Abstract
Background
The definition of bone quality is evolving particularly from the perspective of anabolic agents that can enhance not only bone mineral density but also bone microarchitecture, composition, morphology, amount of microdamage, and remodeling dynamics.
Questions/purposes
This review summarizes the molecular pathways and physiologic effects of current and potential anabolic drugs.
Methods
From a MEDLINE search (1996–2010), articles were identified by the search terms “bone quality” (1851 articles), “anabolic agent” (5044 articles), “PTH or parathyroid hormone” (32,229 articles), “strontium” or “strontium ranelate” (283 articles), “prostaglandin” (77,539 articles), and “statin” or “statins” (14,233 articles). The search strategy included combining each with the phrase “bone quality.” Another more limited search aimed at finding more novel potential agents.
Results
Parathyroid hormone is the only US Food and Drug Administration-approved bone anabolic agent in the United States and has been the most extensively studied in in vitro animal and human trials. Strontium ranelate is approved in Europe but has not undergone Food and Drug Administration trials in the United States. All the studies on prostaglandin agonists have used in vivo animal models and there are no human trials examining prostaglandin agonist effects. The advantages of statins include the long-established advantages and safety profile, but they are limited by their bioavailability in bone. Other potential pathways include proline-rich tyrosine kinase 2 (PYK2) and sclerostin (SOST) inhibition, among others.
Conclusions
The ongoing research to enhance the anabolic potential of current agents, identify new agents, and develop better delivery systems will greatly enhance the management of bone quality-related injuries and diseases in the future.
Introduction
Until recent decades, osteoporosis was defined as a reduced amount of qualitatively normal bone as opposed to osteomalacia, in which bone is qualitatively abnormal. Current technologies to measure bone mass and density at various skeletal sites are designed to aid in the diagnosis of osteoporosis [64] and the assessment of treatment efficacy. Drugs currently available to treat osteoporosis may increase bone mass (ie, the quantity of bone), limit bone loss, and reduce fracture risk. However, various inconsistencies between the extent of increase in bone mass and the extent of reduction in fracture risk with these treatments have led in part to a need to understand the concept of bone quality.
The exact definition of bone quality remains elusive. Perhaps the most structured definition to date is “the characteristics of bone tissue that, in addition to density, contribute to bone strength” [13]. These characteristics include composition, morphology, and architecture, amount of microdamage, and remodeling dynamics. Although the emergence of the concept of bone quality is rooted in the study of osteoporosis, bone quality is also relevant to any instance in which the mechanical integrity of bone tissue is important. As such, this article considers promising anabolic agents for the treatment of bone fragility and the effects of these agents on bone fracture healing.
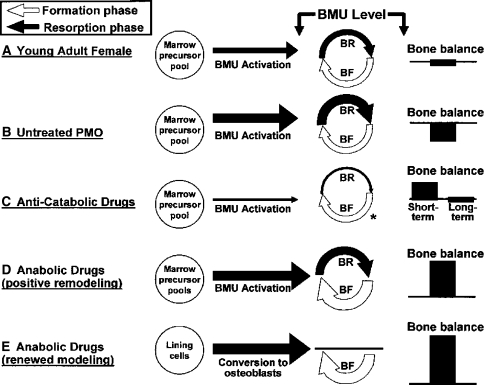
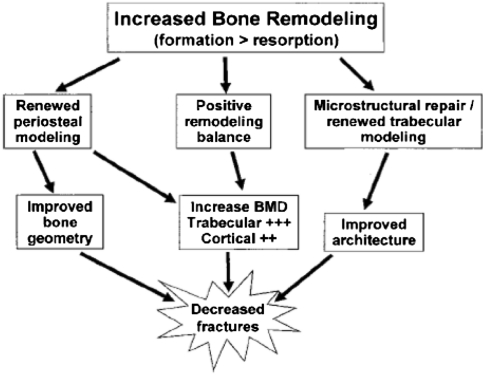
An ideal anabolic drug to treat bone fragility would improve bone strength, defined as the maximum amount of stress (force/area) the bone can withstand, and toughness, defined as the total amount of energy absorbed by the bone before complete failure occurs, thereby reducing fracture risk. For the purpose of this review, an anabolic agent is defined as an agent that increases bone strength by increasing bone mass as a result of an overall increase in bone remodeling (more bone multicellular units [BMUs] are formed) combined with a positive bone balance (the amount of bone formation at a remodeling site is, on average, greater than the amount resorbed) (Fig. 1). Some anabolic drugs may also induce “renewed modeling,” which is a process normally present only during skeletal growth and in which the formation phase of the BMU is not preceded by a resorption phase. Anabolic agents can also increase periosteal apposition and enhance repair of trabecular microstructure; however, these are not required properties (Fig. 2) [70]. Clinically, an increase in bone strength caused by a given anabolic agent can presently be inferred only by an observed reduction in fracture risk.
Fig. 1.
Different drugs and conditions can affect multiple components of bone remodeling. BR = bone resorption; BF = bone formation PMO = postmenopausal osteoporosis. Reprinted with permission of John Wiley & Sons, Inc, and copyright (2005, American Society for Bone and Mineral Research) from Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–184.
Fig. 2.
Treatment with anabolic drugs can increase bone strength and decrease fracture incidence. Reprinted with permission of John Wiley & Sons, Inc, and copyright (2005, American Society for Bone and Mineral Research) from Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–184.
The purposes of this review addressed under each selected drug subheading are to (1) review the bone physiologic effects of the most common anabolic agents either currently on the market or in development in vitro, (2) summarize the molecular pathways and mechanisms of action of these agents, (3) portray the applicability and limitations of these agents and potential methods to make these anabolic agents more efficacious, and (4) identify potential target pathways in future drug trials.
Search Strategy and Criteria
From a MEDLINE search covering the period 1996 to 2010, articles were identified by the search terms “bone quality” (1851 articles), “anabolic agent” (5044 articles), “PTH or parathyroid hormone” (32,229 articles), “strontium” or “strontium ranelate” (283 articles), “prostaglandin” (77,539 articles), and “statin” or “statins” (14,233 articles). The second step of the search strategy included combining each of our initial search terms (by use of “AND”) with the phrase “bone quality,” yielding 476, 66, 12, and two hits for anabolic agents, PTH, strontium ranelate/strontium, and prostaglandin, respectively. We then expanded our search for “prostaglandin” by excluding “quality” from “bone quality” and retrieved 532 articles, which were then limited to only those articles on prostaglandin agonists. We similarly expanded our search for “statins” and obtained 64 hits. When needed, we went back to the original older landmark articles. A few general review articles on bone quality and anabolic agents published in the last 5 years in what we considered high-impact journals were also reviewed to broaden our understanding and definition of bone quality and to have an overview of the most promising agents discussed in the literature. All the articles from our second search were reviewed, with emphasis on those concerning anabolic capacity in vitro, mechanisms of action, and clinical trials in osteoporosis and fracture risk reduction. Finally, a literature search on Google Scholar for the potential effects of proline-rich tyrosine kinase 2 (PYK2), Dickkopf-1 (DKK1), sclerostin (SOST), IGF-I, growth hormone, and hydroxyfasudil on bone quality was performed to identify additional relevant articles selected to be included in our review.
Parathyroid Hormone
Parathyroid hormone (PTH) is a key regulator of calcium and phosphate metabolism that acts by enhancing gastrointestinal calcium absorption and by increasing calcium reabsorption from the kidneys both directly and indirectly through synthesis of 1,25-dihydroxyvitamin D synthesis. Thus, PTH has a conservation effect on plasma calcium balance. In bone, PTH stimulates the release of calcium and phosphate particularly in response to decrements in extracellular calcium.
The molecular mechanism of action of PTH is through the G-protein-coupled PTH/PTH-related protein receptor [40]. PTH stimulates the production of receptor activator of NF-κ-B ligand and macrophage-specific colony-stimulating factor [24]. PTH also increases the number of osteoprogenitor cells [6] by inhibiting apoptosis of preosteoblasts [55], increasing their proliferation, and transforming bone lining cells into active osteoblasts [23]. Thus, the effect of PTH can be anabolic or catabolic depending on the dose, mode of administration, and specific bone site. For example, although chronic administration of PTH leads to increased osteoclastic cell number and activity [61], intermittent administration increases trabecular bone formation [21, 26]. The increase in bone formation seen with intermittent administration likely results from the combination of increased osteoblast function [34] and inhibition of apoptosis that extends the osteoblast’s lifespan [39].
With respect to bone quality, PTH has garnered attention as a result of its effects on bone microarchitecture and mineralization. Teriparatide (PTH(1–34)) is currently the only US Food and Drug Administration (FDA)-approved anabolic agent for the treatment of osteoporosis. In a clinical trial in postmenopausal women, intermittent treatment with PTH(1–34) caused a dose-related increase in spinal bone mineral density (BMD) and a dose-independent decrease in the incidence of new vertebral and nonvertebral fractures [63]. This apparent disassociation between spinal BMD and fracture risk may be the result of effects of PTH(1–34) on microarchitecture [63] or may also be explained by the fact that the lower dose yielded a threshold BMD sufficient to prevent fractures. Recent comparisons of teriparatide and the bisphosphonates alendronate and raloxifene indicate teriparatide is more effective than these two antiresorptive therapies at increasing BMD [71] and reducing vertebral fracture risk [14, 71]. In addition, analysis of bone biopsies from postmenopausal women treated with teriparatide either after previous alendronate treatment or with no prior treatment has indicated teriparatide reduces the amount of microdamage accumulation [22]. As a result of the increase in activation frequency, PTH(1–34) increases bone turnover substantially [17, 44], effectively reducing the mean tissue age, decreasing tissue mineralization, and increasing cortical bone porosity. Lowering mineralization and increased porosity can weaken the bone tissue; however, most of the increase in porosity occurs at the endosteal surface, which is subjected to lower bending stresses than the periosteal surface. This favorable distribution of porosity after PTH(1–34) treatment and the fact that PTH(1–34) increased cortical thickness likely explain the increased flexural stiffness observed with PTH(1–34) therapy [17]. The increase in bone turnover also allows for improvements in bone microarchitecture: treatment with PTH(1–34) increases trabecular connectivity, decreases trabecular separation, and decreases structure model index (resulting in a more platelike than rodlike structure) [38]. These beneficial effects on microarchitecture may also serve to compensate for increases in cortical porosity [73].
Intermittent treatment with PTH also enhances fracture healing. Daily, systemic treatment with PTH(1–34) at doses ranging from 5 to 30 μg/kg per day increases callus formation, and doses of PTH(1–34) ranging from 10 to 200 μg/kg per day also result in increased callus stiffness and strength [2, 3, 36, 45, 62]. Results from human data on distal radial fractures, although less definitive than those of the prior animal studies, suggest PTH(1–34) at the dose normally used for osteoporosis treatment (20 μg) may shorten the time to fracture union [5]. Studies of the mechanisms by which PTH enhances healing indicate PTH stimulates proliferation and differentiation of osteoprogenitor cells, increases synthesis of bone matrix proteins, and enhances osteoclastogenesis during the remodeling phase of repair [62]. A recent study by Kakar et al. [41] indicated PTH primarily enhanced the earliest stages of endochondral bone repair by increasing chondrocyte recruitment and rate of differentiation. In coordination, results from these experiments also showed the anabolic effect of PTH is partly mediated by an increased level of canonical Wnt signaling [41].
The main therapeutic limitation to PTH currently is that its use is restricted to 2 years because of a black box warning that arose from indications of an increased risk of osteosarcoma in rats given systemic exposures to teriparatide ranging from 3 to 60 times the exposure in humans given a 20-μg dose [74, 76]. Other metabolites of PTH are anabolic to the skeleton. These include the full-length molecule PTH(1–84) [35] and the truncated portion of the active form PTH(1–31). Preclinical and clinical reports have confirmed their abilities to increase bone formation [35, 79].
Strontium Ranelate
Strontium ranelate (SR) is an orally active agent consisting of two atoms of stable strontium and an organic moiety (ranelic acid). Treatment with SR results in replacement of some of the calcium in bone with strontium; hence, much of the increase in BMD is purely a result of the presence of strontium in the tissue. This anabolic artifact effect is due to the attenuation effect of strontium on the xray beams of dual-energy xray absorptiometry scans. Furthermore, some have attempted correction of this effect by evaluating the actual percentage of presence of strontium in iliac crest bone biopsies in animal models [12]. Evidence to date suggests SR is in a unique class of drug as a result of its anabolic effect on bone through osteoblast modulation and an antiresorptive effect through osteoclastic inhibition [9, 19, 56]. In ovariectomized (OVX) rats, SR prevented bone loss and microarchitectural degradation and resulted in an increase in mineralizing surface and in intrinsic and extrinsic measures of mechanical competence [7]. However, these beneficial effects may be restricted to higher doses and may be contingent on adequate dietary intake of calcium (Bain 09, Fuchs 09). In normal monkeys, who have active bone remodeling similar to that in humans, SR was found to decrease bone resorption in alveolar bone and increase the extent of mineralizing surfaces [16].
In a large, Phase 3 clinical trial in osteoporotic women, SR (2 g/day) reduced vertebral fractures by 41% and increased BMD in the lumbar spine and femoral neck by 14.4% and 8.3%, respectively, after 3 years of treatment [59]. Serum markers of bone formation (alkaline phosphatase) were higher and those of bone resorption (C-telopeptide) were lower in the treated group as compared with placebo. This divergence between the formation and resorption markers is in contrast to the effects seen for PTH where both formation and resorption markers are increased and for bisphosphonates where both markers are decreased. Other studies in postmenopausal women show relative risk reductions of 16% for all nonvertebral fractures and 19% for major fragility fractures (hip, wrist, pelvis and sacrum, ribs and sternum, clavicle, humerus) [69]. Recent results from 4- and 5-year studies show similar reductions in fracture risk with no increase in adverse events [58, 69].
Analysis of transiliac bone biopsies by histomorphometry and micro-CT suggests SR has a positive effect on bone quality [4]. The biopsies were obtained from postmenopausal women after 1 to 5 years of SR (2 g/day) or placebo and showed a higher mineral apposition rate and improved microarchitecture in the trabecular bone in the treated group. Specifically, the treated group exhibited increased trabecular number (+14%) and decreased structure model index (−22%) and trabecular separation (−16%). Cortical thickness was also increased (+18%) with treatment.
Few data are available on the effects of SR on fracture healing. A recent study comparing SR and PTH(1-34) in their effects on healing of fractures in OVX rats found SR but not PTH restored the work-to-fracture to the level of sham-operated controls [33]. The SR- and PTH-treated groups had equivalent bone volumes in the callus, which, together with the mechanical test results, suggests SR treatment may enhance bone quality in the callus.
Strontium ranelate is approved and marketed in Europe as Protelos® (Les Laboratories Servier, Gidy, France) but has not undergone FDA clinical trials in the United States for the treatment of postmenopausal osteoporosis. FDA approval seems to be the only impediment at this point in time since its efficacy in reducing fracture risk and increasing BMD in postmenopausal women has been established in multiple trials. It is administered orally and has few side effects, although it has been associated with a slight increase in venous thrombosis of the legs and a low incidence of diarrhea as side effects [18, 59].
Prostaglandin Agonists
Prostaglandins belong to a family of unsaturated long-chain fatty acids. They are synthesized as a byproduct of the arachidonic acid pathway by the action of cyclooxygenase enzymes 1 and 2. They are known to have profound osteogenic effects when implanted locally into different bone sites [48], subcutaneously injected [42], or systemically infused [77].
It is well established prostaglandin E2 (PGE2) stimulates both bone resorption and bone formation but favors bone formation, thus increasing bone mass and bone strength [37, 43, 53, 82]. However, a major limitation of PGE2 use in humans is its diffuse systemic distribution. Its variable and extended side effect profile includes diarrhea, lethargy, and flushing [49].
Although it is not clear which receptor subtypes are associated with the anabolic effect of PGE2, multiple in vitro and in vivo animal studies suggest EP2 and EP4 are the most promising [1, 54, 68, 78]. CP-533,536, a nonprostanoid PGE2 E2 receptor agonist, was found to stimulate new bone formation on trabecular, endocortical, and periosteal surfaces in intact rat bones and to increase callus size, density, and strength after fracture [48]. When administered subcutaneously, CP432, a PGE2 E4 receptor agonist, was found to stimulate bone formation through increased osteoblast recruitment and activity on periosteal, endocortical, and trabecular surfaces in OVX rats [42]. These anabolic effects of CP432 were found on both smooth and scalloped endocortical and trabecular surfaces, indicating both bone modeling- and remodeling-dependent bone formation were activated.
Statins
Statins inhibit cholesterol synthesis by reducing 3-hydroxy-3-glutaryl-coenzyme A (HMG-CoA) reductase activity, which is required for the conversion of HMG-CoA to mevalonate. Inhibition of the mevalonate pathway will ultimately result in the inhibition of protein prenylation because of the depletion of farnesyl diphosphate or geranyl diphosphate [83]. The proposed mechanism by which statins stimulate bone formation involves an increase in expression and synthesis of BMP-2 [60, 65] and osteocalcin [65].
Lovastatin was first identified as a possible bone anabolic agent when more than 30,000 compounds were screened for their ability to increase BMP-2 through the inhibition of HMG-CoA reductase activity [60]. Simvastatin increased the compressive strength of rat vertebral bodies after 3 months of treatment [66].
In laboratory studies of the effects of statins on fracture healing, enhancement of healing has been observed with local delivery of the drug to the fracture site [27], transdermal delivery at low doses (0.1 mg/kg [31]), and oral administration at high doses (120 mg/kg) [72, 75]. Collectively, these experiments on fracture repair suggest the in vivo effects of simvastatin on bone are a local phenomenon not related to the established cholesterol-lowering effect and a local delivery system can be as effective as systemic treatment in promoting fracture healing. One major hurdle, however, in the usage of statins would be to devise a suitable delivery system to localize and sustain release in fracture sites and to overcome the liver first-pass effect. Possibilities include copolymerization with ethylene glycol that covalently incorporates into hydrogel networks [11] or transdermal application, which bypasses the first-pass liver effect [32].
Clinical data regarding the effects of statins on BMD and fracture risk also present a mixed picture but do suggest the anabolic potential of these drugs. Statin use in postmenopausal women has been associated with increases (7%–8%) in BMD at the spine and hip, even after controlling for hormone replacement therapy [25]. In a meta-analysis of four prospective cohorts, statins reduced hip fracture risk (odds ratio, 0.43 confidence interval, 0.25–0.75) and, to a lesser extent, nonspine fracture risk (odds ratio, 0.69; confidence interval, 0.55–0.88) [10]. However, meta-analysis of two placebo-controlled clinical trials did not show any benefit [10]. Controlled trials specifically designed to test the effect of the more potent statins cerivastatin and atorvastatin on skeletal metabolism and fracture risk are lacking.
Statins have excellent safety and side effect profiles, and their potential as a therapeutic group is enhanced by the fact that osteoporosis and atherosclerosis affect similar age groups.
Other Potential Anabolic Agents
IGF-I mediates the effects of growth hormone (GH) on longitudinal bone growth [28]. The effect of recombinant human GH on BMD in adult-onset GH deficiency is variable and depends on the duration of the treatment and the time between diagnosis and the start of treatment [30], although the beneficial effects tend to be delayed for 6 months after treatment [20].
Cross-sectional studies have suggested recombinant human GH treatment reduces the risk of vertebral and nonvertebral fractures in GH deficiency [57, 80], but the results are more equivocal for the general population [29] and inconsistent in postmenopausal osteoporosis [18]. The use of GH for the treatment of osteoporosis also is likely to be limited by side effects such as weight gain, carpal tunnel syndrome, glucose intolerance, and edema.
Administration of IGF-I in healthy persons and in patients affected by GH deficiency or IGF-I deficiency causes an anabolic response by increasing bone remodeling [46]. Recombinant human IGF-I is used currently for the treatment of short stature caused by mutations of the GH receptor or the IGF1 gene. However, the long-term efficacy and safety of IGF-I for the treatment of osteoporosis, including the osteoporosis associated with anorexia nervosa, remain to be determined. Potential side effects and the lack of bone tissue specificity are concerns with respect to the long-term administration of IGF-I.
Inhibition of the activity of proline-rich tyrosine kinase 2 (PYK2), a nonreceptor tyrosine kinase related to focal adhesion kinase, may be a novel anabolic therapy. PYK2-null mice were found to exhibit a high bone mass phenotype with marked increases in trabecular number, trabecular thickness, and bone volume fraction [15]. Dynamic histomorphometric data indicated these increases in bone mass and bone quality were a result of increased bone formation rather than reduced bone resorption. Consistent with these data, marrow cultures from the PYK2-null mice showed enhanced osteogenesis. Treatment of OVX rats with PF-431386, a PYK2 inhibitor, counteracted the OVX-induced bone loss by elevating the bone formation rate, resulting in increased mineralizing surface and mineral apposition rate.
Sclerostin (SOST) inhibits osteoblastogenesis and bone formation in vivo by binding to Wnt coreceptors LRP5/6 [51]. Sclerostin is almost exclusively expressed in osteocytes and regulates osteoblastic function [67]. Mutations in SOST cause skeletal dysplasias characterized by increased bone mass, sclerosteosis, and van Buchem’s syndrome [8]. It follows from these studies that antagonism of sclerostin by monoclonal antibodies might be associated with anabolic effects on bone. Indeed, sclerostin antagonists increase bone mass in rodents and nonhuman primates [50]. These observations, if confirmed by definitive studies in patients, might have tremendous clinical applicability. Moreover, recent data suggest, not only is antagonism of SOST anabolic, but any bone gained by the lack of SOST function is not lost by disuse [52]. This sustained effect after withdrawal has not been noted with other anabolic agents such as PTH. Dickkopf-1 (DKK1), expressed solely by osteoblasts and osteocytes, prevents Wnt signaling by interacting with Wnt coreceptors [18]. DKK1 expression restricted to osteoblasts induced severe osteopenia with a severe reduction in bone mass resulting from a 49% reduction in osteoblast numbers and a 45% reduction in serum osteocalcin concentration [47]. Systemic administration of antagonists to DKK1 or SOST may possibly affect only the skeleton, favoring endogenous Wnt signaling and increasing bone formation without affecting Wnt signaling in other organs.
Another very novel therapeutic pathway includes targeting serotonin production by the duodenum. Yadav et al. [81] showed decreasing serotonin blood levels normalizes bone mass in LRP5-deficient mice, and gut- but not osteoblast-specific LRP5 inactivation decreases bone formation. Serotonin acts on osteoblasts through two receptors to inhibit their proliferation [81].
Hydroxyfasudil, a specific inhibitor of ρ-kinase, increases BMP-2 and osteocalcin expression. These mRNA levels are strongly suppressed by dexamethasone but restored by cotreatment with hydroxyfasudil. Therefore, a ρ-kinase inhibitor such as pitavastatin may be a new therapeutic reagent for the treatment of osteoporosis in glucocorticoid-induced osteoporosis [65].
Discussion
In this review, we have attempted to shed light on the efficacy and mechanisms of action of multiple anabolic agents as they relate the emerging definition of bone quality to include measures of bone composition, morphology, and architecture, amount of microdamage, and remodeling dynamic. With this definition as a framework, we have also focused on the potential of various anabolic agents for treatment of osteoporosis by defining the different ways in which anabolic agents can augment bone quantity and strength, such as by increasing bone remodeling combined with a positive bone balance, by inducing “renewed modeling,” increasing periosteal apposition, and enhancing the trabecular microstructure. As such, this review examined the physiologic effects of the most common anabolic agents currently on the market and in the process of development, summarized the molecular pathways/mechanism of action, presented the limitations of these agents, and highlighted potential target pathways in future drug trials. These aims were considered individually in the subsections above, which focused on PTH, strontium, statins, prostaglandin agonists, and other less studied novel agents.
PTH is the only FDA-approved bone anabolic agent in the United States and has been the most extensively studied in in vitro animal and human trials for both osteoporosis and fracture healing models. The main therapeutic limitation to PTH currently is that its use is restricted to 2 years because of a black box warning arising from indications of an increased risk of osteosarcoma in rats given systemic exposures to teriparatide [74, 76]. Long-term followup studies to assess for the long-term osteosarcoma risks in humans are lacking. SR is in a unique class of drug with dual beneficial effects: anabolic and antiresorptive actions on osteoclasts [19, 56]. Treatment with SR results in replacement of some of the calcium in bone with strontium; thus, future reports should develop accurate methods to control for this confounding effect on BMD. SR is approved and marketed in Europe but has not undergone FDA clinical trials in the United States for the treatment of postmenopausal osteoporosis. All the studies on prostaglandin agonists have used in vivo animal models and there are no human trials examining prostaglandin agonist effects in humans. Further animal studies are needed to elucidate the exact receptors and molecular mechanism of action in anabolic bone formation before early human trials can be initiated.
Although many postmenopausal human trials have shown an improvement in the BMD and a fracture risk reduction in a meta-analysis review [25], others have failed to show a benefit [10]. Controlled trials specifically designed to test the effect of the more potent statins cerivastatin and atorvastatin on skeletal metabolism and fracture risk are lacking. The greatest advantages of statins include the long-established advantages and applications in coronary artery disease and hypercholesterolemia, diseases prevalent in older patients with osteoporosis.
Other pathways that have not been translated into clinical human trials yet but might be important clinically include inhibition of the activity of proline-rich tyrosine kinase 2 (PYK2) and sclerostin (SOST) inhibitors whereby bone gained by the lack of SOST function is not lost by disuse [52]. Also, the Dickkopf-1 (DKK1) molecule, expressed solely by osteoblasts and osteocytes, offers an ideal target that affects only the skeleton. Another novel therapeutic pathway includes targeting gut-specific serotonin production, which was found to inhibit osteoblastic proliferation [81] and widens the field of bone biology to include in-depth studies of other organ systems with endocrine capabilities, such as the brain, gastrointestinal tract, kidneys, and liver, to unravel new pathways and functions in regulating bone homeostasis.
This review focused on bone quality as related to osteoporosis and fracture healing and did not address other disease states as rheumatoid arthritis, osteogenesis imperfecta, osteomalacia, and Paget’s disease, among others. Review of the role of anabolic agents in management of these other diseases was beyond the scope of this article and deserves a separate review. Furthermore, the frequency, route of administration, and recommended dosages of the anabolic agents discussed here were not addressed in detail. Finally, this review emphasized summarizing the results of preclinical and clinical studies, when available, over detailed discussion of the signaling interactions among osteoblasts, osteocytes, and osteoclasts.
For the anabolic agents reviewed in this article, the bulk of the data gathered to date relates to the effects of the therapy in question on BMD, bone strength, and fracture risk. However, with the concept of bone quality continuing to gain in prominence, future studies should intensify the focus on whether the therapy can enhance bone strength in manners both dependent and independent of BMD. For example, examining the effect of any of these agents on the organic component of bone tissue and on the structure of bone at length scales ranging from nanoscale to macroscale will add substantially to our understanding of the potential of anabolic mechanisms to augment bone quality in the mature and aging skeleton. Furthermore, causality from an increase in BMUs leading to an increase in BMD cannot be established with the current presented data. The ongoing research to enhance the anabolic potential of current agents, identify new agents, and develop better delivery systems will greatly enhance the management of bone quality-related injuries and diseases in the future.
Acknowledgment
We thank John Wiley & Sons, Inc, for their permission to use published material for our images in the article.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Akhter MP, Cullen DM, Gong G, Recker RR. Bone biomechanical properties in prostaglandin EP1 and EP2 knockout mice. Bone. 2001;29:121–125. doi: 10.1016/S8756-3282(01)00486-0. [DOI] [PubMed] [Google Scholar]
- 2.Alkhiary YM, Gerstenfeld LC, Krall E, Sato M, Westmore M, Mitlak B, Einhorn TA. Parathyroid hormone (1–24; teriparatide) enhances experimental fracture healing. Trans Orthop Res Soc. 2004;29:328. [Google Scholar]
- 3.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960–968. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 4.Arlot ME, Jiang Y, Genant HK, Zhao J, Burt-Pichat B, Roux JP, Delmas PD, Meunier PJ. Histomorphometric and μ-CT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res. 2008;23:215–222. doi: 10.1359/jbmr.071012. [DOI] [PubMed] [Google Scholar]
- 5.Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, García-Hernández PA, Recknor CP, Einhorn TA, Dalsky GP, Mitlak BH, Fierlinger A, Lakshmanan MC. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 6.Aubin J, Triffitt J. Mesenchymal stem cells and osteoblast differentiation. In: Bilezikian J, Raisz L, Rodan G, editors. Principles of Bone Biology. San Diego, CA: Academic Press; 2002. pp. 59–82. [Google Scholar]
- 7.Bain SD, Jerome C, Shen V, Dupin-Roger I, Ammann P. Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int. 2009;20:1417–1428. doi: 10.1007/s00198-008-0815-8. [DOI] [PubMed] [Google Scholar]
- 8.Balemans W, Ebeling M, Patel N, Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Baron R, Tsouderos Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol. 2002;450:11–17. doi: 10.1016/S0014-2999(02)02040-X. [DOI] [PubMed] [Google Scholar]
- 10.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, Klift M, Pols H. Use of statins and fracture results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164:146–152. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 11.Benoit DS, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102–6110. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Blake GM, Fogelman I. The correction of BMD measurements for bone strontium content. J Clin Densitom. 2007;10:259–265. doi: 10.1016/j.jocd.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 13.Bouxsein ML. Bone quality: where do we go from here? Osteoporos Int. 2003;14(Suppl 5):S118–S127. doi: 10.1007/s00198-003-1489-x. [DOI] [PubMed] [Google Scholar]
- 14.Bouxsein ML, Chen P, Glass EV, Kallmes DF, Delmas PD, Mitlak BH. Teriparatide and raloxifene reduce the risk of new adjacent vertebral fractures in postmenopausal women with osteoporosis: results from two randomized controlled trials. J Bone Joint Surg Am. 2009;91:1329–1338. doi: 10.2106/JBJS.H.01030. [DOI] [PubMed] [Google Scholar]
- 15.Buckbinder L, Crawford DT, Qi H, Ke HZ, Olson LM, Long KR, Bonnette PC, Baumann AP, Hambor JE, Grasser WA, 3rd, Pan LC, Owen TA, Luzzio MJ, Hulford CA, Gebhard DF, Paralkar VM, Simmons HA, Kath JC, Roberts WG, Smock SL, Guzman-Perez A, Brown TA, Li M. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc Natl Acad Sci USA. 2007;104:10619–10624. doi: 10.1073/pnas.0701421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buehler J, Chappuis P, Saffar JL, Tsouderos Y, Vignery A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis) Bone. 2001;29:176–179. doi: 10.1016/S8756-3282(01)00484-7. [DOI] [PubMed] [Google Scholar]
- 17.Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM. Intermittently administered human parathyroid hormone(1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001;16:157–165. doi: 10.1359/jbmr.2001.16.1.157. [DOI] [PubMed] [Google Scholar]
- 18.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 19.Canalis E, Hott M, Deloffre P, Tsouderos Y, Marie PJ. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone. 1996;18:517–523. doi: 10.1016/8756-3282(96)00080-4. [DOI] [PubMed] [Google Scholar]
- 20.Degerblad M, Bengtsson BA, Bramnert M, Johnell O, Manhem P, Rosen T, Thoren M. Reduced bone mineral density in adults with growth hormone (GH) deficiency: increased bone turnover during 12 months of GH substitution therapy. Eur J Endocrinol. 1995;133:180–188. doi: 10.1530/eje.0.1330180. [DOI] [PubMed] [Google Scholar]
- 21.Dempster DW, Dosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- 22.Dobnig H, Stepan JJ, Burr DB, Li J, Michalská D, Sipos A, Petto H, Fahrleitner-Pammer A, Pavo I. Teriparatide reduces bone microdamage accumulation in postmenopausal women previously treated with alendronate. J Bone Miner Res. 2009;24:1998–2006. doi: 10.1359/jbmr.090527. [DOI] [PubMed] [Google Scholar]
- 23.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/en.136.8.3632. [DOI] [PubMed] [Google Scholar]
- 24.Edward B, Juppner H. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6. Washington, DC: American Society of Bone and Mineral Research; 2006. pp. 90–93. [Google Scholar]
- 25.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone mineral density in postmenopausal women. Lancet. 2000;355:2218–2219. doi: 10.1016/S0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 26.Finklestein JS. Pharmacological mechanisms of therapeutics parathyroid hormone. In: Bielzikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. New York, NY: Academic Press; 1996. pp. 993–1005. [Google Scholar]
- 27.Garrett IR, Gutierrez GE, Rossini G, Nyman J, McCluskey B, Flores A, Mundy GR. Locally delivered lovastatin nanoparticles enhance fracture healing in rats. J Orthop Res. 2007;25:1351–1357. doi: 10.1002/jor.20391. [DOI] [PubMed] [Google Scholar]
- 28.Gazzerro E, Canalis E. Skeletal actions of insulin-like growth factors. Expert Rev Endocrinol Metab. 2006;1:47–56. doi: 10.1586/17446651.1.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Ghiron LJ, Thompson JL, Holloway L, Hintz RL, Butterfield GE, Hoffman AR, Marcus R. Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res. 1995;10:1844–1852. doi: 10.1002/jbmr.5650101203. [DOI] [PubMed] [Google Scholar]
- 30.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez GE, Edwards JR, Garrett IR, Nyman JS, McCluskey B, Rossini G, Flores A, Neidre DB, Mundy GR. Transdermal lovastatin enhances fracture repair in rats. J Bone Miner Res. 2008;23:1722–1730. doi: 10.1359/jbmr.080603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez GE, Lalka D, Garrett IR, Rossini G, Mundy GR. Transdermal application of lovastatin to rats causes profound increases in bone formation and plasma concentrations. Osteoporos Int. 2006;17:1033–1042. doi: 10.1007/s00198-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 33.Habermann B, Kafchitsas K, Olender G, Augat P, Kurth A. Strontium ranelate enhances callus strength more than PTH 1–34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int. 2010;86:82–89. doi: 10.1007/s00223-009-9317-8. [DOI] [PubMed] [Google Scholar]
- 34.Hock JM. Stemming bone loss by suppressing apoptosis. J Clin Invest. 1999;104:371–373. doi: 10.1172/JCI7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, Lindsay R. Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88:5212–5220. doi: 10.1210/jc.2003-030768. [DOI] [PubMed] [Google Scholar]
- 36.Holzer G, Majeska RJ, Lundy MW, Hartke JR, Einhorn TA. Parathyroid hormone enhances fracture healing: a preliminary report. Clin Orthop Relat Res. 1999;366:258–263. doi: 10.1097/00003086-199909000-00033. [DOI] [PubMed] [Google Scholar]
- 37.Jee WS, Ma YF. The in vivo anabolic actions of prostaglandins in bone. Bone. 1997;21:297–304. doi: 10.1016/S8756-3282(97)00147-6. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 39.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juppner H, Gardella T, Brown E, Kronenberg H, Potts J., Jr . Parathyroid hormone and parathyroid hormone-related peptide in the regulation of calcium homeostasis and bone development. In: DeGroot L, Jameson J, editors. Endocrinology. Philadelphia, PA: Saunders; 2005. pp. 1377–1417. [Google Scholar]
- 41.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–1912. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 42.Ke HZ, Crawford DT, Qi H, Simmons HA, Owen TA, Paralkar VM, Mei Li M, Lu B, Grasser WA, Cameron KO, Lefker BA, DaSilva-Jardine P, Scott DO, Zhang Q, Tian XY, Jee WS, Brown TA, Thompson DD. A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Miner Res. 2006;21:565–575. doi: 10.1359/jbmr.051110. [DOI] [PubMed] [Google Scholar]
- 43.Ke HZ, Shen V, Qi H, Crawford DT, Wu DD, Liang XG, Chidsey-Frink KL, Pirie CM, Simmons HA, Thompson DD. Prostaglandin E2 increases bone strength in intact rats and in ovariectomized rats with established osteopenia. Bone. 1998;23:249–255. doi: 10.1016/S8756-3282(98)00102-1. [DOI] [PubMed] [Google Scholar]
- 44.Kneissel M, Boyde A, Gasser JA. Bone tissue and its mineralization in aged estrogen-depleted rats after long-term intermittent treatment with parathyroid hormone (PTH) analog SDZ PTS 893 or human PTH(1–34) Bone. 2001;28:237–250. doi: 10.1016/S8756-3282(00)00448-8. [DOI] [PubMed] [Google Scholar]
- 45.Komatsubara S, Mori S, Mashiba T, Nonaka K, Seki A, Akiyama T, Miyamoto K, Cao Y, Manabe T, Norimatsu H. Human parathyroid hormone (1-34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone. 2005;36:678–687. doi: 10.1016/j.bone.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–4262. doi: 10.1210/jc.83.12.4257. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Ke HZ, Qi H, Healy DR, Li Y, Crawford DT, Paralkar VM, Owen TA, Cameron KO, Lefker BA, Brown TA, Thompson DD. A novel, nonprostanoid EP2 receptor selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res. 2003;18:2033–2042. doi: 10.1359/jbmr.2003.18.11.2033. [DOI] [PubMed] [Google Scholar]
- 49.Li M, Thompson DD, Paralkar VM. Prostaglandin E2 receptors in bone formation. Int Orthop. 2007;31:767–772. doi: 10.1007/s00264-007-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 52.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/b-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 53.Ma YF, Li XJ, Jee WS, McOsker J, Liang XG, Setterberg R, Chow SY. Effects of prostaglandin E2 and F2 on the skeleton of osteopenic ovariectomized rats. Bone. 1995;17:549–554. doi: 10.1016/8756-3282(95)00387-8. [DOI] [PubMed] [Google Scholar]
- 54.Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, Hutchins S, Lachance N, Sawyer N, Slipetz D, Metters KM, Rodan SB, Young R, Rodan GA. Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- 55.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/er.21.2.115. [DOI] [PubMed] [Google Scholar]
- 56.Marie PJ. Strontium ranelate: a physiological approach for optimizing bone formation and resorption. Bone. 2006;38(Suppl 1):S10–S14. doi: 10.1016/j.bone.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 57.Mazziotti G, Bianchi A, Bonadonna S, Nuzzo M, Cimino V, Fusco A, Marinis L, Giustina A. Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy. J Bone Miner Res. 2006;21:520–528. doi: 10.1359/jbmr.060112. [DOI] [PubMed] [Google Scholar]
- 58.Meunier PJ, Roux C, Ortolani S, Diaz-Curiel M, Compston J, Marquis P, Cormier C, Isaia G, Badurski J, Wark JD, Collette J, Reginster JY. Effects of long-term strontium ranelate treatment on the risk of nonvertebral and vertebral fractures in postmenopausal osteoporosis: results of a five-year, randomized, placebo-controlled trial. Osteoporos Int. 2009;20:1663–1673. doi: 10.1007/s00198-008-0825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK, Reginster JY. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 60.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 61.Mundy GR, Roodman GD, editors. Osteoclast Ontogeny and Function. Amsterdam, The Netherlands: Elsevier; 1987. [Google Scholar]
- 62.Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J Bone Miner Res. 2002;17:2038–2047. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 63.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 64.Nelson BW. Bone quality: getting closer to a definition. J Bone Miner Res. 2002;17:1148–1150. doi: 10.1359/jbmr.2002.17.7.1148. [DOI] [PubMed] [Google Scholar]
- 65.Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, Takayanagi R. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337–342. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 66.Oxlund H, Dalstra M, Andreassen TT. Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tissue Int. 2001;69:299–304. doi: 10.1007/s00223-001-2027-5. [DOI] [PubMed] [Google Scholar]
- 67.Poole KE, Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 68.Raisz LG, Woodiel FN. Effects of selective prostaglandin EP2 and EP4 receptor agonists on bone resorption and formation in fetal rat organ cultures. Prostaglandin Other Lipid Mediat. 2003;71:287–292. doi: 10.1016/S1098-8823(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 69.Reginster JY, Felsenberg D, Boonen S, Diez-Perez A, Rizzoli R, Brandi ML, Spector TD, Brixen K, Goemaere S, Cormier C, Balogh A, Delmas PD, Meunier PJ. Effects of long-term strontium ranelate treatment on the risk of nonvertebral and vertebral fractures in postmenopausal osteoporosis: results of a five-year, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:1687–1695. doi: 10.1002/art.23461. [DOI] [PubMed] [Google Scholar]
- 70.Riggs BL, Michael AP. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–183. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- 71.Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–3355. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]
- 72.Saraf SK, Singh A, Garbyal RS, Singh V. Effect of simvastatin on fracture healing—an experimental study. Indian J Exp Biol. 2007;45:444–449. [PubMed] [Google Scholar]
- 73.Sato M, Westmore M, Ma YL, Schmidt A, Zeng QQ, Glass EV, Vahle J, Brommage R, Jerome CP, Turner CH. Teriparatide [PTH(1–34)] strengthens the proximal femur of ovariectomized nonhuman primates despite increasing porosity. J Bone Miner Res. 2004;19:623–629. doi: 10.1359/JBMR.040112. [DOI] [PubMed] [Google Scholar]
- 74.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 75.Skoglund B, Forslund C, Aspenberg P. Simvastatin improves fracture healing in mice. J Bone Miner Res. 2002;17:2004–2008. doi: 10.1359/jbmr.2002.17.11.2004. [DOI] [PubMed] [Google Scholar]
- 76.Tashjian AH, Jr, Gagel RF. Teriparatide [human PTH(1–34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res. 2006;21:354–436. doi: 10.1359/JBMR.051023. [DOI] [PubMed] [Google Scholar]
- 77.Ueda K, Saito A, Nakano H, Aoshima M, Yokota M, Muraoka R, Iwaya T. Cortical hyperostosis following long-term administration of prostaglandin E1 in infants with cyanotic congenital heart disease. J Pediatr. 1980;97:834–836. doi: 10.1016/S0022-3476(80)80282-4. [DOI] [PubMed] [Google Scholar]
- 78.Weinreb M, Grosskopf A, Shir N. The anabolic effect of PGE2 in rat bone marrow cultures is mediated via EP4 receptor subtype. Am J Physiol. 1999;276:E376–E383. doi: 10.1152/ajpendo.1999.276.2.E376. [DOI] [PubMed] [Google Scholar]
- 79.Whitfield JF, Morley P, Willick G, Langille R, Ross V, MacLean S, Barbier JR. Cyclization by a specific lactam increases the ability of human parathyroid hormone (hPTH)-(1–31)NH2 to stimulate bone growth in ovariectomized rats. J Bone Miner Res. 1997;12:1246–1252. doi: 10.1359/jbmr.1997.12.8.1246. [DOI] [PubMed] [Google Scholar]
- 80.Wuster C, Abs R, Bengtsson BA, Bennmarker H, Feldt-Rasmussen U, Hernberg-Stahl E, Monson JP, Westberg B, Wilson P, KIMS Study Group and the KIMS International Board The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res. 2001;16:398–405. doi: 10.1359/jbmr.2001.16.2.398. [DOI] [PubMed] [Google Scholar]
- 81.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang RS, Liu TK, Lin-Shiau SY. Increased bone growth by local prostaglandin E2 in rat. Calcif Tissue Int. 1993;52:57–61. doi: 10.1007/BF00675627. [DOI] [PubMed] [Google Scholar]
- 83.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]