Abstract
Background
The skeleton plays a critical structural role in bearing functional loads, and failure to do so results in fracture. As we evaluate new therapeutics and consider treatments to prevent skeletal fractures, understanding the basic mechanics underlying whole bone testing and the key principles and characteristics contributing to the structural strength of a bone is critical.
Questions/purposes
We therefore asked: (1) How are whole bone mechanical tests performed and what are the key outcomes measured? (2) How do the intrinsic characteristics of bone tissue contribute to the mechanical properties of a whole bone? (3) What are the effects of extrinsic characteristics on whole bone mechanical behavior? (4) Do environmental factors affect whole bone mechanical properties?
Methods
We conducted a PubMed search using specific search terms and limiting our included articles to those related to in vitro testing of whole bones. Basic solid mechanics concepts are summarized in the context of whole bone testing and the determinants of whole bone behavior.
Results
Whole bone mechanical tests measure structural stiffness and strength from load-deformation data. Whole bone stiffness and strength are a function of total bone mass and the tissue geometric distribution and material properties. Age, sex, genetics, diet, and activity contribute to bone structural performance and affect the incidence of skeletal fractures.
Conclusions
Understanding and preventing skeletal fractures is clinically important. Laboratory tests of whole bone strength are currently the only measures for in vivo fracture prediction. In the future, combined imaging and engineering models may be able to predict whole bone strength noninvasively.
Introduction
The adverse outcome of many skeletal diseases is fracture or failure of the skeleton. Fracture is an inherently mechanical event indicating the load-bearing capacity of a particular skeletal element has been exceeded. To determine whether a bone will fail under loading, one should consider the loads or forces applied to the skeleton and the structural strength or resistance to fracture of the bone itself. In particular, if the applied loads exceed the structural strength of the bone, fracture will occur. The forces generated during various daily activities have been measured at the hip and ranged from less than body weight to many multiples of body weight. The lowest forces occurred during nonweightbearing rehabilitation exercises and the highest forces while rising from a chair or climbing stairs [7, 74]. For any given set of applied loads, the characteristics of the bone, joint, or other skeletal structure of interest determine whether the skeleton can carry these loads successfully or fracture will result. In this article, we focus on considerations influencing the structural capacity of the skeleton rather than the dynamic mechanical forces applied to the skeleton.
The term “bone quality” generally refers to all characteristics beyond bone mass influencing the ability of the skeleton to bear loads. Although bone mass and its changes with aging and disease are well correlated with fracture risk [30, 59, 62, 83, 86, 109], the bone mass of populations who fracture and those who do not fracture overlaps considerably, and bone mass alone is insufficient to predict whether an individual will fracture [109, 116]. Many critical properties are encompassed by the term “quality,” including cortical geometry, cancellous architecture, material microstructure, and tissue composition. In general, the contribution of macro- and mesoscale properties to bone strength has been examined extensively and is the best understood among the different levels of analysis, whereas the role of microscale features is more difficult to study experimentally, especially in vivo, and has many open questions remaining to be explored.
We address the following four questions regarding the mechanical properties of the skeleton: (1) How are whole bone mechanical tests performed and what are the key outcomes measured? (2) How do the intrinsic characteristics of bone tissue contribute to the mechanical properties of a whole bone? (3) What are the effects of extrinsic characteristics on whole bone mechanical behavior? (4) Do environmental factors affect whole bone mechanical properties?
Search Strategy and Criteria
The fundamental basis of using mechanical testing to measure bone mechanical behavior comes from engineering mechanics, in particular solid mechanics. The determinants of structural performance are defined by basic mechanics and apply to all load-bearing structures. To relate these concepts to bone and the intrinsic and extrinsic contributors to skeletal performance, literature searches were performed using PubMed. Searching on [bone AND strength] yielded 18,327 articles; [bone AND strength AND mechanical testing] produced 2274 articles. We applied the exclusion [NOT (tendon OR ligament OR disc OR implant OR screw OR pin OR cement OR alloy OR graft OR scaffold OR polymer OR composite OR healing OR tumor)], producing 522 articles. We reviewed the titles and abstracts of all of these articles to focus on in vitro testing of tissue samples and relating determinants at this length scale to whole bone behavior. Using our inclusion criteria, we identified 143 relevant articles.
Whole Bone Mechanical Tests to Assess Bone Strength
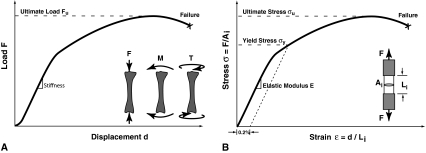
Whole bone tests can be performed in axial, bending, or torsional loading. For all loading modes, when a monotonically increasing load is applied to a whole bone until failure, deformations or displacements are produced throughout the structure (Fig. 1A). The load-deformation behavior is characterized by an initial linear (elastic) region before yield, a postyield nonlinear region containing the maximum (ultimate) load, and the failure point at which the whole bone fractures and can no longer carry the load. The slope of the preyield elastic region is the structural stiffness, and the ultimate load is the whole bone strength for a given loading. The area under the load-deformation curve is the energy absorbed to failure. The deformation occurring postyield is known as ductility. Structures lacking postyield deformation and failing before the onset of yield are known as brittle. Nondestructive tests can be performed at low loads in the elastic region without creating permanent deformation; however, the data obtained do not provide information about failure properties. The strength of a structure can only be measured by destructive testing to failure and thus can only be directly obtained ex vivo.
Fig. 1A–B.
(A) Load-displacement behavior for a whole bone structural test is shown. The structural stiffness is determined from the initial linear region, where F is load and d is displacement. The structural strength is the load required to fail the whole bone. These parameters depend on the loading mode; a compressive load (F) is shown, but loading can also be a bending (M) or torsional moment (T). (B) Stress-strain behavior for a materials test is shown. These measurements are independent of specimen size and shape but do depend on the loading mode (tension, compression, bending, or shear). The modulus of elasticity (E) is determined from the initial linear elastic region; yield stress (σy) is the transition from linear to nonlinear behavior and is based on 0.2% offset of the linear region (dashed line); and ultimate strength (σu) is the maximum stress (strength). For the tensile specimen shown, F is the tensile load, Ai is the initial area, and Li is the initial specimen length.
Our discussion is focused on whole bone mechanical behavior. However, analogous mechanical tests can be performed to characterize the tissue level mechanical properties. The material characteristics of the tissue are independent of bone size and shape and are measured on homogeneous samples of known geometry. The material properties are similar to the load-displacement data but are normalized for the known geometric characteristics, producing a stress-strain curve (Fig. 1B). Stress is defined as the applied load per cross-sectional area. Strain is the change in length per initial length. Analogous to a whole bone test, the slope of the stress-strain curve is the tissue stiffness and is known as the modulus of elasticity. The maximum (ultimate) stress is the strength of the bone tissue for given loading conditions. The energy absorbed to failure is the area under the stress-strain curve and is known as the toughness.
In vivo loading conditions should be considered when designing laboratory tests to measure whole bone strength to ensure clinically relevant loading and failure modes. In vivo, the long bones of the appendicular skeleton experience high stresses and strains primarily from loads that induce bending and torsion [8, 51, 76]. Under these loading conditions, the cortical diaphysis experiences the highest loads and deformations, whereas the cancellous bone at the bone ends transfers the load from the joint surface to the cortex. In contrast, cancellous bone is critical to the mechanical performance of whole bones such as the vertebra and calcaneus that have less cortical bone and primarily experience compression and bending loads [23, 48, 101].
Intrinsic Influences on Whole Bone Mechanics
When an individual performs normal activities of daily living, loads are created that are borne by the bones and joints of the skeleton. In vivo activities such as walking produce complex loading conditions that are combinations of more well-defined loading modes, including axial (tension or compression), bending, and torsional loads. For each loading mode, whole bone tests in the laboratory can measure the structural stiffness and strength.
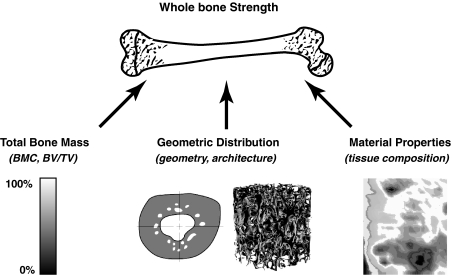
From a mechanics perspective, the ability of a structure such as a whole bone to bear applied loads is a function of three characteristics: the total mass, the geometric distribution of the mass, and the material properties (Fig. 2). Therefore, bone strength can be improved through several different mechanisms: by increasing the total bone mass, by distributing the bone mass to locations that experience high loads, or by enhancing the material properties of the constituent tissue. Cellular metabolic activity distinguishes the skeleton from manmade structures and not only creates but also adapts and repairs the structural characteristics during growth and aging and with other conditions such as exercise, injury, and disease. In some animal models, for example, reduced tissue material properties are compensated for by altered geometry, resulting in comparable structural properties and minimizing the risk of fracture [13, 61].
Fig. 2.
Determinants of whole bone strength include how much tissue is present (bone mass), where the tissue is located (cancellous architecture or cortical geometry), and the properties of the tissue (material). BMC = bone mineral content.
Bone mass is the most studied determinant of bone structural behavior. Clinically, bone mass is reported as bone mineral content and is measured using a variety of in vivo imaging techniques [65]. For cortical bone from a healthy adult skeleton, geometric measures such as cross-sectional area directly reflect cortical bone mass. Cancellous bone architecture is more complex and spatially varying, so bone mass is generally measured in small homogeneous volumes of tissue. Bulk cancellous bone mass is quantified as bone volume fraction, the volume of bone present in the total volume of interest (BV/TV), or apparent density, the mass of bone present in the total volume of interest. These measures include bulk porosity and are distinct from the material level properties of the tissue. Spatial variations in bone mass can produce 100-fold differences in cancellous bone stiffness within the tibial metaphysis, ranging from 4 to 453 MPa in a single individual [49]. BV/TV and apparent density can explain 60% to 85% of the experimentally measured variability in compressive apparent stiffness and strength of human cancellous bone [66, 73, 79, 80, 88]. Mechanical properties scale with bone mass by a power law relationship, so small changes in mass effect large changes in bone strength [24, 47, 100]. Measures of bone mass can only capture the total tissue quantity and do not reflect where the tissue is located, which will impact the fracture resistance of bone.
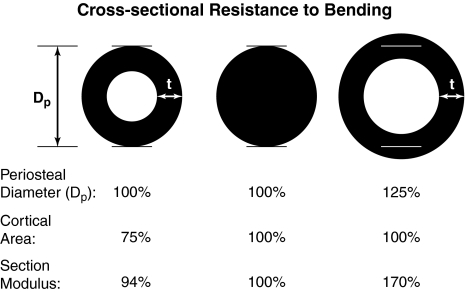
In cortical bone, the bone size and tissue distribution influence fracture resistance under all loading modes. If one assumes constant tissue material properties, geometric parameters such as cross-sectional area, moments of inertia, and section modulus quantify the distribution of the material and determine the response to applied forces. The relevant geometric measure depends on the particular loading mode [124]. In bending and torsion, resistance to the applied load is increased for tissue located further from the plane of bending or axis of torsion. Altering mass at critical locations will have disproportionate effects on the structural performance (Fig. 3). For example, the periosteal surface of a solid cylinder resists the bending load more than the central core, so coring out the solid cylinder only reduces the bending strength by 6% despite removing 25% of the total bone mass. Similarly, the bending strength of a hollow cylinder will be 70% greater than a solid cylinder with the same bone mass despite only a 25% increased periosteal diameter.
Fig. 3.
Variations in the size and distribution of bone mass in a cortical cross-section influence the section modulus, which is proportional to the bending failure strength of the whole bone. Bone resorption on the endosteal surface or apposition on the periosteal surface will change the cortical thickness (t) or the distribution of bone about the loading axis, thereby altering the ability of the bone to resist fracture. For example, compared with the reference bone (center), a bone of the same girth but with 25% less material in its core (left) will be only 6% weaker, but a bone with the same amount of material distributed further away from the bending plane of the bone (right) will be 70% stronger.
In cancellous bone, the size and spatial distribution of trabeculae determine the structural contribution of the architecture. As with bone mass, small homogenous samples of cancellous bone are generally examined. Architectural indices explained 10% to 70% of the variation in compressive strength in sheep femoral bone [87]. Sites with similar bone mineral density (BMD) but different architectures exhibited differences in strength and stiffness of up to 50% [45, 122]. In addition, the architecture of cancellous bone often develops a preferred orientation in response to habitual loading, resulting in substantially different strength values when loaded in different directions [43]. In human vertebrae, for example, alignment of the trabeculae along the axis of the spine makes the bone nearly twice as strong when loaded along the superior-inferior axis as when loaded in either the AP or left-right direction [45, 91]. Combined imaging and computational modeling advances allow cancellous bone architecture to be captured (see articles by Donnelly [35] and Burghardt et al. [18] in this issue) and input into mechanics-based models that can be used to understand the effect of architecture on stiffness of whole bones such as the vertebral body, distal radius, or proximal femur [42, 57, 98, 126]. These computational modeling approaches will be essential to identify trabecular failure mechanisms in whole bones [41], especially at clinically important sites.
The final determinant of whole bone strength is the properties of the constituent material, which is currently the least understood. Both cortical and cancellous bone consist of lamellar tissue with differing microstructural organizations, which is generally considered part of the material phase. At the material level, bone tissue is a composite of an organic matrix containing primarily Type I collagen and an inorganic phase of crystalline mineral. The characteristics of the two phases and their interactions determine the material properties of bone tissue. The latter point is emphasized by the fact that the strength of the composite tissue is greater than that of either of the individual constituents. In addition, the collagen and mineral phases contribute differently to the mechanical behavior of the tissue. Bone mineral content is directly related to the material stiffness of the tissue and whole bone stiffness [4, 22, 31]. At the micro- and nanoscale, mineral characteristics such as crystallinity and carbonate substitution determine material properties that contribute directly to whole bone stiffness and strength; however, the precise nature of these relationships is not yet clear [3, 37]. Collagen characteristics in the extracellular matrix, such as collagen content and maturity or crosslinking, contribute primarily to the toughness and postyield deformation of the tissue [14, 19, 22]. Microdamage, which occurs naturally in response to the cyclic loading of daily activities [20], alters mineral stoichiometry and collagen structure [107, 117]. Water present within the bone tissue, both mobile in vascular-lacunar-canalicular spaces and bound to the collagen and mineral phases, appears to impact bone tissue toughness and strength [94], although this effect on mechanical properties has not been examined at the whole bone level. Matrix stoichiometry and the structure of proteoglycans and other matrix proteins are critical in diseases such as osteogenesis imperfecta and contribute to increased fragility [52]. The complex organization and the inherent and adapted variability of bone tissue make fully elucidating these structure-function relationships challenging.
Similar to other natural materials, bone tissue is spatially heterogeneous in material properties and composition [36, 50]. Osteoporotic tissue exhibits reduced spatial variation in compositional measures [96, 102], but a direct link to fracture remains to be established. Engineering analyses used to analyze whole bone mechanical tests assume homogeneous material properties, so computational models are necessary to capture and understand the functional implications of these material property variations on structural performance [29, 67]. These models generally include either architectural variation or material property distributions but generally not both. In both cases, the advantage of these engineering-based approaches over clinical bone density assessments is the ability to probe intrinsic mechanisms of structural performance.
Our focus on whole bone mechanics leads us to view these structures in terms of their bone mass, geometry, and material properties, the metrics relevant to engineering analyses. However, these three characteristics are the net product of coordinated biologic processes such as bone modeling in growing individuals or bone remodeling and turnover in adults. Any breakdown in these processes can lead to excessive microdamage accumulation, insufficient repair, and eventually fracture. Diseases such as osteoporosis, osteomalacia, and osteogenesis imperfecta alter biologic processes, which then alter the structural determinants, as do the pharmacologic agents used to treat these conditions. As stated earlier, compensatory mechanisms also actively sense and adapt to the applied mechanical loads in vivo.
Extrinsic Influences on Whole Bone Mechanics
Many external factors contribute to whole bone structure and strength and clinical fracture risk, including aging, sex, disease, and genetics. Understanding the effects of these factors on whole bone and bone tissue mechanical performance is merely the first step to developing effective clinical diagnostics and preventive therapies to minimize fracture risk.
Both men and women experience a progressive decline in bone structure and mechanical properties with age, thereby compromising the load-bearing capacity. The compressive strength of bone declines throughout adulthood, decreasing by 50% to 75% in whole vertebrae, vertebral trabecular bone, and distal femoral trabecular bone [84, 89, 90]. Lifetime losses also occur in tissue modulus and toughness (81%–85%) and ultimate strain or compressibility (26%) in vertebral trabecular bone [90]. Such deteriorated mechanical properties contribute to the increased incidence of osteoporosis and susceptibility to fragility fractures with aging [58, 104, 105].
The mechanisms of bone acquisition during growth and bone loss with aging differ between women and men. Decreased volume fraction results primarily from trabecular loss in women but trabecular thinning in men [1]. When these sex-specific mechanisms of bone loss were explored using computer models designed to mimic a 10% decline in bone density, bone strength was reduced by only 20% for trabecular thinning but 70% for trabecular loss and 77% for both thinning and loss [111]. Even when normal bone density was restored by trabecular thickening, strength was still reduced by 63%, indicating the mechanism of bone loss in women is more detrimental to the mechanical properties, which may explain the higher clinical incidence of fractures in women. Although the cortical thickness in long bones is similar for boys and girls during growth, boys experience greater periosteal expansion and thus increased section modulus, which provides more resistance to applied loads [123]. This geometric deficit persists and worsens with aging for women [39, 82]. With aging, endocortical resorption occurs in both sexes, although the cortical thinning is more pronounced in women as a result of less compensatory periosteal apposition [106, 108], which makes the long bones more susceptible to fracture.
Musculoskeletal diseases such as osteoporosis, osteomalacia, and osteogenesis imperfecta disrupt bone metabolism and can be detrimental to the load-bearing capacity of the skeleton. Osteoporosis is characterized by reduced bone mass and deteriorated architecture and material properties, which contribute to increased fracture risk. Trabecular thinning is more pronounced at the spine and iliac crest in patients with vertebral fractures than in those without fractures [68, 95]. Similarly, bone volume fraction and trabecular number are reduced, and the trabeculae are more aligned along the primary loading direction in female patients with hip fractures [27]. Osteoporosis is also associated with altered tissue composition [96, 97] and reduced bone tissue strength and stiffness [27].
Many genetic factors contribute to bone morphology and structure, which influence the mechanical functionality and fracture susceptibility (as reviewed in [12, 99, 103]). Bone mass is influenced highly by genetics (eg, [33, 64, 112, 121]), although this impact appears to diminish with aging [53, 112]. Bone morphology, particularly hip axis length and femoral head width, is highly heritable [44, 71]. Using inbred mouse strains, whole bone properties have been linked to different combinations of bone morphologic and compositional traits that are strongly heritable [60, 118]. To better elucidate the genetic etiology of these phenotypic traits and of skeletal fragility, various quantitative trait loci and regions within quantitative trait loci have been linked to specific measures of bone density [5, 6, 69], morphology and structure [17, 38, 70, 72, 119, 129, 132], and mechanical properties [72, 75, 78, 128, 132].
Environmental Factors Affecting Whole Bone Mechanics
Environmental factors also contribute to whole bone mechanical behavior and clinical fracture risk, including diet and mechanical loading. The degree of control over these factors varies, but understanding their role in whole bone performance is critical.
The intake and absorption of essential nutrients, especially calcium and vitamin D, are critical for the formation and maintenance of bone and the reduction in remodeling rate and osteoporosis risk [25, 32]. Calcium intake, particularly through dairy foods (reviewed in [55, 56]), can substantially decrease the risk for osteoporosis and fractures [54, 93, 110]. Similarly, vitamin D supplementation of 700 to 800 IU per day can reduce the risk of hip and nonvertebral fractures by approximately 25% [11], likely as a result of both increased BMD [10] and a decreased risk for falls [9]. In rodent models, low-calcium diets have been associated with reduced BV/TV in the proximal ulna [130] and ash content, breaking strength, and elastic modulus in the femur [46]. Calcium and vitamin D supplementation increased whole bone stiffness, strength, and toughness in the femur [2] and increased stiffness but decreased maximum load and energy to maximum load in the lumbar spine [92].
Dynamic physical forces are an established environmental regulator of whole bone mechanical behavior and mass and structure in cortical and cancellous bone. In adults, normal skeletal mass is maintained with mechanical loading, as can be demonstrated by increasing or decreasing normal levels of activity with exercise [63, 113, 114] or disuse [34, 77, 127]. In these human studies, whole bone strength cannot be measured directly, so much of our knowledge regarding load-based functional adaptation comes from cortical bone in animal models. In young adult animals, increased loading enhances bone mass and strength [131], and decreased functional loads decrease bone mass and strength [125]. Increasing bone strength is more difficult than either decreasing strength in the adult or reducing the increase in growing animals. In most animal models of altered loading, strength changes were primarily effected through altered geometry, although space flight modified tissue level bone properties [120].
Discussion
Failure of the load-bearing function of the skeleton manifests clinically as fracture. In the laboratory, the mechanical performance of the skeleton can be tested under a variety of different loading conditions to understand the structural capacity of whole bones. These tests are a critical functional assay of skeletal performance and depend on the total tissue mass, the geometric distribution of the tissue, and its material properties. Furthermore, individual age, sex, genetics, and disease modulate these intrinsic properties and whole bone behavior. In this review, we addressed the following four questions regarding the mechanical properties of the skeleton: (1) How are whole bone mechanical tests performed and what are the key outcomes measured? (2) How do the intrinsic characteristics of bone tissue contribute to the mechanical properties of a whole bone? (3) What are the effects of extrinsic characteristics on whole bone mechanical behavior? (4) Do environmental factors affect whole bone mechanical properties?
This review has several limitations. First, whole bone strength is a broad topic. We did not present a systematic review. Rather, this article is intended to be a tutorial with representative references and cannot comprehensively include all studies. Second, many extrinsic and environmental factors contribute to whole bone mechanical behavior. While some of the major contributors have been highlighted here, a myriad of other factors contribute to the variability of bone properties and can impact structural performance. Most notably, biologic pathways that drive bone cell structure, function, and adaptation and are known to influence the tissue properties likely also influence whole bone characteristics. Finally, we have not discussed specific whole bone stiffness and strength values, given the dependence on site tested, loading mode, and other factors described.
The research question must be the driving consideration for whole bone testing. When designing tests and interpreting data, one must ask whether the test and testing parameters are representative of the conditions of interest. For example, most tests are performed under quasistatic loading conditions; however, the material properties of bone tissue are known to depend on strain rate [28, 85]. If one is interested in traumatic failure, these conditions may not adequately address the research objectives.
While methods for these physical tests are reasonably standard, the data should be interpreted in the context of their limitations. The variability of whole bone strength measurements is often 20% to 30% of the mean value. Factors contributing to this variation include biologic variability across individuals and testing methodology [40]. Inbred animal models have a more uniform genetic background that can reduce interspecimen variability. Testing considerations include specimen fixturing, loading mode, loading rate, and boundary conditions [21, 85]. The mean value obtained from whole bone tests will vary as a function of these loading conditions and the extrinsic and environmental factors discussed here.
Computational models incorporating intrinsic whole bone characteristics from imaging are becoming more prevalent for nondestructive prediction of bone mechanical performance [26]. These approaches hold promise for noninvasive patient-specific determination of fracture risk [15, 81]. A considerable strength of these engineering models is the ability to incorporate clinical imaging data from the patient into model geometry and material properties, thereby allowing a more direct assessment of the patient’s whole bone properties. In addition, models can be used to vary the load and bone parameters parametrically to examine the impact of different loading scenarios. However, capturing appropriate boundary conditions and applied loading characteristics to mimic precisely the in vivo state for a particular patient will be challenging. This consideration especially applies to the spine, which has little experimental data for in vivo loads and experiences a wide range of loading states due to the complex kinematics. Furthermore, experimental validation of structural behavior remains difficult and is a key limitation of engineering models. The ability to relate characteristics of tissue level composition to material properties for inclusion in whole bone models is limited but rapidly evolving.
This review has summarized methods and outcomes for mechanical tests of whole bones and the intrinsic, extrinsic, and environmental factors contributing to whole bone mechanical behavior. Much remains to be learned about the effects of material composition on whole bone behavior beyond the contributions of bone mass and geometry. In the future, multiscale analyses combining imaging, compositional, and mechanical analyses will help to understand the role of localized variations in material properties on whole bone stiffness and strength and develop methods of modulating these properties in vivo. For clinical investigations, laboratory tests of whole bone strength are often used as surrogate measures for in vivo fracture prediction. Although these in vitro methods have been used to assess skeletal stiffness and strength for many decades and will likely continue to do so, methods to predict whole bone strength noninvasively under a variety of loading modes are limited [16, 29, 115] and highly desirable for improving human skeletal health.
Footnotes
One or more of the authors received funding from the National Institutes of Health (F32-AR056186 (JHC) and R01-AR053571 (MCHM)).
References
- 1.Aaron JE, Makins NB, Sagreiya K. The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res. 1987;215:260–271. [PubMed] [Google Scholar]
- 2.Aerssens J, Audekercke R, Talalaj M, Vlasselaer P, Bramm E, Geusens P, Dequeker J. Effect of 1 alpha-vitamin D3 on bone strength and composition in growing rats with and without corticosteroid treatment. Calcif Tissue Int. 1994;55:443–450. doi: 10.1007/BF00298558. [DOI] [PubMed] [Google Scholar]
- 3.Akkus O, Adar F, Schaffler MB. Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone. 2004;34:443–453. doi: 10.1016/j.bone.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia TC, Tsou AC, Taylor EA, Mikić B. Ash content modulation of torsionally derived effective material properties in cortical mouse bone. J Biomech Eng. 2003;125:615–619. doi: 10.1115/1.1611513. [DOI] [PubMed] [Google Scholar]
- 5.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 6.Benes H, Weinstein RS, Zheng W, Thaden JJ, Jilka RL, Manolagas SC, Shmookler Reis RJ. Chromosomal mapping of osteopenia-associated quantitative trait loci using closely related mouse strains. J Bone Miner Res. 2000;15:626–633. doi: 10.1359/jbmr.2000.15.4.626. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech. 1993;26:969–990. doi: 10.1016/0021-9290(93)90058-m. [DOI] [PubMed] [Google Scholar]
- 8.Biewener AA. Musculoskeletal design in relation to body size. J Biomech. 1991;24(Suppl 1):19–29. doi: 10.1016/0021-9290(91)90374-v. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–22564. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 12.Blank RD. Breaking down bone strength: a perspective on the future of skeletal genetics. J Bone Miner Res. 2001;16:1207–1211. doi: 10.1359/jbmr.2001.16.7.1207. [DOI] [PubMed] [Google Scholar]
- 13.Bonadio J, Jepsen KJ, Mansoura MK, Jaenisch R, Kuhn JL, Goldstein SA. A murine skeletal adaptation that significantly increases cortical bone mechanical properties: implications for human skeletal fragility. J Clin Invest. 1993;92:1697–1705. doi: 10.1172/JCI116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J Bone Miner Res. 1999;14:330–335. doi: 10.1359/jbmr.1999.14.3.330. [DOI] [PubMed] [Google Scholar]
- 15.Boutroy S, Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–399. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 16.Bouxsein ML, Melton LJ, 3rd, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH, Khosla S. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. J Bone Miner Res. 2006;21:1475–1482. doi: 10.1359/jbmr.060606. [DOI] [PubMed] [Google Scholar]
- 17.Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, Churchill G, Adamo ML, Powell DR, Turner RT, Müller R, Beamer WG. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res. 2002;17:570–579. doi: 10.1359/jbmr.2002.17.4.570. [DOI] [PubMed] [Google Scholar]
- 18.Burghardt AJ, Link TM, Majumdar S. High-resolution Computed Tomography for Clinical Imaging of Bone Microarchitecture. Clin Orthop Relat Res. 2010. DOI 10.1007/s11999-010-1766-x. [DOI] [PMC free article] [PubMed]
- 19.Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 20.Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18:189–200. doi: 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 21.Burstein AH, Frankel VH. A standard test for laboratory animal bone. J Biomech. 1971;4:155–158. doi: 10.1016/0021-9290(71)90026-1. [DOI] [PubMed] [Google Scholar]
- 22.Burstein AH, Zika JM, Heiple K, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg Am. 1975;57:956–961. [PubMed] [Google Scholar]
- 23.Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech Bristol, Avon. 1999;14:203–216. doi: 10.1016/s0268-0033(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 24.Carter DR, Hayes WC. The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am. 1977;59:954–962. [PubMed] [Google Scholar]
- 25.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 26.Christen D, Webster DJ, Müller R. Multiscale modelling and nonlinear finite element analysis as clinical tools for the assessment of fracture risk. Philos Transact A Math Phys Eng Sci. 2010;368:2653–2668. doi: 10.1098/rsta.2010.0041. [DOI] [PubMed] [Google Scholar]
- 27.Ciarelli TE, Fyhrie DP, Schaffler MB, Goldstein SA. Variations in three-dimensional cancellous bone architecture of the proximal femur in female hip fractures and in controls. J Bone Miner Res. 2000;15:32–40. doi: 10.1359/jbmr.2000.15.1.32. [DOI] [PubMed] [Google Scholar]
- 28.Courtney AC, Wachtel EF, Myers ER, Hayes WC. Effects of loading rate on strength of the proximal femur. Calcif Tissue Int. 1994;55:53–58. doi: 10.1007/BF00310169. [DOI] [PubMed] [Google Scholar]
- 29.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33:744–750. doi: 10.1016/s8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 30.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 31.Currey JD. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech. 1988;21:131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 32.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 33.Dequeker J, Nijs J, Verstraeten A, Geusens P, Gevers G. Genetic determinants of bone mineral content at the spine and radius: a twin study. Bone. 1987;8:207–209. doi: 10.1016/8756-3282(87)90166-9. [DOI] [PubMed] [Google Scholar]
- 34.Donaldson CL, Hulley SB, Vogel JM, Hattner RS, Bayers JH, McMillan DE. Effect of prolonged bed rest on bone mineral. Metabolism. 1970;19:1071–1084. doi: 10.1016/0026-0495(70)90032-6. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly E. Methods for assessing bone quality: A review. Clin Orthop Relat Res. 2010 November 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 36.Donnelly E, Boskey AL, Baker SP, Meulen MC. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A. 2010;92:1048–1056. doi: 10.1002/jbm.a.32442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnelly E, Chen DX, Boskey AL, Baker SP, Meulen MC. Contribution of mineral to bone structural behavior and tissue mechanical properties. Calcif Tissue Int. 2010;87:450–460. doi: 10.1007/s00223-010-9404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake TA, Hannani K, Kabo JM, Villa V, Krass K, Lusis AJ. Genetic loci influencing natural variations in femoral bone morphometry in mice. J Orthop Res. 2001;19:511–517. doi: 10.1016/S0736-0266(00)00056-5. [DOI] [PubMed] [Google Scholar]
- 39.Duan Y, Beck TJ, Wang XF, Seeman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res. 2003;18:1766–1774. doi: 10.1359/jbmr.2003.18.10.1766. [DOI] [PubMed] [Google Scholar]
- 40.Eckstein F, Wunderer C, Boehm H, Kuhn V, Priemel M, Link TM, Lochmüller EM. Reproducibility and side differences of mechanical tests for determining the structural strength of the proximal femur. J Bone Miner Res. 2004;19:379–385. doi: 10.1359/JBMR.0301247. [DOI] [PubMed] [Google Scholar]
- 41.Eswaran SK, Gupta A, Keaveny TM. Locations of bone tissue at high risk of initial failure during compressive loading of the human vertebral body. Bone. 2007;41:733–739. doi: 10.1016/j.bone.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fields AJ, Eswaran SK, Jekir MG, Keaveny TM. Role of trabecular microarchitecture in whole-vertebral body biomechanical behavior. J Bone Miner Res. 2009;24:1523–1530. doi: 10.1359/JBMR.090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fields AJ, Lee GL, Liu XS, Jekir MG, Guo XE, Keaveny TM. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2010 August 16 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 44.Flicker L, Faulkner KG, Hopper JL, Green RM, Kaymacki B, Nowson CA, Young D, Wark JD. Determinants of hip axis length in women aged 10–89 years: a twin study. Bone. 1996;18:41–45. doi: 10.1016/8756-3282(95)00418-1. [DOI] [PubMed] [Google Scholar]
- 45.Galante J, Rostoker W, Ray RD. Physical properties of trabecular bone. Calcif Tissue Res. 1970;5:236–246. doi: 10.1007/BF02017552. [DOI] [PubMed] [Google Scholar]
- 46.Galfsky I, Wolinsky I, Simkin A, Guggenheim K. Effect of repletion with dietary calcium on composition and mechanical properties of bone of calcium-deprived rats. Nutr Metab. 1975;18:99–104. doi: 10.1159/000175581. [DOI] [PubMed] [Google Scholar]
- 47.Gibson LJ. The mechanical behavior of cancellous bone. J Biomech. 1985;18:317–328. doi: 10.1016/0021-9290(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 48.Giddings VL, Beaupré GS, Whalen RT, Carter DR. Calcaneal loading during walking and running. Med Sci Sports Exerc. 2000;32:627–634. doi: 10.1097/00005768-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein SA, Wilson DL, Sonstegard DA, Matthews LS. The mechanical properties of human tibial trabecular bone as a function of metaphyseal location. J Biomech. 1983;16:965–969. doi: 10.1016/0021-9290(83)90097-0. [DOI] [PubMed] [Google Scholar]
- 50.Gourion-Arsiquaud S, Burket JC, Havill LM, DiCarlo E, Doty SB, Mendelsohn R, Meulen MC, Boskey AL. Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res. 2009;24:1271–1281. doi: 10.1359/JBMR.090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gross TS, McLeod KJ, Rubin CT. Characterizing bone strain distributions in vivo using three triple rosette strain gages. J Biomech. 1992;25:1081–1087. doi: 10.1016/0021-9290(92)90044-2. [DOI] [PubMed] [Google Scholar]
- 52.Grzesik WJ, Frazier CR, Shapiro JR, Sponseller PD, Robey PG, Fedarko NS. Age-related changes in human bone proteoglycan structure: impact of osteogenesis imperfecta. J Biol Chem. 2002;277:43638–43647. doi: 10.1074/jbc.M202124200. [DOI] [PubMed] [Google Scholar]
- 53.Gueguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G. Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res. 1995;10:2017–2022. doi: 10.1002/jbmr.5650101223. [DOI] [PubMed] [Google Scholar]
- 54.Heaney RP. Calcium in the prevention and treatment of osteoporosis. J Intern Med. 1992;231:169–180. doi: 10.1111/j.1365-2796.1992.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 55.Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr. 2000;19:83S–99S. doi: 10.1080/07315724.2000.10718088. [DOI] [PubMed] [Google Scholar]
- 56.Heaney RP. Dairy and bone health. J Am Coll Nutr. 2009;28(Suppl 1):82S–90S. doi: 10.1080/07315724.2009.10719808. [DOI] [PubMed] [Google Scholar]
- 57.Homminga J, Rietbergen B, Lochmüller EM, Weinans H, Eckstein F, Huiskes R. The osteoporotic vertebral structure is well adapted to the loads of daily life, but not to infrequent ‘error’ loads. Bone. 2004;34:510–516. doi: 10.1016/j.bone.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hui SL, Slemenda CW, Johnston CC., Jr Baseline measurement of bone mass predicts fracture in white women. Ann Intern Med. 1989;111:355–361. doi: 10.7326/0003-4819-111-5-355. [DOI] [PubMed] [Google Scholar]
- 60.Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14:97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- 61.Jepsen KJ, Goldstein SA, Kuhn JL, Schaffler MB, Bonadio J. Type-I collagen mutation compromises the post-yield behavior of Mov13 long bone. J Orthop Res. 1996;14:493–499. doi: 10.1002/jor.1100140320. [DOI] [PubMed] [Google Scholar]
- 62.Johnell O, Kanis JA, Oden A, Johansson H, Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 63.Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59:204–208. [PubMed] [Google Scholar]
- 64.Jouanny P, Guillemin F, Kuntz C, Jeandel C, Pourel J. Environmental and genetic factors affecting bone mass: similarity of bone density among members of healthy families. Arthritis Rheum. 1995;38:61–67. doi: 10.1002/art.1780380110. [DOI] [PubMed] [Google Scholar]
- 65.Kazakia GJ, Majumdar S. New imaging technologies in the diagnosis of osteoporosis. Rev Endocr Metab Disord. 2006;7:67–74. doi: 10.1007/s11154-006-9004-2. [DOI] [PubMed] [Google Scholar]
- 66.Keaveny TM, Pinilla TP, Crawford RP, Kopperdahl DL, Lou A. Systematic and random errors in compression testing of trabecular bone. J Orthop Res. 1997;15:101–110. doi: 10.1002/jor.1100150115. [DOI] [PubMed] [Google Scholar]
- 67.Keyak JH, Rossi SA, Jones KA, Skinner HB. Prediction of femoral fracture load using automated finite element modeling. J Biomech. 1998;31:125–133. doi: 10.1016/s0021-9290(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 68.Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 69.Klein RF, Carlos AS, Vartanian KA, Chambers VK, Turner EJ, Phillips TJ, Belknap JK, Orwoll ES. Confirmation and fine mapping of chromosomal regions influencing peak bone mass in mice. J Bone Miner Res. 2001;16:1953–1961. doi: 10.1359/jbmr.2001.16.11.1953. [DOI] [PubMed] [Google Scholar]
- 70.Klein RF, Turner RJ, Skinner LD, Vartanian KA, Serang M, Carlos AS, Shea M, Belknap JK, Orwoll ES. Mapping quantitative trait loci that influence femoral cross-sectional area in mice. J Bone Miner Res. 2002;17:1752–1760. doi: 10.1359/jbmr.2002.17.10.1752. [DOI] [PubMed] [Google Scholar]
- 71.Koller DL, Liu G, Econs MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC, Jr, Foroud T, Peacock M. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16:985–991. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- 72.Koller DL, Schriefer J, Sun Q, Shultz KL, Donahue LR, Rosen CJ, Foroud T, Beamer WG, Turner CH. Genetic effects for femoral biomechanics, structure, and density in C57BL/6J and C3 h/HeJ inbred mouse strains. J Bone Miner Res. 2003;18:1758–1765. doi: 10.1359/jbmr.2003.18.10.1758. [DOI] [PubMed] [Google Scholar]
- 73.Kopperdahl DL, Keaveny TM. Yield strain behavior of trabecular bone. J Biomech. 1998;31:601–608. doi: 10.1016/s0021-9290(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 74.Kotzar GM, Davy DT, Goldberg VM, Heiple KG, Berilla J, Heiple KG, Jr, Brown RH, Burstein AH. Telemeterized in vivo hip joint force data: a report on two patients after total hip surgery. J Orthop Res. 1991;9:621–633. doi: 10.1002/jor.1100090502. [DOI] [PubMed] [Google Scholar]
- 75.Lang DH, Sharkey NA, Mack HA, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, McClearn GE. Quantitative trait loci analysis of structural and material skeletal phenotypes in C57BL/6J and DBA/2 second-generation and recombinant inbred mice. J Bone Miner Res. 2005;20:88–99. doi: 10.1359/JBMR.041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lanyon LE, Hampson WG, Goodship AE, Shah JS. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand. 1975;46:256–268. doi: 10.3109/17453677508989216. [DOI] [PubMed] [Google Scholar]
- 77.LeBlanc A, Schneider V, Krebs J, Evans H, Jhingran S, Johnson P. Spinal bone mineral after 5 weeks of bed rest. Calcif Tissue Int. 1987;41:259–261. doi: 10.1007/BF02555226. [DOI] [PubMed] [Google Scholar]
- 78.Li X, Masinde G, Gu W, Wergedal J, Mohan S, Baylink DJ. Genetic dissection of femur breaking strength in a large population (MRL/MpJ x SJL/J) of F2 mice: single QTL effects, epistasis, and pleiotropy. Genomics. 2002;79:734–740. doi: 10.1006/geno.2002.6760. [DOI] [PubMed] [Google Scholar]
- 79.Linde F, Gøthgen CB, Hvid I, Pongsoipetch B. Mechanical properties of trabecular bone by a non-destructive compression testing approach. Eng Med. 1988;17:23–29. doi: 10.1243/emed_jour_1988_017_008_02. [DOI] [PubMed] [Google Scholar]
- 80.Linde F, Hvid I, Pongsoipetch B. Energy absorptive properties of human trabecular bone specimens during axial compression. J Orthop Res. 1989;7:432–439. doi: 10.1002/jor.1100070316. [DOI] [PubMed] [Google Scholar]
- 81.Liu XS, Cohen A, Shane E, Stein E, Rogers H, Kokolus SL, Yin PT, McMahon DJ, Lappe JM, Recker RR, Guo XE. Individual trabeculae segmentation (ITS)-based morphological analysis of high-resolution peripheral quantitative computed tomography images detects abnormal trabecular plate and rod microarchitecture in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2010;25:1496–1505. doi: 10.1002/jbmr.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? J Bone Miner Res. 2001;16:1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 83.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ (Int Ed) 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCalden RW, McGeough JA, Court-Brown CM. Age-related changes in the compressive strength of cancellous bone: the relative importance of changes in density and trabecular architecture. J Bone Joint Surg Am. 1997;79:421–427. doi: 10.2106/00004623-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 85.McElhaney JH. Dynamic response of bone and muscle tissue. J Appl Physiol. 1966;21:1231–1236. doi: 10.1152/jappl.1966.21.4.1231. [DOI] [PubMed] [Google Scholar]
- 86.Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Wahner HW, Riggs BL. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8:1227–1233. doi: 10.1002/jbmr.5650081010. [DOI] [PubMed] [Google Scholar]
- 87.Mittra E, Rubin C, Qin YX. Interrelationship of trabecular mechanical and microstructural properties in sheep trabecular bone. J Biomech. 2005;38:1229–1237. doi: 10.1016/j.jbiomech.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 88.Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36:897–904. doi: 10.1016/s0021-9290(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 89.Mosekilde L, Mosekilde L. Normal vertebral body size and compressive strength: relations to age and to vertebral and iliac trabecular bone compressive strength. Bone. 1986;7:207–212. doi: 10.1016/8756-3282(86)90019-0. [DOI] [PubMed] [Google Scholar]
- 90.Mosekilde L, Mosekilde L, Danielsen CC. Biomechanical competence of vertebral trabecular bone in relation to ash density and age in normal individuals. Bone. 1987;8:79–85. doi: 10.1016/8756-3282(87)90074-3. [DOI] [PubMed] [Google Scholar]
- 91.Mosekilde L, Viidik A. Correlation between the compressive strength of iliac and vertebral trabecular bone in normal individuals. Bone. 1985;6:291–295. doi: 10.1016/8756-3282(85)90317-5. [DOI] [PubMed] [Google Scholar]
- 92.Naghii MR, Torkaman G, Mofid M. Effects of boron and calcium supplementation on mechanical properties of bone in rats. Biofactors. 2006;28:195–201. doi: 10.1002/biof.5520280306. [DOI] [PubMed] [Google Scholar]
- 93.Nieves JW, Barrett-Connor E, Siris ES, Zion M, Barlas S, Chen YT. Calcium and vitamin D intake influence bone mass, but not short-term fracture risk, in Caucasian postmenopausal women from the National Osteoporosis Risk Assessment (NORA) study. Osteoporos Int. 2008;19:673–679. doi: 10.1007/s00198-007-0501-2. [DOI] [PubMed] [Google Scholar]
- 94.Nyman JS, Roy A, Shen XM, Acuna RL, Tyler JH, Wang XD. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39:931–938. doi: 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis: implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paschalis EP, Betts F, DiCarlo E, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 97.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pistoia W, Rietbergen B, Rüegsegger P. Mechanical consequences of different scenarios for simulated bone atrophy and recovery in the distal radius. Bone. 2003;33:937–945. doi: 10.1016/j.bone.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 99.Recker RR, Deng HW. Role of genetics in osteoporosis. Endocrine. 2002;17:55–66. doi: 10.1385/ENDO:17:1:55. [DOI] [PubMed] [Google Scholar]
- 100.Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988;21:155–168. doi: 10.1016/0021-9290(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 101.Rohlmann A, Graichen F, Kayser R, Bender A, Bergmann G. Loads on a telemeterized vertebral body replacement measured in two patients. Spine (Phila Pa 1976) 2008;33:1170–1179. doi: 10.1097/BRS.0b013e3181722d52. [DOI] [PubMed] [Google Scholar]
- 102.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 103.Rosen CJ, Beamer WG, Donahue LR. Defining the genetics of osteoporosis: using the mouse to understand man. Osteoporos Int. 2001;12:803–810. doi: 10.1007/s001980170030. [DOI] [PubMed] [Google Scholar]
- 104.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 105.Ross PD, Davis JW, Vogel JM, Wasnich RD. A critical review of bone mass and the risk of fractures in osteoporosis. Calcif Tissue Int. 1990;46:149–161. doi: 10.1007/BF02555036. [DOI] [PubMed] [Google Scholar]
- 106.Ruff CB, Hayes WC. Sex differences in age-related remodeling of the femur and tibia. J Orthop Res. 1988;6:886–896. doi: 10.1002/jor.1100060613. [DOI] [PubMed] [Google Scholar]
- 107.Ruppel ME, Burr DB, Miller LM. Chemical makeup of microdamaged bone differs from undamaged bone. Bone. 2006;39:318–324. doi: 10.1016/j.bone.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 108.Russo CR, Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Volpato S, Guralnik JM, Harris T, Ferrucci L. Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 109.Schuit SC, Klift M, Weel AE, Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, Leeuwen JP, Pols HA. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Shea B, Wells G, Cranney A, Zytaruk N, Robinson V, Griffith L, Ortiz Z, Peterson J, Adachi J, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23:552–559. doi: 10.1210/er.2001-7002. [DOI] [PubMed] [Google Scholar]
- 111.Silva MJ, Gibson LJ. Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone. 1997;21:191–199. doi: 10.1016/s8756-3282(97)00100-2. [DOI] [PubMed] [Google Scholar]
- 112.Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC., Jr Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–567. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- 113.Smith EL, Gilligan C, McAdam M, Ensign CP, Smith PE. Deterring bone loss by exercise intervention in premenopausal and postmenopausal women. Calcif Tissue Int. 1989;44:312–321. doi: 10.1007/BF02556310. [DOI] [PubMed] [Google Scholar]
- 114.Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res. 1992;7:761–769. doi: 10.1002/jbmr.5650070706. [DOI] [PubMed] [Google Scholar]
- 115.Snyder BD, Hauser-Kara DA, Hipp JA, Zurakowski D, Hecht AC, Gebhardt MC. Predicting fracture through benign skeletal lesions with quantitative computed tomography. J Bone Joint Surg Am. 2006;88:55–70. doi: 10.2106/JBJS.D.02600. [DOI] [PubMed] [Google Scholar]
- 116.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 117.Timlin JA, Carden A, Morris MD, Rajachar RM, Kohn DH. Raman spectroscopic imaging markers for fatigue-related microdamage in bovine bone. Anal Chem. 2000;72:2229–2236. doi: 10.1021/ac9913560. [DOI] [PubMed] [Google Scholar]
- 118.Turner CH, Hsieh YF, Müller R, Bouxsein ML, Baylink DJ, Rosen CJ, Grynpas MD, Donahue LR, Beamer WG. Genetic regulation of cortical and trabecular bone strength and microstructure in inbred strains of mice. J Bone Miner Res. 2000;15:1126–1131. doi: 10.1359/jbmr.2000.15.6.1126. [DOI] [PubMed] [Google Scholar]
- 119.Turner CH, Sun Q, Schriefer J, Pitner N, Price R, Bouxsein ML, Rosen CJ, Donahue LR, Shultz KL, Beamer WG. Congenic mice reveal sex-specific genetic regulation of femoral structure and strength. Calcif Tissue Int. 2003;73:297–303. doi: 10.1007/s00223-002-1062-1. [DOI] [PubMed] [Google Scholar]
- 120.Turner RT, Bell NH, Duvall P, Bobyn JD, Spector M, Holton EM, Baylink DJ. Spaceflight results in formation of defective bone. Proc Soc Exp Biol Med. 1985;180:544–549. doi: 10.3181/00379727-180-42215. [DOI] [PubMed] [Google Scholar]
- 121.Tylavsky FA, Bortz AD, Hancock RL, Anderson JJ. Familial resemblance of radial bone mass between premenopausal mothers and their college-age daughters. Calcif Tissue Int. 1989;45:265–272. doi: 10.1007/BF02556017. [DOI] [PubMed] [Google Scholar]
- 122.Ulrich D, Hildebrand T, Rietbergen B, Müller R, Rüegsegger P. The quality of trabecular bone evaluated with micro-computed tomography, FEA and mechanical testing. Stud Health Technol Inform. 1997;40:97–112. [PubMed] [Google Scholar]
- 123.Meulen MC, Ashford MW, Jr, Kiratli BJ, Bachrach LK, Carter DR. Determinants of femoral geometry and structure during adolescent growth. J Orthop Res. 1996;14:22–29. doi: 10.1002/jor.1100140106. [DOI] [PubMed] [Google Scholar]
- 124.Meulen MC, Jepsen KJ, Mikić B. Understanding bone strength: size isn’t everything. Bone. 2001;29:101–104. doi: 10.1016/s8756-3282(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 125.Meulen MC, Morey-Holton ER, Carter DR. Hindlimb suspension diminishes femoral cross-sectional growth in the rat. J Orthop Res. 1995;13:700–707. doi: 10.1002/jor.1100130509. [DOI] [PubMed] [Google Scholar]
- 126.Rietbergen B, Huiskes R, Eckstein F, Rüegsegger P. Trabecular bone tissue strains in the healthy and osteoporotic human femur. J Bone Miner Res. 2003;18:1781–1788. doi: 10.1359/jbmr.2003.18.10.1781. [DOI] [PubMed] [Google Scholar]
- 127.Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 128.Volkman SK, Galecki AT, Burke DT, Miller RA, Goldstein SA. Quantitative trait loci that modulate femoral mechanical properties in a genetically heterogeneous mouse population. J Bone Miner Res. 2004;19:1497–1505. doi: 10.1359/JBMR.040506. [DOI] [PubMed] [Google Scholar]
- 129.Volkman SK, Galecki AT, Burke DT, Paczas MR, Moalli MR, Miller RA, Goldstein SA. Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. J Bone Miner Res. 2003;18:1497–1505. doi: 10.1359/jbmr.2003.18.8.1497. [DOI] [PubMed] [Google Scholar]
- 130.Welch JM, Turner CH, Devareddy L, Arjmandi BH, Weaver CM. High impact exercise is more beneficial than dietary calcium for building bone strength in the growing rat skeleton. Bone. 2008;42:660–668. doi: 10.1016/j.bone.2007.12.220. [DOI] [PubMed] [Google Scholar]
- 131.Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, Akeson WH. The effect of prolonged physical training on the properties of long bone: a study of Wolff’s law. J Bone Joint Surg Am. 1981;63:780–787. [PubMed] [Google Scholar]
- 132.Yershov Y, Baldini TH, Villagomez S, Young T, Martin ML, Bockman RS, Peterson MG, Blank RD. Bone strength and related traits in HcB/Dem recombinant congenic mice. J Bone Miner Res. 2001;16:992–1003. doi: 10.1359/jbmr.2001.16.6.992. [DOI] [PubMed] [Google Scholar]