16) | Dyspepsia | NA | + | |
| 1997 | Ozgur O (27) | 51 ± 40 | 70% (54) | 64% (100) | Symptoms | + | + | |
| 1999 | Yildiz A (29) | 38.6% (57) | 73% (55) | Healthy | NA | − | ||
| 2007 | Khedmat H (9) | 52 ± 16 | 40% (25) | 35% (305) | Dyspepsia | + | + | |
| CRF | ||||||||
| 1990 | Shousha S (53) | 24% (50)a | 42% (120) | Symptoms | − | + | ||
| 1991 | Ala-Kaila K (54) | 17% (89) | NA | NA | − | |||
| 1993 | Neithercut WD (55) | 48% (23) | NA | NA | + | |||
| 1995 | Jaspcrson D (20) | 24% (59)a | 37% (127) | Symptoms | + | + | ||
| 1997 | Moustafa FE (56) | 40% (70) | NA | NA | + | |||
| 1999 | Misra V (57) | 56% (50)a | 78% (50) | Healthy | − | + | ||
| 2000 | Emir S (58) | 63% (16) | NA | + | + | |||
| 2002 | Schoonjans R (59) | 46% (28) | NA | − | − | |||
| 2004 | Strid H (60) | 26% (31) | NA | NA | − | |||
| 2004 | Nakajima F (41) | 53.3 (30) | 62% (138) | Healthy | NA | − | ||
| 2007 | Khedmat H (9) | 66% (71)b | 35% (305) | Dyspepsia | + | + |
P < 0.05 when significantly lower compared with control subjects;
P < 0.05 when significantly higher compared with control subjects.
Treatment period was determined as median (range) or mean ± SE or SD according to reports. Gastrointestinal endoscopy (GIF)-positive (+) indicates that GIF was performed in the study, and peptic ulcer-positive (+) meant reports included peptic ulcer in both/either patient group and/or control group with normal renal function. CAPD, continuous ambulatory peritoneal dialysis; CRF, chronic renal failure; PU, peptic ulcer; NA, not available; Tx, kidney transplant.
Patients with chronic renal failure often have gastrointestinal symptoms caused not only by H. pylori infection, but also by high urea levels, decline of gastrointestinal motility, amyloid protein deposition (59,60), and decreased sensory disturbance. Therefore, the quality of life in patients with chronic real failure is usually poor, which affects their nutrition status leading to the development of malnutrition, which is a potent predictor of morbidity and mortality. Moreover, patients with chronic renal failure may have higher risks of gastric mucosal damages compared with individuals with normal renal function because of systemic and/or local chronic circulatory failure (61,62), hypergastrinemia (32), high ammonia levels (55), and enhanced inflammation. Especially, 25–75% of those patients suffer from a number of gastrointestinal lesions and complications including gastric erosions, peptic ulcers, angiodysplasia and gastrointestinal bleeding (3–12,17).
It is unclear about the actual condition of gastroduodenal diseases in hemodialysis and CAPD patients related with H. pylori infection, because previous reports have controversial results due to small sample size and short observation periods. In this review article, we therefore initially reviewed the association between H. pylori infection and chronic renal failure, the natural history of H. pylori infection according to the treatment periods, and then summarize the H. pylori eradication therapy for chronic renal failure patients.
Helicobacter pylori infection in patients receiving hemodialysis and CAPD
Helicobacter pylori infection has an influence on intake of proton pump inhibitors (PPI) and antibiotics and eradication therapy of H. pylori. The prevalence of H. pylori infection in chronic renal failure patients receiving CAPD or hemodialysis was shown to be equal or lower compared to subjects with normal renal function in various different geographic populations irrespective to presence/absence of gastric symptoms (4–9,16,18–32,34,36–45,47–60) (Table 1). Recently, in the investigation of 539 Japanese hemodialysis patients with treatment periods of mean 8.4 ± 0.3 years, the prevalence of H. pylori infection was reported to be 48.6% ([95% CI: 44.3–52.9%]), which was significantly lower than in dyspepsia patients with normal renal function (78.5% [74.1–82.4%], 314/400, P < 0.001) and individuals with normal renal function receiving annual health exams (69.4% [60.3–77.5%], 84/121, P < 0.001) (48). Moreover, the prevalence of H. pylori infection in hemodialysis patients is significantly lower (27.5%) compared with non-hemodialysis chronic renal failure patients (56.0%) (36), and the prevalence in individuals with normal renal function is similar with patients receiving hemodialysis treatment for less than 1-year period (48). These data suggest that hemodialysis treatment, but not uremia by chronic renal failure itself, plays a role in the lower prevalence of H. pylori infection. The start of hemodialysis treatment is thought trigger the decrease in the prevalence of H. pylori infection.
Previously, eight studies showed significantly lower prevalence of H. pylori infection in patients receiving hemodialysis (8/36 studies, 22.2%) (20,21,25,36,38,48,53,57) compared with controls and one study has found a significantly lower prevalence of H. pylori infection in patients receiving CAPD (1/5 studies, 20.0%) (21); whereas only one study reported that Iranian hemodialysis patients (63.0%) and chronic renal failure patients (66.2%) had significantly higher prevalence of H. pylori infection compared with normal individuals (27.5%) (Table 1) (9). However, since the prevalence of H. pylori infection in the Iranian population is reported to be more than 60% in other reports (63,64), further studies are required to clarify this discrepancy in the Iranian population. In a combined analysis using previous reports (4–9,16,18–32,34,36–45,48–60), the prevalence of H. pylori infection in patients receiving chronic hemodialysis and CAPD are 43.9% ([95% CI: 42.2–45.6%], 1435/3 272) and 34.8% ([29.6–40.2%], 113/325), respectively, which is significantly lower than in individuals with normal renal function both with and without gastrointestinal symptoms (49.8% [48.0–51.7%], 1476/2961, P < 0.001).
There is variation in H. pylori infection rates among different countries. It may, therefore, be better to evaluate the infection rate in various countries. In East Asian countries, the prevalence of H. pylori infection in patients receiving chronic hemodialysis is 44.5% ([95% CI: 41.5–47.6%], 474/1065), which is significantly lower than in individuals with normal renal function (54.0% [50.9–57.1%], 560/1038, P < 0.001). On the other hand, because the prevalence of H. pylori in other areas, such as Europe, Middle East, and South Asia has a wide variation, it is difficult to evaluate the prevalence of H. pylori infection in those areas.
The prevalence of H. pylori infection in patients receiving a kidney transplant was 39.0% ([33.9–44.2%], 143/367) (Table 2). Kidney transplant patients with sufficient renal function at present also have a lower H. pylori infection rate, as well as hemodialysis and CAPD patients. This may be explained by a situation in which most patients received hemodialysis and/or CAPD anytime before kidney transplantation.
The evaluation of an association between H. pylori infection and causes of chronic renal failure is limited. The prevalence in patients whose chronic renal failure was caused by diabetic nephropathy (60.1%) is reported to be significantly higher than that in chronic glomerulonephritis patients (46.1%) irrespective of the decreased immune system of diabetes patients (48). This suggests that disorders of the immune system, such as severe diabetic nephropathy, does not lose the maintenance of H. pylori infection.
Treatment periods of hemodialysis and H. pylori infection
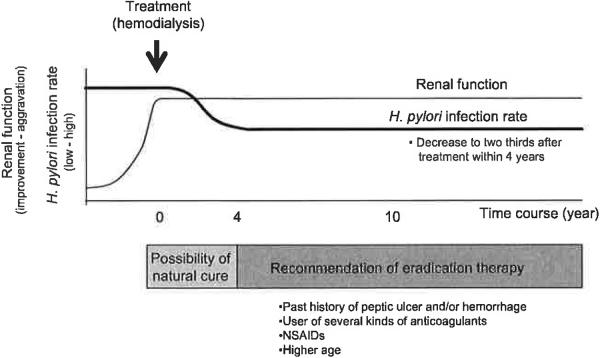
Only a few reports have evaluated the relationship between H. pylori infection and dialysis treatment periods, and the mean dialysis duration in H. pylori-positive patients was significantly shorter than in uninfected patients (28,29,36,41,48). Munoz de Bustillo et al. (28) reported that mean hemodialysis periods in H. pylori negative patients receiving dialysis was 66.5 months, which was significantly longer than in positive patients (24.7 months). Nakajima et al. (36) reported that the prevalence of H. pylori infection markedly decreases when the treatment duration is two years or more. Recently, when the treatment period of hemodialysis and the H. pylori infection rate were simply measured at one time in a hospital, the infection rate was reported to gradually decrease according to dialysis treatment periods until four years after starting hemodialysis followed by a plateau (Table 3) (48). The prevalence of H. pylori infection is inversely associated with treatment periods of hemodialysis and the decreasing pattern is characteristic (Table 3). However, there are no data on the relationship between H. pylori infection and the time course of CAPD treatment periods.
TABLE 3.
Changes in Helicobacter pylori infection rate at the beginning, two years later, and 4 years later among total patients, chronic glomerulonephritis (CGN) patients, and patients with diabetes and others (48)
| Total | CGN | Diabetes | Others | |
|---|---|---|---|---|
| Beginning | 51.7% | 48.8% | 64.0 %b | 48.8% |
| 2 years later | 42.9%a | 41.3% | 47.2% | 46.5% |
| 4 years later | 38.3%a | 38.2% | 41.6% | 32.6% |
P < 0.05 (vs. Beginning);
P < 0.05 (vs. CGN and others groups).
Others included lupus nephritis, polycystic kidney, chronic pyelonephritis and nephrosclerosis.
The follow-up survey of H. pylori infection
To investigate whether H. pylori infection was actually eradicated after beginning hemodialysis treatment, a follow-up survey using the same dialysis patients with H. pylori infection is required. When the natural history of H. pylori infection was investigated using more than 300 patients for a 4 year-follow up survey, although nobody received eradication therapy, the prevalence of H. pylori infection was reported to be 51.6% at first year, 42.9% two years later, and 38.3% four years later, indicating that the infection rate gradually decreased during dialysis treatment (Table 3). In other words, 26.7% of dialysis patients naturally cured H. pylori infection over four years. Patients who received hemodialysis and CAPD are expected to eradicate H. pylori not only naturally but also by environmental improvement and H. pylori eradication.
The mechanisms of lower prevalence of H. pylori infection in hemodialysis and CAPD patients
There are at least three explanations as to why dialysis patients have low prevalence of H. pylori infection: (i) Patients receiving dialysis have higher levels of pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α, from activated inflammatory cells infiltrating the gastric mucosa (65). As a result, gastric atrophy progresses, accompanied by increased pH, and finally H. pylori are not able to live in gastric mucosa (66). (ii) Blood urea levels as well as urea nitrogen levels in gastric secretions are higher in dialysis patients than in patients with normal renal function, and high urea levels may inhibit H. pylori growth in the stomach (67). (iii) H. pylori infection might be cured upon antibiotic treatment, both because antibiotics are commonly used during the initial treatment periods and most dialysis patients had many chances of bacterial infection during the treatment, and because antibiotic concentrations are higher and more prolonged in patients with renal failure than in individuals with normal renal function. Importantly, there was no significant difference in the prevalence of H. pylori infection between patients with normal renal function and patients receiving less than one year of dialysis treatment (48). Therefore, urea concentration and antibiotic usage are unlikely to be contributors in decreasing the prevalence of infection. One possible explanation for decreased H. pylori prevalence is therefore up-regulation of pro-inflammatory cytokine resulting from dialysis enhanced severe inflammation of gastric mucosa, and progression to gastric atrophy. However, further studies to investigate the histopathology of gastric inflammation and atrophy are necessary.
Necessity of H. pylori eradication therapy for chronic renal failure patients
The prevalence of peptic ulcer in hemodialysis and CAPD patients with H. pylori infection is higher than in individuals with normal renal function (9). Moreover, the incidence of tumor death is 2–4 times higher in dialysis patients than in the general population, and it is known that the most frequently detected malignant tumor is gastric cancer (68).
For hemodialysis and CAPD patients, one of the severe gastroduodenal events is a hemorrhage from gastroduodenal lesions. Because most chronic renal failure patients receive anti-coagulant drugs and/or anti-platelet drugs for prevention of hypertension and brain infarction, and thus anti-coagulant drugs, such as heparin sodium, are usually used during hemodialysis treatment, hemorrhage from gastroduodenal lesions easily occurs in such patients and often causes fatal blood loss. Moreover, gastric mucosa is known to be fragile in hemodialysis and CAPD patients. Therefore, to prevent hemorrhage from gastrointestinal diseases, intragastric pH in patients receiving hemodialysis and CAPD should be kept at a higher level by doses of acid inhibitory drugs (e.g. proton pump inhibitors) and by limiting doses of anticoagulant drugs.
One important question is whether H. pylori eradication therapies are necessary for H. pylori-infected hemodialysis and CAPD patients to prevent gastroduodenal disease development. As described above, more than one-third of the patients receiving approximately four years of dialysis treatment have naturally cured H. pylori infections, especially in hemodialysis patients (48). However, the frequency of peptic ulcer in patients receiving dialysis treatment is known to be higher in H. pylori infected patients than in uninfected patients, as observed in patients with normal renal function (48). We therefore recommend eradication therapies for H. pylori-infected hemodialysis patients and for patients receiving hemodialysis treatment. Moreover, it is better to eradicate at an early time for patients with a higher risk of disease development, such as past history of peptic ulcer and/or gastroduodenal hemorrhage, usage of several kinds of anticoagulants and non-steroidal anti-inflammatory drugs (NSAIDs, Fig. 1).
FIG. 1.
The schema of natural history of Helicobacter pylori infection in hemodialysis patients and treatment strategy.
Present condition otH. pylori eradication therapy for patients with chronic renal failure
One of the merits for H. pylori eradication therapy in hemodialysis and CAPD patients is that they keep a high plasma concentration of PPI and antibiotics even with low doses of drugs, although overdose of drugs must be carefully checked. On the other hand, one of the demerits for H. pylori eradication therapy in hemodialysis and CAPD patients is that there is a possibility that those patients are often infected with antibiotic-resistant strains since they have many occurrences of antibiotic intake due to an impaired immune system. In fact, 36.4% of patients with end-stage renal disease are reported to have clarithromycin-resistant strains, which is significantly higher than in non-uremic controls (15.2%) (69).
As summarized in Table 4 (4,28,31,32,35,38,46,70–75), each eradication regimen previously reported use of different PPIs (omeprazole, lansopprazole, esomeprazole) and/or antibiotics (clarithromycin, amoxicillin and metronidazole), and variation of the duration of treatment from 7 days to more than 3 weeks. However, there is no significant difference in the overall cure rates among the different studies listed. In multivariate analysis for eradication failure (age, gender, endoscopic findings, dialysis therapy, and previous eradication treatment), only history of previous treatment remained (38). This suggests that antibiotic resistance influences eradication success/failure, as observed in healthy non-dialysis patients. However, there are currently no data on antibiotic-resistant strains in eradication studies with dialysis patients (Table 4).
TABLE 4.
Helicobacter pylori eradication therapy for chronic renal failure patients
| Year | Author | Country | Number | Regimen | Eradication rate | Determine methods | Resistance |
|---|---|---|---|---|---|---|---|
| 1997 | Tamura H (70) | Japan | 14 | LPZ (30) oid/ 8 weeks, AMPC (500) oid/ 3 weeks, plaunotol (80) tid/ 24 weeks | 78.6% | RUT, Culture, Histology | No data |
| 1998 | Munos de Bustillo E (28) | Spain | 23 | OPZ (20) bid, AMPC (500) tid/ 14 days | 60.8% | UBT | No data |
| 23 | Plus OPZ (20) bid, CAM (500) bid/ 14 days | 82.6% | |||||
| 1998 | Tokushima H (71) | Japan | 17 | LPZ (30) oid/ 8 weeks, AMPC (500)/3 weeks | 76.5% | RUT, Culture, Histology | No data |
| 10 | LPZ (30) oid, AMPC (250), MNZ (250) bid/ 7 days | 90.0% | |||||
| 1999 | Araki H (31) | Japan | 17 | OPZ (20) oid/ 8 weeks, AMPC (250) oid, CAM (200) oid/ 3 weeks, polaprizinc (0.5) bid/ 24 weeks | 88.2% | IgG, Histology | No data |
| 1999 | Gur G (32) | Turkey | 25 | FAM (40) oid, CAM (500) bid, MNZ (250) bid/ 15 days | 80.0% | Histology, RUT | No data |
| 2001 | Wang YL (35) | China | 38 | OPZ (20), AMPC (1000), CAM (500) bid/ 7 days | 86.8% | Stool | No data |
| 2002 | Mak SK (72) | China | 21 (CRF) | OPZ (20), AMPC (1000), CAM (500) bid/ 7 days | 90.5% | RUT | No data |
| 2002 | Tsukada K (38) | Japan | 39 | OPZ (30) bid, AMPC (500) tid, CAM (400) bid/ 7 days | 82.1% | UBT | No data |
| 2003 | Mak SK (73) | China | 25 (CRF) | OPZ (20) or LPZ (30), AMPC (1000), CAM (5O0) bid/ 7 days | 96.0% | Histology | No data |
| 2003 | Sheu BS (74) | China | 38 (CRF) | LPZ (30), AMPC (750), CAM (500) bid/ 7 days | 76.3% | ||
| 40 (CRF) | LPZ (30), CAM (500), MNZ (500) bid/ 7 days | 92.5% | Stool | No data | |||
| 2004 | Sezer S (4) | Turkey | 17 | OPZ (20), AMPC (1000), CAM (500) bid/ 14 days | 94.1% | Endoscopy | No data |
| 2007 | Tseng GY (75) | China | 34 (CRF) | ESO (40) or OPZ (20) bid, AMPC (1000) bid, CAM (500) bid/ 7 days | 94.1% | UBT | No data |
| 2007 | Itatsu T (46) | Japan | 11 | LPZ (60), AMPC (750), CAM (400)/ 7 days | 72.7% | RUT | No data |
| 9 | LPZ (60), CAM (400)/ 7 days | 33.3% |
AMPC, amoxicillin; CAM, clarithromycin; Eso, Esomeprazole; FAM, famotidine; LPZ, lansoprazole; MNZ, metronidazole; OPZ, omeprazole; RUT, rapid urease test; UBT, urea breath test.
Antibiotics are known to have the possibility to progress renal dysfunction. Sheu et al. (74) reported that among chronic renal failure non-dialysis patients, those in the lansoprazole–clarithromycin–metronidazole regimen had a lower risk of acute renal failure than those in the lansoprazole–clarithromycin–amoxicillin regimen (2% vs. 18%, P < 0.05; relative risk, 0.128, 95%CI, 0.016–0.979). Because amoxicillin is mainly excreted from the kidney, toxic effects of amoxicillin on renal function in patients with chronic renal failure have been reported in various studies (76,77). However, most previous reports showed no severe adverse effects of amoxicillin in hemodialysis patients who received eradication therapy containing amoxicillin (4,28,31,32,35,38,70,71). Moreover, since PPI and antibiotics are removed by hemodialysis, plasma concentration of PPI and antibiotics is expected to decrease to a concentration that is less than effective. Therefore, it is necessary to set an optimal dosing plan in hemodialysis patients to increase cure rate and to use antibiotics more safely by using established pharmacological methods, such as monitored concentration.
Most patients who received eradication therapy were prevented from recurrence of peptic ulcer and had decreased risk of gastric cancer development (78–80). With eradication therapy in patients treated for early gastric cancer by endoscopic mucosal resection, the odds ratio for metachronous gastric cancer decreased significantly [0.353 (95% CI 0.161–0.775; P = 0.009)] (79). Patients who received hemodialysis often encountered gastric mucosal injuries, such as gastric erosion and gastric ulcer, irrespective to H. pylori infection due to NSAID intake and arteriosclerosis. Therefore, for patients receiving hemodialysis and CAPD, the most important benefit is the prevention of gastric cancer development.
CONCLUSION
Chronic renal failure patients receiving hemodialysis and CAPD have a low prevalence of H. pylori infection. More than one-third (36.2%) of patients receiving less than four years' dialysis had naturally cured H. pylori infection within the four-year observation period. However, because chronic renal failure patients have a higher risk of gastroduodenal disorders, it is recommended that all hemodialysis and CAPD patients receive endoscopic check-ups to reduce the chance of developing peptic ulcers. Moreover, patients with H. pylori infection should receive eradication therapy to prevent peptic ulcer and hemorrhage. The clinical protocol for a detailed endoscopic check-up and eradication therapy for dialysis patients should be evaluated in future studies.
Acknowledgments
The project described was supported by Grant Number R01 DK62813 from the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol. 2002;13(Suppl l):S37–40. [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Ala-Kaila K. Upper gastrointestinal findings in chronic renal failure. Scand J Gastroenterol. 1987;22:372–6. doi: 10.3109/00365528709078607. [DOI] [PubMed] [Google Scholar]
- 4.Sezer S, Ibis A, Ozdemir BH, et al. Association of Helicobacter pylori infection with nutritional status in hemodialysis patients. Transplant Proc. 2004;36:47–9. doi: 10.1016/j.transproceed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Sotoudehmanesh R, Ali Asgari A, Ansari R, Nouraie M. Endoscopic findings in end-stage renal disease. Endoscopy. 2003;35:502–5. doi: 10.1055/s-2003-39672. [DOI] [PubMed] [Google Scholar]
- 6.Prakash J, Agrawal BK. Upper gastrointestinal mucosal lesions in chronic renal failure. Indian J Gastroenterol. 1991;10:131–2. [PubMed] [Google Scholar]
- 7.Gheissari A, Rajyaguru V, Kumashiro R, Matsumoto T. Gastrointestinal hemorrhage in end stage renal disease patients. Int Surg. 1990;75:93–5. [PubMed] [Google Scholar]
- 8.Tsai CJ, Hwang JC. Investigation of upper gastrointestinal hemorrhage in chronic renal failure. J Clin Gastroenterol. 1996;22:2–5. doi: 10.1097/00004836-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Khedmat H, Ahmadzad-Asl M, Amini M, et al. Gastroduodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc. 2007;39:1003–7. doi: 10.1016/j.transproceed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Milito G, Taccone-Gallucci M, Brancaleone C, et al. Assessment of the upper gastrointestinal tract in hemodialysis patients awaiting renal transplantation. Am J Gastroenterol. 1983;78:328–31. [PubMed] [Google Scholar]
- 11.Milito G, Taccone-Gallucci M, Brancaleone C, et al. The gastrointestinal tract in uremic patients on long-term hemodialysis. Kidney Int Suppl. 1985;17:S157–60. [PubMed] [Google Scholar]
- 12.Musola R, Franzin G, Mora R, Manfrini C. Prevalence of gastroduodenal lesions in uremic patients undergoing dialysis and after renal transplantation. Gastrointest Endosc. 1984;30:343–6. doi: 10.1016/s0016-5107(84)72450-3. [DOI] [PubMed] [Google Scholar]
- 13.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–15. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 14.Rocha GA, Queiroz DM, Mendes EN, et al. Indirect immunofluorescence determination of the frequency of anti-H. pylori antibodies in Brazilian blood donors. Braz J Med Biol Res. 1992;25:683–9. [PubMed] [Google Scholar]
- 15.Perez-Perez GI, Taylor DN, Bodhidatta L, et al. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis. 1990;161:1237–1. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- 16.Offerhaus GJ, Kreuning J, Valentijn RM, et al. Campylobacter pylori: prevalence and significance in patients with chronic renal failure. Clin Nephrol. 1989;32:239–41. [PubMed] [Google Scholar]
- 17.Wee A, Kang JY, Ho MS, Choong HL, Wu AY, Sutherland IH. Gastroduodenal mucosa in uraemia: endoscopic and histological correlation and prevalence of Helicobacter-like organisms. Gut. 1990;31:1093–6. doi: 10.1136/gut.31.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport A, Shallcross TM, Crabtree JE, Davison AM, Will EJ, Heatley RV. Prevalence of Helicobacter pylori in patients with end-stage renal failure and renal transplant recipients. Nephron. 1991;59:597–601. doi: 10.1159/000186650. [DOI] [PubMed] [Google Scholar]
- 19.Loffeld RJ, Peltenburg HG, vd Oever H, Stobberingh E. Prevalence of Helicobacter pylori antibodies in patients on chronic intermittent haemodialysis. Nephron. 1991;59:250–3. doi: 10.1159/000186560. [DOI] [PubMed] [Google Scholar]
- 20.Jaspersen D, Fassbinder W, Heinkele P, et al. Significantly lower prevalence of Helicobacter pylori in uremic patients than in patients with normal renal function. J Gastroenterol. 1995;30:585–8. doi: 10.1007/BF02367783. [DOI] [PubMed] [Google Scholar]
- 21.De Vecchi AF, Quatrini M, Boni F, et al. Epidemiology of Helicobacter pylori in dialysis patients. Pent Dial Int. 1995;15:178–9. [PubMed] [Google Scholar]
- 22.Luzza F, Imeneo M, Maletta M, et al. Helicobacter pylori-specific IgG in chronic haemodialysis patients: relationship of hypergastrinaemia to positive serology. Nephrol Dial Transplant. 1996;11:120–4. [PubMed] [Google Scholar]
- 23.Seyrek N, Kocabas E, Hazar S, Paydas S, Aksaray N, Sagliker Y. Helicobacter pylori antibodies in patients on chronic hemodialysis. Nephron. 1996;72:725–6. doi: 10.1159/000188978. [DOI] [PubMed] [Google Scholar]
- 24.Krawczyk W, Gorna E, Suwala J, et al. Frequency of Helicobacter pylori infection in uremic hemodialyzed patients with antral gastritis. Nephron. 1996;74:621–2. doi: 10.1159/000189465. [DOI] [PubMed] [Google Scholar]
- 25.Abu Farsakh NA, Roweily E, Rababaa M, Butchoun R. Brief report: evaluation of the upper gastrointestinal tract in uraemic patients undergoing haemodialysis. Nephrol Dial Transplant. 1996;11:847–50. doi: 10.1093/oxfordjournals.ndt.a027411. [DOI] [PubMed] [Google Scholar]
- 26.Hruby Z, Myszka-Bijak K, Gosciniak G, et al. Helicobacter pylori in kidney allograft recipients: high prevalence of colonization and low incidence of active inflammatory lesions. Nephron. 1997;75:25–9. doi: 10.1159/000189495. [DOI] [PubMed] [Google Scholar]
- 27.Ozgur O, Boyacioglu S, Ozdogan M, Gur G, Telatar H, Haberal M. Helicobacter pylori infection in haemodialysis patients and renal transplant recipients. Nephrol Dial Transplant. 1997;12:289–91. doi: 10.1093/ndt/12.2.289. [DOI] [PubMed] [Google Scholar]
- 28.Munoz de Bustillo E, Sanchez Tomero JA, Sanz JC, et al. Eradication and follow-up of Helicobacter pylori infection in hemodialysis patients. Nephron. 1998;79:55–60. doi: 10.1159/000044992. [DOI] [PubMed] [Google Scholar]
- 29.Yildiz A, Besisik F, Akkaya V, et al. Helicobacter pylori antibodies in hemodialysis patients and renal transplant recipients. Clin Transplant. 1999;13:13–16. doi: 10.1034/j.1399-0012.1999.t01-1-130102.x. [DOI] [PubMed] [Google Scholar]
- 30.Fabrizi F, Martin P, Dixit V, et al. Epidemiology of Helicobacter pylori in chronic haemodialysis patients using the new RIBA H. pylori SIA. Nephrol Dial Transplant. 1999;14:1929–33. doi: 10.1093/ndt/14.8.1929. [DOI] [PubMed] [Google Scholar]
- 31.Araki H, Miyazaki R, Matsuda T, Gejyo F, Koni I. Significance of serum pepsinogens and their relationship to Helicobacter pylori infection and histological gastritis in dialysis patients. Nephrol Dial Transplant. 1999;14:2669–75. doi: 10.1093/ndt/14.11.2669. [DOI] [PubMed] [Google Scholar]
- 32.Gur G, Boyacioglu S, Gul C, et al. Impact of Helicobacter pylori infection on serum gastrin in haemodialysis patients. Nephrol Dial Transplant. 1999;14:2688–91. doi: 10.1093/ndt/14.11.2688. [DOI] [PubMed] [Google Scholar]
- 33.Boran M, Cetin S. Helicobacter pylori and Giardia lamblia infection in hemodialysis patients. Nephrol Dial Transplant. 1999;14:1803–4. doi: 10.1093/ndt/14.7.1803. [DOI] [PubMed] [Google Scholar]
- 34.Huang JJ, Huang CJ, Ruaan MK, Chen KW, Yen TS, Sheu BS. Diagnostic efficacy of (13)C-urea breath test for Helicobacter pylori infection in hemodialysis patients. Am J Kidney Dis. 2000;36:124–9. doi: 10.1053/ajkd.2000.8284. [DOI] [PubMed] [Google Scholar]
- 35.Wang YL, Sheu BS, Huang JJ, Yang HB. Noninvasive stool antigen assay can effectively screen Helicobacter pylori Infection and assess success of eradication therapy in hemodialysis patients. Am J Kidney Dis. 2001;38:98–103. doi: 10.1053/ajkd.2001.25200. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima F, Sakaguchi M, Amemoto K, et al. Helicobacter pylori in patients receiving long-term dialysis. Am J Nephrol. 2002;22:468–72. doi: 10.1159/000065278. [DOI] [PubMed] [Google Scholar]
- 37.Fabbian F, Catalano C, Bordin V, Balbi T, Di Landro D. Esophagogastroduodenoscopy in chronic hemodialysis patients: 2-year clinical experience in a renal unit. Clin Nephrol. 2002;58:54–9. doi: 10.5414/cnp58054. [DOI] [PubMed] [Google Scholar]
- 38.Tsukada K, Miyazaki T, Katoh H, et al. Seven-day triple therapy with omeprazole, amoxycillin and clarithromycin for Helicobacter pylori infection in haemodialysis patients. Scand J Gastroenterol. 2002;37:1265–8. doi: 10.1080/003655202761020524. [DOI] [PubMed] [Google Scholar]
- 39.Marsenic O, Peco-Antic A, Perisic V, Virijevic V, Kruscic D, Kostic M. Upper gastrointestinal lesions in children on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:2687–8. doi: 10.1093/ndt/gfg468. [DOI] [PubMed] [Google Scholar]
- 40.Lopez T, Quesada M, Almirall J, Sanfeliu I, Segura F, Calvet X. Usefulness of non-invasive tests for diagnosing Helicobacter pylori infection in patients undergoing dialysis for chronic renal failure. Helicobacter. 2004;9:674–80. doi: 10.1111/j.1083-4389.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima F, Sakaguchi M, Oka H, et al. Prevalence of Helicobacter pylori antibodies in long-term dialysis patients. Nephrology. 2004;9:73–6. doi: 10.1111/j.1440-1797.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 42.Al-Mueilo SH. Gastroduodenal lesions and Helicobacter pylori infection in hemodialysis patients. Saudi Med J. 2004;25:1010–14. [PubMed] [Google Scholar]
- 43.Trimarchi H, Forrester M, Schropp J, Pereyra H, Freixas EA. Low initial vitamin B12 levels in Helicobacter pylori—positive patients on chronic hemodialysis. Nephron Clin Pract. 2004;96:C28–32. doi: 10.1159/000075569. [DOI] [PubMed] [Google Scholar]
- 44.Blusiewicz K, Rydzewska G, Rydzewski A. Gastric juice ammonia and urea concentrations and their relation to gastric mucosa injury in patients maintained on chronic hemodialysis. Rocz Akad Med Bialymst. 2005;50:188–92. [PubMed] [Google Scholar]
- 45.Lentine KL, Parsonnet J, Taylor I, Wrone EM, Lafayette RA. Associations of serologic markers of infection and inflammation with vascular disease events and mortality in American dialysis patients. Clin Exp Nephrol. 2006;10:55–62. doi: 10.1007/s10157-005-0392-5. [DOI] [PubMed] [Google Scholar]
- 46.Itatsu T, Miwa H, Nagahara A, et al. Eradication of Helicobacter pylori in hemodialysis patients. Ren Fail. 2007;29:97–102. doi: 10.1080/08860220601039122. [DOI] [PubMed] [Google Scholar]
- 47.Gioe FP, Cudia B, Romano G, et al. Role and clinical importance of Helicobacter pylori infection in hemodialysis patients. G Chir. 2008;29:81–4. [PubMed] [Google Scholar]
- 48.Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96–103. doi: 10.1038/ki.2008.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoniou S, Dimitriadis A, Kliridou M, Pavlitou K, Batzili H, Malaka E. Prevalence of Helicobacter pylori antibodies in CAPD patients. Nephron. 1997;75:358–9. doi: 10.1159/000189563. [DOI] [PubMed] [Google Scholar]
- 50.Aguilera A, Codoceo R, Bajo MA, et al. Helicobacter pylori infection: a new cause of anorexia in peritoneal dialysis patients. Perit Dial Int. 2001;21(Suppl 3):S152–6. [PubMed] [Google Scholar]
- 51.Lui SL, Wong WM, Ng SY, Chan TM, Lai KN, Lo WK. Sero-prevalence of Helicobacter pylori in Chinese patients on continuous ambulatory peritoneal dialysis. Nephrology. 2005;10:21–4. doi: 10.1111/j.1440-1797.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 52.Altay M, Turgut F, Akay H, et al. Dyspepsia in Turkish patients on continuous ambulatory peritoneal dialysis. Int Urol Nephrol. 2008;40:211–17. doi: 10.1007/s11255-007-9324-5. [DOI] [PubMed] [Google Scholar]
- 53.Shousha S, Arnaout AH, Abbas SH, Parkins RA. Antral Helicobacter pylori in patients with chronic renal failure. J Clin Pathol. 1990;43:397–9. doi: 10.1136/jcp.43.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ala-Kaila K, Vaajalahti P, Karvonen AL, Kokki M. Gastric Helicobacter and upper gastrointestinal symptoms in chronic renal failure. Ann Med. 1991;23:403–6. doi: 10.3109/07853899109148082. [DOI] [PubMed] [Google Scholar]
- 55.Neithercut WD, Rowe PA, el Nujumi AM, Dahill S, McColl KE. Effect of Helicobacter pylori infection on intragastric urea and ammonium concentrations in patients with chronic renal failure. J Clin Pathol. 1993;46:544–7. doi: 10.1136/jcp.46.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moustafa FE, Khalil A, Abdel Wahab M, Sobh MA. Helicobacter pylori and uremic gastritis: a histopathologic study and a correlation with endoscopic and bacteriologic findings. Am J Nephrol. 1997;17:165–71. doi: 10.1159/000169092. [DOI] [PubMed] [Google Scholar]
- 57.Misra V, Misra SP, Dwivedi M, et al. Decreased sensitivity of the ultrarapid urease test for diagnosing Helicobacter pylori in patients with chronic renal failure. Pathology. 1999;31:44–6. doi: 10.1080/003130299105520. [DOI] [PubMed] [Google Scholar]
- 58.Emir S, Bereket G, Boyacioglu S, Varan B, Tunali H, Haberal M. Gastroduodenal lesions and Helicobacter pylori in children with end-stage renal disease. Pediatr Nephrol. 2000;14:837–40. doi: 10.1007/s004679900254. [DOI] [PubMed] [Google Scholar]
- 59.Schoonjans R, Van VB, Vandamme W, et al. Dyspepsia and gastroparesis in chronic renal failure: the role of Helicobacter pylori. Clin Nephrol. 2002;57:201–7. doi: 10.5414/cnp57201. [DOI] [PubMed] [Google Scholar]
- 60.Strid H, Simren M, Stotzer PO, Abrahamsson H, Bjomsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39:516–20. doi: 10.1080/00365520410004505. [DOI] [PubMed] [Google Scholar]
- 61.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura S, Sasaki O, Nakahama H, Inenaga T, Kawano Y. Clinical characteristics and survival in end-stage renal disease patients with arteriosclerosis obliterans. Am J Nephrol. 2002;22:422–8. doi: 10.1159/000065267. [DOI] [PubMed] [Google Scholar]
- 63.Jafarzadeh A, Rezayati MT, Nemati M. Specific serum immunoglobulin G to H pylori and CagA in healthy children and adults (south-east of Iran) World J Gastroenterol. 2007;13:3117–21. doi: 10.3748/wjg.v13.i22.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashemi MR, Rahnavardi M, Bikdeli B, Dehghani Zahedani M. H pylori infection among 1000 southern Iranian dyspeptic patients. World J Gastroenterol. 2006;12:5479–82. doi: 10.3748/wjg.v12.i34.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 66.Wesdorp RI, Falcao HA, Banks PB, Martino J, Fischer JE. Gastrin and gastric acid secretion in renal failure. Am J Surg. 1981;141:334–8. doi: 10.1016/0002-9610(81)90190-2. [DOI] [PubMed] [Google Scholar]
- 67.Gladziwa U, Haase G, Handt S, et al. Prevalence of Helicobacter pylori in patients with chronic renal failure. Nephrol Dial Transplant. 1993;8:301–6. [PubMed] [Google Scholar]
- 68.Ota K, Yamashita N, Suzuki T, Agishi T. Malignant tumours in dialysis patients: a nationwide survey. Proc Eur Dial Transplant Assoc. 1981;18:724–30. [PubMed] [Google Scholar]
- 69.Aydemir S, Boyacioglu S, Gur G, et al. Helicobacter pylori infection in hemodialysis patients: susceptibility to amoxicillin and clarithromycin. World J Gastroenterol. 2005;11:842–5. doi: 10.3748/wjg.v11.i6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamura H, Tokushima H, Murakawa M, et al. Eradication of Helicobacter pylori in patients with end-stage renal disease under dialysis treatment. Am J Kidney Dis. 1997;29:86–90. doi: 10.1016/s0272-6386(97)90012-3. [DOI] [PubMed] [Google Scholar]
- 71.Tokushima H, Tamura H, Murakawa M, et al. Eradication of Helicobacter pylori restores elevation of serum gastrin concentrations in patients with end-stage renal disease. Intern Med. 1998;37:435–9. doi: 10.2169/internalmedicine.37.435. [DOI] [PubMed] [Google Scholar]
- 72.Mak SK, Loo CK, Wong AM, et al. Efficacy of a 1-week course of proton-pump inhibitor-based triple therapy for eradicating Helicobacter pylori in patients with and without chronic renal failure. Am J Kidney Dis. 2002;40:576–81. doi: 10.1053/ajkd.2002.34916. [DOI] [PubMed] [Google Scholar]
- 73.Mak SK, Loo CK, Wong PN, et al. A retrospective study on efficacy of proton-pump inhibitor-based triple therapy for eradication of Helicobacter pylori in patients with chronic renal failure. Singapore Med J. 2003;44:74–8. [PubMed] [Google Scholar]
- 74.Sheu BS, Huang JJ, Yang HB, Huang AH, Wu JJ. The selection of triple therapy for Helicobacter pylori eradication in chronic renal insufficiency. Aliment Pharmacol Ther. 2003;17:1283–90. doi: 10.1046/j.1365-2036.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- 75.Tseng GY, Lin HJ, Fang CT, et al. Recurrence of peptic ulcer in uraemic and non-uraemic patients after Helicobacter pylori eradication: a 2-year study. Aliment Pharmacol Ther. 2007;26:925–33. doi: 10.1111/j.1365-2036.2007.03438.x. [DOI] [PubMed] [Google Scholar]
- 76.Arancibia A, Drouguett MT, Fuentes G, et al. Pharmacokinetics of amoxicillin in subjects with normal and impaired renal function. Int J Clin Pharmacol Ther Toxicol. 1982;20:447–53. [PubMed] [Google Scholar]
- 77.Jones DP, Gaber L, Nilsson GR, Brewer ED, Stapleton FB. Acute renal failure following amoxicillin overdose. Clin Pediatr. 1993;32:735–9. doi: 10.1177/000992289303201205. [DOI] [PubMed] [Google Scholar]
- 78.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 79.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 80.Take S, Mizuno M, Ishiki K, et al. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–42. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]