Abstract
Background
Osteolysis due to wear of UHMWPE limits the longevity of joint arthroplasty. Oxidative degradation of UHMWPE gamma-sterilized in air increases its wear while decreasing mechanical strength. Vitamin E stabilization of UHMWPE was proposed to improve oxidation resistance while maintaining wear resistance and fatigue strength.
Questions/purposes
We reviewed the preclinical research on the development and testing of vitamin E-stabilized UHMWPE with the following questions in mind: (1) What is the rationale behind protecting irradiated UHMWPE against oxidation by vitamin E? (2) What are the effects of vitamin E on the microstructure, tribologic, and mechanical properties of irradiated UHMWPE? (3) Is vitamin E expected to affect the periprosthetic tissue negatively?
Methods
We performed searches in PubMed, Scopus, and Science Citation Index to review the development of vitamin E-stabilized UHMWPEs and their feasibility as clinical implants.
Results
The rationale for using vitamin E in UHMWPE was twofold: improving oxidation resistance of irradiated UHMWPEs and fatigue strength of irradiated UHMWPEs with an alternative to postirradiation melting. Vitamin E-stabilized UHMWPE showed oxidation resistance superior to that of irradiated UHMWPEs with detectable residual free radicals. It showed equivalent wear and improved mechanical strength compared to irradiated and melted UHMWPE. The biocompatibility was confirmed by simulating elution, if any, of the antioxidant from implants.
Conclusions
Vitamin E-stabilized UHMWPE offers a joint arthroplasty technology with good mechanical, wear, and oxidation properties.
Clinical Relevance
Vitamin E-stabilized, irradiated UHMWPEs were recently introduced clinically. The rationale behind using vitamin E and in vitro tests comparing its performance to older materials are of great interest for improving longevity of joint arthroplasties.
Introduction
Reducing osteolysis has been the driving force behind the development of a new generation of UHMWPEs. The occurrence of osteolysis in cementless hip implants has led to its realization as a clinical problem associated mainly with UHMWPE implant wear particles [35].
Sterilization of UHMWPE with gamma radiation in air was common practice until the late 1990s, but it was proven to induce severe oxidation of the polymer. Oxidation and subsequent embrittlement of UHMWPE decrease the abrasive wear resistance and increase the wear debris associated with the polymer [9, 27, 28, 49]. Radiation crosslinking of UHMWPE reduces wear in vitro [48, 50, 51, 53] and in vivo [29, 47] but also induces free radicals, which can cause oxidation. Postirradiation melting renders crosslinked UHMWPE oxidation-resistant by allowing these residual free radicals trapped in the crystalline regions [8, 40] to recombine. However, the postirradiation melting step reduces the fatigue strength of irradiated UHMWPE [34], which is already decreased by crosslinking [58], due to a decrease in crystallinity that accompanies postirradiation melting.
To develop an oxidation-resistant UHMWPE and improve the fatigue properties of crosslinked UHMWPE by avoiding postirradiation melting, an alternative method is the stabilization of the radiation-induced free radicals by using the antioxidant vitamin E (α-tocopherol). The major physiologic role of vitamin E [64] is to react with free radicals in cell membranes and protect polyunsaturated fatty acids from degradation due to oxidation [14, 41, 76, 78, 80]. Oxidation reactions in polyethylene, which contains very long, mostly saturated aliphatic chains, are believed to follow a mechanism similar to that in lipids [1, 27].
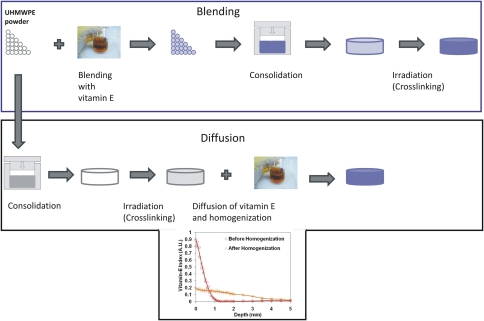
There are two methods of incorporating vitamin E into UHMWPE (Fig. 1). One is to blend vitamin E with UHMWPE powder before consolidation. Once consolidated, the blend can be crosslinked with the use of ionizing radiation. The presence of vitamin E in UHMWPE during irradiation protects the polymer from oxidation but reduces the efficiency of crosslinking [56, 57, 65] while the vitamin E itself is reacted; therefore, the vitamin E concentration and the subsequent radiation dose must be optimized to obtain a simultaneously wear- and oxidation-resistant UHMWPE. The alternative method is the diffusion of vitamin E into UHMWPE after radiation crosslinking [62, 63]. The crosslinking efficiency of UHMWPE is not adversely affected in this method since vitamin E is not present during irradiation. Therefore, the amount of vitamin E that can be incorporated into the material is not limited by concerns for crosslink density. On the other hand, the polymer remains unprotected against oxidation during irradiation and after storage until vitamin E is incorporated. Furthermore, a homogenization step is required after incorporation to obtain adequate antioxidant concentration throughout the implants.
Fig. 1.
A flowchart shows the processing steps for the incorporation of vitamin E into UHMWPE: blending versus diffusion.
We reviewed the vitamin E-stabilized UHMWPE development with the following questions in mind: (1) What is the rationale behind protecting irradiated UHMWPE against oxidation by vitamin E? (2) What are the effects of vitamin E on the microstructure, tribologic, and mechanical properties of irradiated UHMWPE? (3) Is vitamin E expected to affect the periprosthetic tissue negatively?
Search Strategy and Criteria
We performed parallel searches of PubMed, Science Citation Indices, and Scopus, the latter ones including also more specialized, not strictly biomedical, literature (ie, on polymer chemistry). A search of PubMed for “UHMWPE AND vitamin E” returned 29 papers; the same search yielded 32 results in Scopus and 73 results in Science Citation Indices. These three searches resulted in 73 unique articles. We excluded 34 articles focused on antioxidants other than vitamin E and forms of polyethylene not clinically relevant at this time (eg, high-pressure crystallized UHMWPE or polyethylene nanocomposites). A total of 39 references were included from this search.
To cover the function of vitamin E in biologic systems, we performed a search for “vitamin E AND function AND humans” and restricted our search to reviews. The search returned 132 results of which we included six. Our exclusion criteria were transport mechanisms, animal-centered articles, discussion on tocotrienols specifically, and overlap from authors from the same group.
To compare vitamin E-stabilized materials to previously available irradiated and melted UHMWPE materials without free radicals, a search for “UHMWPE AND crosslinking effects,” “UHMWPE AND irradiation AND melting,” “UHMWPE AND oxidation AND crosslinked,” and “UHMWPE AND free radicals AND trapped” were performed, returning a total of 183 results. We analyzed all abstracts and included 29 articles on the irradiation effects on crosslinking and postirradiation oxidation. We excluded 154 articles overlapping with other searches, repeat publications from same group, and those on oxidation effects during irradiation. Additionally, we added four book chapters, three patents, and one thesis we thought relevant to the topic or contained otherwise unpublished results. Also, there was one US Food and Drug Administration report and one ASTM standard included.
Thus, we read 143 full articles, 10 book chapters, and 25 patents and included 85 references. We describe concepts, methods, results, and statistical analyses from these references where appropriate.
The Rationale for Using the Antioxidant Vitamin E
The rationale for using vitamin E was twofold: improving oxidation resistance of irradiated UHMWPEs and improving the fatigue strength of irradiated UHMWPEs using an alternative to postirradiation melting [9, 11, 42, 44, 55, 62, 82].
Tocopherol compounds were proposed as stabilizers for polyolefins in the 1980s [31] but did not include orthopaedic implants. In 1994, Hoechst researchers (now Ticona, Kelsterbach, Germany) described consolidated forms of UHMWPE with antioxidants for orthopaedic implants [7]. To stabilize UHMWPE, Brach del Prever et al. [13] also described vitamin E-blended UHMWPE for orthopaedics. In 1998, a gamma-sterilized UHMWPE blended with 0.1% wt% α-tocopherol was developed by Sulzer (Winterthur, Switzerland) in collaboration with Lederer et al. [65, 82–85]. For reasons unknown to the authors, this UHMWPE (VITASUL®) was never released. In Japan, the research of Tomita et al. [71–73, 75, 77] resulted in a vitamin E-blended UHMWPE for total knee implants (Nakashima Medical Ltd, Okayama, Japan), which have been in clinical use in Japan since 2006 [71]. In 2007, ASTM published a standard specification for medical-grade UHMWPE blended with vitamin E [3] followed, in 2009, by commercialization by Ticona of the first vitamin E-containing UHMWPE resins for use in orthopaedics. Also in 2007, the first vitamin E-diffused, irradiated UHMWPE hip implant was clinically introduced in the United States (Biomet Inc, Warsaw, IN), followed by knee implants in 2008.
Under accelerated aging at elevated temperatures and/or in the presence of pure oxygen, vitamin E-stabilized, irradiated UHMWPE was oxidatively more stable than gamma-sterilized or high-dose irradiated UHMWPE [11, 55, 60, 62, 84]. Thus, in vitro studies corroborated the hypothesis that vitamin E would increase the oxidative stability of irradiated UHMWPEs.
Effects of Vitamin E on Microstructure, Tribologic, and Mechanical Properties of Irradiated UHMWPE
The mechanical and fatigue strength of vitamin E-stabilized, crosslinked UHMWPEs was improved compared to irradiated and melted UHMWPE (Table 1) [33, 62]. The strength of vitamin E-stabilized UHMWPE remained unchanged when accelerated aged, while that of gamma-sterilized UHMWPE deteriorated considerably [55, 59]. Vitamin E alone had no effect on the mechanical properties of UHMWPE [75].
Table 1.
Mechanical properties and fatigue strength of crosslinked UHMWPEs
| UHMWPE | UTS (MPa) | EAB (%) | WF (kJ/m2) | ∆Ki (MPam1/2) |
|---|---|---|---|---|
| Conventional (25 kGy) | 46 ± 2 | 376 ± 20 | 2237 ± 148 | 1.19 ± 0.04 |
| First-generation crosslinked (100 kGy + melting) | 39 ± 3 | 225 ± 9 | 1206 ± 67 | 0.54 ± 0.02 |
| Second-generation crosslinked (100 kGy + vitamin E diffusion) | 43 ± 2 | 256 ± 17 | 1240 ± 151 | 0.72 ± 0.02 |
Values are expressed as mean ± SD; UTS = ultimate tensile strength; EAB = elongation at break; WF = work to failure; ∆Ki = stress factor range at fatigue crack inception.
For virgin and vitamin E-doped UHMWPE with an initial radiation dose of 85 to 100 kGy and a terminal gamma sterilization [55, 62], the wear reduction in irradiated/vitamin E-diffused UHMWPE compared to conventional UHMWPE was comparable to that observed previously with irradiated and melted UHMWPE compared to conventional UHMWPE [50, 52]. A study comparing the wear of vitamin E-blended (0.3 wt%), unirradiated UHMWPE versus conventional UHMWPE in a knee simulator showed lower wear volume and different debris for the former [75], suggesting vitamin E alone may improve the wear/delamination resistance [77].
Effects of Vitamin E on Periprosthetic Tissue
Wolf et al. [83] have determined there were no cytotoxic or genotoxic effects of vitamin E from vitamin E-blended and gamma-sterilized UHMWPE containing 0.8 wt% vitamin E in vitro. To investigate the local toxicity of vitamin E, an emulsion (10 mg vitamin E) was injected into knees in a rabbit model. At 2 and 12 weeks, the synovial tissue had a normal appearance and there were no signs of inflammation or sterile pus [39].
Discussion
One approach of vitamin E stabilization of UHMWPE focuses on reducing the oxidation of gamma-sterilized UHMWPE, while the alternative introduces vitamin E into highly crosslinked UHMWPE, to improve its mechanical properties compared to clinically available irradiated and melted UHMWPE. We reviewed the vitamin E-stabilized UHMWPE development with the following questions in mind: (1) What is the rationale behind protecting irradiated UHMWPE against oxidation by vitamin E? (2) What are the effects of vitamin E on the microstructure, tribologic, and mechanical properties of irradiated UHMWPE? (3) Is vitamin E expected to affect the periprosthetic tissue negatively?
Our review is subject to a number of limitations. First, there are no published clinical studies on vitamin E-stabilized UHMWPE and we are limited to in vitro results. Second, our review is intended to be directed to a broad audience; we generally omitted discussions on polymer chemistry. Third, this compilation is related only to the use of vitamin E as a stabilizer of UHMWPE for biomedical implants. Fourth, we could not include recent developments in this rapidly evolving field, which are not yet fully published.
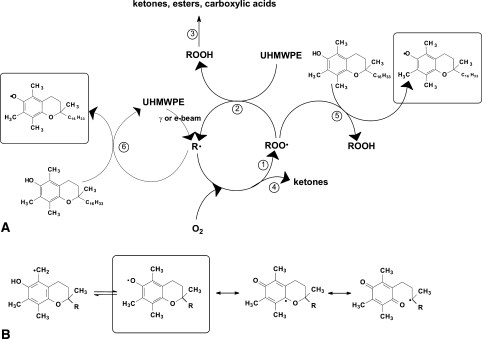
The main purpose of using vitamin E in UHMWPE is to prevent oxidative degradation. Due to radiolytic bond scission, free radicals are produced in irradiated UHMWPE [15, 18, 20, 30, 38], which react with oxygen and trigger the oxidation cascade [2, 16, 17, 20, 22, 25–27, 54, 69] (Fig. 2A, Reactions 1–4). Oxidation is accompanied by chain scissioning, deteriorating its mechanical properties [19, 24, 43]. The stabilization mechanism of α-tocopherol in UHMWPE has been studied [11, 20, 23, 85]. It is believed α-tocopherol can stabilize peroxy radicals formed by oxidation and can also directly react with alkyl macroradicals (Fig. 2A, Reactions 5 and 6) [11, 23]. The formed tocopheryl product (Fig. 2B) can in turn interact with another alkyl macroradical, furthering the stabilizing effect [11]. The oxidation cascade in irradiated polyethylene is therefore hindered, even in the presence of a small amount of vitamin E (0.05% wt%) [42, 44].
Fig. 2A–B.
A diagram illustrates the oxidation scheme of UHMWPE and the stabilizing mechanisms of vitamin E. (A) Due to radiolytic bond scission, free radicals are produced in irradiated UHMWPE, which react with oxygen and trigger the oxidation cascade (Reactions 1–4). Oxidation is accompanied by chain scissioning, deteriorating its mechanical properties. It is believed α-tocopherol can stabilize peroxy radicals formed by oxidation and can also directly react with alkyl macroradicals (Reactions 5 and 6). (B) The formed tocopheryl product can in turn interact with another alkyl macroradical, furthering the stabilizing effect.
When radiation crosslinking UHMWPE is blended with vitamin E, there is a decrease in crosslinking efficiency. Since 50 to 100 kGy of radiation is required for wear resistance of virgin UHMWPE, low concentrations of vitamin E would be recommended to minimize the adverse effect on crosslinking, but a sufficient amount of antioxidant is necessary for effective stabilization. Concentrations in the range of 0.05% to 0.1% wt%, along with a small/moderate increase in the radiation dose commonly used for crosslinking of unstabilized material [43, 56], can represent an optimum balance between stabilization and crosslinking efficiency. The main goal of diffusing vitamin E into radiation-crosslinked UHMWPE is to obtain enough vitamin E throughout implants to protect against long-term oxidation. A two-step diffusion process at elevated temperatures below the melting point was developed involving doping of UHMWPE with vitamin E with subsequent homogenization [63]. Vitamin E improves the oxidative resistance of irradiated UHMWPE in vitro using either method although there are differences in concentration and radiation exposure.
The fatigue strength increases with crystallinity and decreases with crosslinking [5, 34, 45, 79, 81]. While conventional UHMWPE has high crystallinity and fatigue strength in its unaged form, its fatigue resistance is severely deteriorated due to oxidation [58, 66]. Irradiated and melted UHMWPEs have low fatigue strength caused by crosslinking and the loss of crystallinity during melting [4, 46, 53, 62]. The fatigue strength loss due to postirradiation melting has been recovered by vitamin E stabilization due to the maintenance of crystallinity. The fatigue strength of a vitamin E-blended UHMWPE with an equivalent crosslink density to 100-kGy irradiated and melted virgin UHMWPE is improved approximately 30% [33, 58, 62]. Tensile mechanical and fatigue testing after accelerated aging of vitamin E-stabilized UHMWPE corroborated the lack of oxidative degradation of these properties [59].
It appears crosslink density is the major factor affecting the wear resistance of UHMWPE, and vitamin E incorporation did not detrimentally affect the wear resistance of crosslinked UHMWPE. Wear debris of conventional UHMWPE has been associated with osteolysis and implant loosening [6, 36, 74]. Currently, there is no consensus on the differences in the biologic activity of the wear debris from crosslinked UHMWPE compared to conventional UHMWPE. While the average particle size from highly crosslinked UHMWPEs appears smaller than that of conventional UHMWPE, the number of particles is also lower. The oxidative state of wear particles may also play a role in inciting an inflammatory response [10, 37, 67, 68]. Conventional UHMWPE has been associated with high oxidation not observed in irradiated and melted UHMWPE [12, 21]. The effect of vitamin E on the immunogenicity of UHMWPE wear particles is not yet fully investigated; however, it is widely accepted the oxidation level of the particles will be less than the particles from conventional UHMWPE.
One concern is the elution of vitamin E in vivo and its local/systemic effects. Vitamin E is abundant naturally [70], but the vitamin E commonly used for the stabilization of UHMWPE is produced synthetically (97% pure α-tocopherol). Both are safe for human consumption in prepared food [32], but their intra-articular effects are not known. Simulated manufacturing conditions showed no detectable elution from vitamin E-diffused UHMWPE [20, 59], but there was measurable elution in water at 40°C. The concentration profile became uniform at approximately 0.7 wt% at 3 years, suggesting saturation at this concentration at 40°C. These components did not show any detectable oxidation at 3 years. Also, similar samples extracted harshly to remove all detectable vitamin E [61] were exposed to accelerated aging and did not oxidize. This suggests, under clinically relevant conditions, the complete removal of vitamin E is unlikely and the components would be protected even under adverse conditions. Small animal studies [39] have also suggested the elution of vitamin E from the components, even under adverse conditions, is unlikely to cause substantial periprosthetic effects in vivo.
In conclusion, vitamin E-stabilized UHMWPEs are good alternative bearing surfaces. Vitamin E protected UHMWPE from oxidative degradation in vitro resulting in improved mechanical and wear properties. In vitro and animal studies did not register adverse biologic responses to vitamin E-stabilized UHMWPE. The clinical performance of these materials will best be determined by long-term prospective randomized clinical studies.
Acknowledgments
This review originates from the work on vitamin E-stabilized UHMWPEs performed by the authors in collaboration with numerous researchers. The authors thank their collaborators and especially their mentors, Drs. Luigi Costa and Orhun Muratoglu, respectively.
Footnotes
One or more of the authors or of the authors’ institutions have received funding from NIH Grant AR051142 (EO); Biomet, Inc, Warsaw, IN (EO); Bioster SpA, Seriate, Italy (PB); Lima Lto, San Daniele del Friuli, Italy (PB); Zimmer, Inc, Warsaw, IN (EO, PB); and Harris Orthopaedic Laboratory funds (EO).
This work was performed at Università di Torino and Massachusetts General Hospital.
References
- 1.Al-Malaika S. Autoxidation. In: Scott G, ed. Atmospheric Oxidation and Antioxidants. Volume I. Amsterdam, The Netherlands: Elsevier Science Publishers BV; 1993:45–82.
- 2.Assink R, Celina M, Dunbar T, Alam T, Clough R, Gillen K. Analysis of hydroperoxides in solid polyethylene by MAS 13C NMR and EPR. Macromolecules. 2000;33:4023–4029. doi: 10.1021/ma991970d. [DOI] [Google Scholar]
- 3.Standard Specification for Ultra-high Molecular Weight Polyethylene Powder Blended With alpha-Tocopherol (Vitamin E) and Fabricated Forms for Surgical Implant Applications. West Conshohocken, PA: ASTM International; 2007. [Google Scholar]
- 4.Baker DA, Bellare A, Pruitt L. The effect of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res A. 2003;66:146–154. doi: 10.1002/jbm.a.10606. [DOI] [PubMed] [Google Scholar]
- 5.Baker DA, Hastings RS, Pruitt L. Study of fatigue resistance of chemical and radiation crosslinked medical grade UHMWPE. J Biomed Mater Res. 1999;46:573–581. doi: 10.1002/(SICI)1097-4636(19990915)46:4<573::AID-JBM16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Bauer T, Schils J. The pathology of total joint arthroplasty. II. Mechanisms of implant failure. Skeletal Radiol. 1999;28:483–497. doi: 10.1007/s002560050552. [DOI] [PubMed] [Google Scholar]
- 7.Berzen J, Luketic D. Stabilised polyethylene moulding materials, for implants containing polyethylene with molecular wt. 105–107, antioxidant, and tocopherol, α-tocopherol or vitamin E, as stabiliser. European Patent. 1994;613:923. [Google Scholar]
- 8.Bhateja S, Duerst R, Aus E, Andrews E. Free radicals trapped in polyethylene crystals. J Macromol Sci Phys B. 1995;34:263–272. doi: 10.1080/00222349508215534. [DOI] [Google Scholar]
- 9.Blunn G, Brach del Preva EM, Costa L, Fisher J, Freeman MA. Ultra high molecular-weight polyethylene (UHMWPE) in total knee replacement: fabrication, sterilisation and wear. J Bone Joint Surg Br. 2002;84:946–949. doi: 10.1302/0301-620X.84B7.13647. [DOI] [PubMed] [Google Scholar]
- 10.Bosetti M, Zanardi L, Bracco P, Costa L, Cannas M. In vitro evaluation of the inflammatory activity of UHMWPE. Biomaterials. 2003;24:1419–1426. doi: 10.1016/S0142-9612(02)00526-4. [DOI] [PubMed] [Google Scholar]
- 11.Bracco P, Brunella V, Zanetti M, Luda MP, Costa L. Stabilisation of UHMWPE with vitamin E. Polym Degrad Stab. 2007;92:2155–2162. doi: 10.1016/j.polymdegradstab.2007.02.023. [DOI] [Google Scholar]
- 12.Brach del Prever EM, Bistolfi A, Costa L, Bracco P, Linari A, Botto Micca F, Crova M, Gallinaro P. The biological reaction to polyethylene wear debris can be related with oxidation of the UHMWPE cups. Chir Organi Mov. 2003;88:291–303. [PubMed] [Google Scholar]
- 13.Brach del Prever EM, Camino G, Costa L, Crova M, Dallera A, Gallinaro P. Impianto protesico contenente un componente di materiale plastico. Italian Patent 1271590. 1994.
- 14.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 15.Brunella V, Bracco P, Carpentieri I, Paganini MC, Zanetti M, Costa L. Lifetime of alkyl macroradicals in irradiated UHMWPE. Polym Degrad Stab. 2007;92:1498–1503. doi: 10.1016/j.polymdegradstab.2007.05.005. [DOI] [Google Scholar]
- 16.Carlsson D, Chmela S, Lacoste J. On the structures and yields of the first peroxyl radicals in γ-irradiated polyolefins. Macromolecules. 1990;23:4934–4938. doi: 10.1021/ma00225a009. [DOI] [Google Scholar]
- 17.Carlsson D, Dobbin C, Wiles D. Direct observations of macroperoxyl radical propagation and termination by electron spin resonance and infrared spectroscopies. Macromolecules. 1985;18:2092–2094. doi: 10.1021/ma00152a053. [DOI] [Google Scholar]
- 18.Charlesby A, Libby D, Ormerod M. Radiation damage in polyethylene as studied by electron spin resonance. Proc R Soc Lond A. 1961;262:207–218. doi: 10.1098/rspa.1961.0113. [DOI] [Google Scholar]
- 19.Collier J, Sutula L, Currier B, John H, Wooding R, Williams I, Farber K, Mayor M. Overview of polyethylene as a bearing material: comparison of sterilization methods. Clin Orthop Relat Res. 1996;333:76–86. doi: 10.1097/00003086-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Costa L, Bracco P. Mechanisms of crosslinking, oxidative degradation and stabilization of UHMWPE. In: Kurtz SM, editor. The UHMWPE Biomaterials Handbook. Burlington, MA: Elsevier Academic Press; 2009. pp. 309–323. [Google Scholar]
- 21.Costa L, Bracco P, Brach Del Prever EM. Physicochemical and mechanical properties of UHMWPE: 45 years’ experience. Interact Surg. 2007;2:169–173. doi: 10.1007/s11610-007-0052-4. [DOI] [Google Scholar]
- 22.Costa L, Carpentieri I, Bracco P. Post electron-beam irradiation oxidation of orthopaedic UHMWPE. Polym Degrad Stab. 2008;93:1695–1703. doi: 10.1016/j.polymdegradstab.2008.06.003. [DOI] [Google Scholar]
- 23.Costa L, Carpentieri I, Bracco P. Post electron-beam irradiation oxidation of orthopaedic UHMWPE stabilized with vitamin E. Polym Degrad Stab. 2009;94:1542–1547. doi: 10.1016/j.polymdegradstab.2009.04.023. [DOI] [Google Scholar]
- 24.Costa L, Jacobson K, Bracco P, Brach del Prever EM. Oxidation of orthopaedic UHMWPE. Biomaterials. 2002;23:1613–1624. doi: 10.1016/S0142-9612(01)00288-5. [DOI] [PubMed] [Google Scholar]
- 25.Costa L, Luda MP, Trossarelli L. Ultra-high molecular weight polyethylene. I. Mechano-oxidative degradation. Polym Degrad Stab. 1997;55:329–338. doi: 10.1016/S0141-3910(96)00170-X. [DOI] [Google Scholar]
- 26.Costa L, Luda MP, Trossarelli L. Ultra-high molecular weight polyethylene. II. Thermal- and photo-oxidation. Polym Degrad Stab. 1997;58:41–54. doi: 10.1016/S0141-3910(97)00010-4. [DOI] [Google Scholar]
- 27.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. Oxidation in orthopaedic UHMWPE sterilized by gamma radiation and ethylene oxide. Biomaterials. 1998;19:659–668. doi: 10.1016/S0142-9612(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 28.Costa L, Luda MP, Trossarelli L, Brach Del Prever EM, Crova M, Gallinaro P. In vivo UHMWPE biodegradation of retrieved prosthesis. Biomaterials. 1998;19:1371–1385. doi: 10.1016/S0142-9612(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 29.Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly crosslinked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis. Acta Orthop. 2007;78:746–754. doi: 10.1080/17453670710014518. [DOI] [PubMed] [Google Scholar]
- 30.Dole M. Free radicals in irradiated polyethylene. In: Dole M, editor. The Radiation Chemistry of Macromolecules. New York, NY: Academic Press; 1972. pp. 335–348. [Google Scholar]
- 31.Dolezel B, Adamirova L. Method of hygienically safe stabilization of polyolefines against thermoxidative and photooxidative degradation. Czechoslovakian Socialist Republic Patent. 1982;221:403. [Google Scholar]
- 32.Food and Drug Administration, Department of Health and Human Services. Code of Federal Regulations 184.1890: Direct Food Substances Affirmed as Generally Recognized as Safe–[alpha]-tocopherols. Title 21, Volume 3. Revised April 1, 2004.
- 33.Furmanski J. Mechanistic and Clinical Aspects of Fatigue of Ultrahigh Molecular Weight Polyethylene [dissertation] Berkeley, CA: University of California, Berkeley; 2008. [Google Scholar]
- 34.Gencur SJ, Rimnac CM, Kurtz SM. Fatigue crack propagation resistance of virgin and highly crosslinked thermally treated UHMWPE. Biomaterials. 2006;27:1550–1557. doi: 10.1016/j.biomaterials.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Harris W. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 36.Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng H. 2000;214:21–37. doi: 10.1243/0954411001535219. [DOI] [PubMed] [Google Scholar]
- 37.Ingram JH, Stone M, Fisher J, Ingham E. The influence of molecular weight, crosslinking and counterface roughness on TNF-alpha production by macrophages in response to UHMWPE particles. Biomaterials. 2004;25:3511–3522. doi: 10.1016/j.biomaterials.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 38.Jahan MS, King MC, Haggard WO, Sevo KL, Parr JE. A study of long-lived free radicals in gamma-irradiated medical grade polyethylene. Radiat Phys Chem. 2001;62:141–144. doi: 10.1016/S0969-806X(01)00431-5. [DOI] [Google Scholar]
- 39.Jarrett B, Cofske J, Rosenberg A, Oral E, Muratoglu O, Malchau H. In vivo biological response to vitamin E and vitamin E-doped polyethylene. J Bone Joint Surg Am. 2010;92:2672–2681. doi: 10.2106/JBJS.I.00068. [DOI] [PubMed] [Google Scholar]
- 40.Keller A, Ungar G. Radiation effects and crystallinity in polyethylene. J Phys Chem. 1983;22:155–181. [Google Scholar]
- 41.Kiyose C, Ueda T. Distribution and metabolism of tocopherols and tocotrienols in vivo. J Clin Biochem Nutr. 2004;35:47–52. doi: 10.3164/jcbn.35.47. [DOI] [Google Scholar]
- 42.Kurtz SM, Dumbleton J, Siskey RS, Wang A, Manley M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J Biomed Mater Res A. 2009;90:549–563. doi: 10.1002/jbm.a.32122. [DOI] [PubMed] [Google Scholar]
- 43.Kurtz SM, Hozack W, Marcolongo M, Turner J, Rimnac C, Edidin A. Degradation of mechanical properties of UHMWPE acetabular liners following long-term implantation. J Arthroplasty. 2003;18(7 Suppl 1):68–78. doi: 10.1016/S0883-5403(03)00292-4. [DOI] [PubMed] [Google Scholar]
- 44.Lerf R, Zurbrugg D, Delfosse D. Use of vitamin E to protect cross-linked UHMWPE from oxidation. Biomaterials. 2010;31:3643–3648. doi: 10.1016/j.biomaterials.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 45.Luisetto Y, Wesslen B, Maurer F, Lidgren L. The effect of irradiation, annealing temperature and artificial aging on oxidation, mechanical properties and fracture mechanisms of UHMWPE. J Biomed Mater Res A. 2003;67:908–917. doi: 10.1002/jbm.a.10090. [DOI] [PubMed] [Google Scholar]
- 46.Lyons B. Radiolytic unsaturation decay in polyethylene. Part II. The effect of irradiation temperature, thermal history and orientation. Radiat Phys Chem. 2004;69:503–510. doi: 10.1016/j.radphyschem.2003.10.005. [DOI] [Google Scholar]
- 47.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 Suppl 1):55–59. doi: 10.1016/S0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 48.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear resistant ultra-high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17:157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 49.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene: a hip-simulator study. J Bone Joint Surg Am. 2000;82:1708–1725. doi: 10.2106/00004623-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH. 1999 HAP Paul Award. A novel method of crosslinking UHMWPE to improve wear, reduce oxidation and retain mechanical properties. J Arthroplasty. 2001;16:149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 51.Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE) Biomaterials. 1999;20:1463–1470. doi: 10.1016/S0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 52.Muratoglu OK, Bragdon CR, O’Connor DO, Perinchief RS, Estok DM, Jasty M, Harris WH. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty. 2001;16(8 Suppl):24–30. doi: 10.1054/arth.2001.28376. [DOI] [PubMed] [Google Scholar]
- 53.Muratoglu OK, Merrill EW, Bragdon CR, O’Connor D, Hoeffel D, Burroughs B, Jasty M, Harris WH. Effect of radiation, heat, and aging on in vitro wear resistance of polyethylene. Clin Orthop Relat Res. 2003;417:253–262. doi: 10.1097/01.blo.0000093004.90435.d1. [DOI] [PubMed] [Google Scholar]
- 54.Ohnishi S, Sugimoto S, Nitta I. Electron spin resonance study of radiation oxidation of polymers. IIIA. Results for polyethylene and some general remarks. J Polym Sci A Poly Chem. 1963;1:605–623. [Google Scholar]
- 55.Oral E, Christensen S, Malhi A, Wannomae K, Muratoglu O. Wear resistance and mechanical properties of highly crosslinked UHMWPE doped with vitamin E. J Arthroplasty. 2006;21:580–591. doi: 10.1016/j.arth.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oral E, Godleski Beckos C, Malhi A, Muratoglu O. The effects of high dose irradiation on the cross-linking of vitamin E-blended UHMWPE. Biomaterials. 2008;29:3557–3560. doi: 10.1016/j.biomaterials.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oral E, Greenbaum ES, Malhi AS, Harris WH, Muratoglu OK. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials. 2005;26:6657–6663. doi: 10.1016/j.biomaterials.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oral E, Malhi A, Muratoglu O. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27:917–925. doi: 10.1016/j.biomaterials.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oral E, Malhi A, Wannomae K, Muratoglu O. 2006 HAP Paul Award. Highly cross-linked UHMWPE with improved fatigue resistance for total joint arthroplasty. J Arthroplasty. 2008;23:1037–1044. doi: 10.1016/j.arth.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oral E, Rowell SL, Muratoglu OK. The effect of α-tocopherol on the oxidation and free radical decay in irradiated UHMWPE. Biomaterials. 2006;27:5580–5587. doi: 10.1016/j.biomaterials.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oral E, Wannomae K, Rowell S, Muratoglu O. Migration stability of alpha-tocopherol in irradiated UHMWPE. Biomaterials. 2006;27:2434–2439. doi: 10.1016/j.biomaterials.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oral E, Wannomae KK, Hawkins NE, Harris WH, Muratoglu OK. α-Tocopherol doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials. 2004;25:5515–5522. doi: 10.1016/j.biomaterials.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 63.Oral E, Wannomae KK, Rowell SL, Muratoglu OK. Diffusion of vitamin E in ultra-high molecular weight polyethylene. Biomaterials. 2007;28:5225–5237. doi: 10.1016/j.biomaterials.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 65.Parth M, Aust N, Lederer K. Studies on the effect of electron beam radiation on the molecular structure of ultra-high molecular weight polyethylene under the influence of alpha-tocopherol with respect to its application in medical implants. J Mater Sci Mater Med. 2002;13:917–921. doi: 10.1023/A:1019892004830. [DOI] [PubMed] [Google Scholar]
- 66.Pruitt LA. Deformation, yielding, fracture and fatigue behavior of conventional and highly cross-linked ultra high molecular weight polyethylene. Biomaterials. 2005;26:905–915. doi: 10.1016/j.biomaterials.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 67.Reno F, Lombardi F, Cannas M. UHMWPE oxidation increases granulocyte activation: a role in tissue response after prosthesis implant. Biomaterials. 2003;24:2895–2900. doi: 10.1016/S0142-9612(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 68.Reno F, Sabbatini M, Cannas M. Surface oxidation of UHMWPE for orthopaedic use increases apoptosis and necrosis in human granulocytes. J Mater Sci Mater Med. 2003;14:241–245. doi: 10.1023/A:1022832707221. [DOI] [PubMed] [Google Scholar]
- 69.Seguchi T, Tamura N. Mechanism of decay of alkyl radicals in irradiated polyethylene on exposure to air as studied by electron spin resonance. J Phys Chem. 1973;77:40–44. doi: 10.1021/j100620a008. [DOI] [Google Scholar]
- 70.Sheppard AJ, Pennington JA, Weihrauch JL. Analysis and distribution of vitamin E in vegetable oils and foods. In: Packer L, Fuchs J, editors. Vitamin E in Health and Disease. New York, NY: Marcel Dekker, Inc; 1993. pp. 9–31. [Google Scholar]
- 71.Shibata N, Kurtz SM, Tomita N. Advances of mechanical performance and oxidation stability in ultrahigh molecular weight polyethylene for total joint replacement: highly crosslinked and alpha-tocopherol doped. J Biomech Sci Eng. 2006;1:107–123. doi: 10.1299/jbse.1.107. [DOI] [Google Scholar]
- 72.Shibata N, Tomita N. The anti-oxidative properties of alpha tocopherol in gamma-irradiated UHMWPE with respect to fatigue and oxidation resistance. Biomaterials. 2005;26:5755–5762. doi: 10.1016/j.biomaterials.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 73.Shibata N, Tomita N, Onmori N, Kato K, Ikeuchi K. Defect initiation at subsurface grain boundary as a precursor of delamination in ultrahigh molecular weight polyethylene. J Biomed Mater Res A. 2003;67:276–284. doi: 10.1002/jbm.a.10133. [DOI] [PubMed] [Google Scholar]
- 74.Sochart DH. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop Relat Res. 1999;363:135–150. doi: 10.1097/00003086-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 75.Teramura S, Sakoda H, Terao T, Endo MM, Fujiwara K, Tomita N. Reduction of wear volume from ultrahigh molecular weight polyethylene knee components by the addition of vitamin E. J Orthop Res. 2008;26:460–464. doi: 10.1002/jor.20514. [DOI] [PubMed] [Google Scholar]
- 76.Thomas MJ. Physiological aspects of low-density lipoprotein oxidation. Curr Opin Lipidol. 2000;11:297–301. doi: 10.1097/00041433-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 77.Tomita N, Kitakura T, Onmori N, Ikada Y, Aoyama E. Prevention of fatigue cracks in ultrahigh molecular weight polyethylene joint components by the addition of vitamin E. J Biomed Mater Res B. 1999;48:474–478. doi: 10.1002/(SICI)1097-4636(1999)48:4<474::AID-JBM11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 78.Traber MG, Sies H. Vitamin E in humans: demand and delivery. Ann Rev Nutr. 1996;16:321–347. doi: 10.1146/annurev.nu.16.070196.001541. [DOI] [PubMed] [Google Scholar]
- 79.Wang A, Zeng H, Yau SS, Essner A, Manely M, Dumbleton J. Wear, oxidation and mechanical properties of a sequentially irradiated and annealed UHMWPE in total joint replacement. J Phys D Appl Phys. 2006;39:3213–3219. doi: 10.1088/0022-3727/39/15/S12. [DOI] [Google Scholar]
- 80.Wang XY, Quinn PJ. The location and function of vitamin E in membranes. Mol Membr Biol. 2000;17:143–156. doi: 10.1080/09687680010000311. [DOI] [PubMed] [Google Scholar]
- 81.Wannomae KK, Christensen SD, Freiberg AA, Bhattacharyya S, Harris WH, Muratoglu OK. The effect of real-time aging on the oxidation and wear of highly crosslinked UHMWPE acetabular liners. Biomaterials. 2006;27:1980–1987. doi: 10.1016/j.biomaterials.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Wolf C, Krivec T, Blassnig J, Lederer K, Schneider W. Examination of the suitability of alpha-tocopherol as a stabilizer for ultra-high molecular weight polyethylene used for articulating surfaces in joint endoprostheses. J Mater Sci Mater Med. 2002;13:185–189. doi: 10.1023/A:1013834113967. [DOI] [PubMed] [Google Scholar]
- 83.Wolf C, Lederer K, Muller U. Tests of biocompatibility of α-tocopherol with respect to the use as a stabilizer in ultrahigh molecular weight polyethylene for articulating surfaces in joint endoprostheses. J Mater Sci Mater Med. 2002;13:701–705. doi: 10.1023/A:1015750112343. [DOI] [PubMed] [Google Scholar]
- 84.Wolf C, Macho C, Lederer K. Accelerated ageing experiments with crosslinked and conventional ultra-high molecular weight polyethylene (UHMW-PE) stabilised with alpha-tocopherol for total joint arthroplasty. J Mater Sci Mater Med. 2006;17:1333–1344. doi: 10.1007/s10856-006-0608-6. [DOI] [PubMed] [Google Scholar]
- 85.Wolf C, Maninger J, Lederer K, Fruhwirth-Smounig H, Gamse T, Marr R. Stabilisation of crosslinked ultra-high molecular weight polyethylene (UHMW-PE)-acetabular components with alpha-tocopherol. J Mater Sci Mater Med. 2006;17:1323–1331. doi: 10.1007/s10856-006-0607-7. [DOI] [PubMed] [Google Scholar]