Abstract
Background
Highly crosslinked and thermally treated polyethylenes were clinically introduced to reduce wear and osteolysis. Although the crosslinking process improves the wear performance, it also introduces free radicals into the polymer that can subsequently oxidize. Thermal treatments have been implemented to reduce oxidation; however, the efficacy of these methods with regard to reducing in vivo oxidative degradation remains to be seen. Polyethylene oxidation is a concern because it can compromise the ultimate strength and ductility of the material.
Questions/purposes
We analyzed the oxidation, oxidation potential, and mechanical behavior of thermally treated highly crosslinked polyethylene retrieved acetabular liners.
Methods
Three hundred seven acetabular liners were collected from consecutive revision surgeries at six institutions over a 10-year period. Twenty-four were sterilized using nonionizing methods, 43 were sterilized in an inert environment, 80 were highly crosslinked and annealed, and 160 were highly crosslinked and remelted. Oxidation and oxidation potential were assessed by Fourier transmission infrared spectroscopy. Mechanical behavior was assessed by the small punch test.
Results
Oxidation and hydroperoxide (oxidation potential) indices were elevated in the annealed and gamma inert sterilized groups compared with those of the remelted liners and uncrosslinked gas sterilized controls, particularly at the rim. We also detected an increase in oxidation over time at the bearing surface of the remelted group. Ultimate strength of the polyethylene at the bearing surface was negatively correlated with implantation time for the annealed liners.
Conclusions
Within the first decade of implantation, the clinical outlook for first-generation highly crosslinked polyethylene remains promising. However, ongoing research continues to be warranted for first-generation highly crosslinked polyethylene bearings to monitor the implications of elevated oxidation at the rim of annealed liners as well as to better understand the subtle changes in oxidation at the bearing surface of remelted liners that occur in vivo.
Introduction
Conventional UHMWPE (hereafter, polyethylene) may be sterilized by gamma radiation (usually 25–40 kGy dose) or by gas (eg, ethylene oxide [EtO]). In the past decade, highly crosslinked and thermally treated polyethylenes were introduced to reduce wear and osteolysis [3, 17, 22]. First-generation highly crosslinked polyethylenes were thermally stabilized by annealing (heating below the melting point) or by remelting (heating above the melting point) [17] to reduce free radical concentrations and thereby reduce oxidation. Many clinical studies of first-generation highly crosslinked polyethylenes have reported superior wear performance of both annealed and remelted materials in the first decade of use [4, 9–14, 16, 24, 28] when compared with controls. Comparatively few studies have explored their long-term oxidative stability in vivo [8, 18, 20, 30].
In vivo oxidative stability of polyethylene is believed to depend primarily on the free radical content after irradiation and sterilization [17]. For example, uncrosslinked ethylene oxide sterilized components that have never been exposed to radiation contain no residual macroradicals and thus would likely be oxidatively stable in vivo. Remelted polyethylene, with reportedly undetectable free radical concentrations [27], would likewise be considered relatively oxidatively stable. First-generation annealed and highly crosslinked polyethylene, which contain a higher concentration of macroradicals as compared with gamma-sterilized conventional polyethylene liners [5], would be expected to exhibit greater in vivo oxidation than uncrosslinked gas-sterilized liners and remelted highly crosslinked liners. At elevated levels, oxidation may compromise the ultimate strength and ductility of the polyethylene, leading to the complete loss of mechanical integrity [8, 17, 19].
Because of the substantially lower clinical wear rates reported in the literature (20%–95% depending on measurement method, control material, and length of followup [4, 9–14, 16, 24, 28]) of first-generation crosslinked polyethylenes, the clinical importance of in vivo oxidation for hip arthroplasty remains open to debate. For annealed and gamma inert sterilized highly crosslinked components, in vivo oxidation occurs preferentially at the rim of the liner, which has the greatest exposure to oxygen-containing body fluids and tissues [17]. Previous studies have reported lower oxidation of the bearing surface for annealed and gamma inert sterilized highly crosslinked liners [7, 18] as compared with the rim and may explain the general preservation of wear properties seen in clinical studies [9, 18, 28]. However, in the case of a malfunctioning hip such as chronic instability with dislocation, rim oxidation may contribute to localized delamination or rim fracture [18]. Previous studies of remelted liners, including short-term retrievals, demonstrated low levels of oxidation [5, 30]. Additional longer-term studies are necessary to monitor the oxidative stability of these materials as well as to assess the potential impact of in vivo oxidation on wear and mechanical behavior.
We asked whether (1) annealed and gamma inert sterilized polyethylene acetabular components would exhibit greater oxidation and oxidation potential than the remelted and EtO sterilized components; (2) as a result of the lack of free radicals, both the remelted and gas sterilized components would exhibit undetectable oxidation; and (3) mechanical properties would be unimpaired at the articulating surface of the polyethylene hip bearings regardless of formulation.
Materials and Methods
We collected 307 acetabular liners from consecutive revision surgeries at six institutions (with Institutional Review Board approval) over a 10-year period. The implant cleaning and storage for the multicenter retrieval program was carried out using institutional protocols. The liners were analyzed continuously over the course of the last 10 years and were expeditiously stored in a −80°C freezer to prevent ex vivo degradation from occurring before testing.
Of the 307 acetabular liners, 24 were sterilized using nonionizing methods (“nonionized”; n = 16 [EtO sterilization], Smith & Nephew, Memphis, TN, and n = 8 [gas plasma sterilization], DePuy, Warsaw, IN), 43 were gamma sterilized in an inert environment (“gamma inert”; n = 40, Zimmer, Warsaw, IN; n = 3, DePuy), 80 were annealed (“annealed”; Crossfire; Stryker, Mahwah, NJ), and 160 were remelted from four formulations (Durasul; Zimmer, n = 7; Marathon; DePuy, n = 25; XLPE; Smith & Nephew, n = 15; and Longevity; Zimmer, n = 113; Table 1). The nonionized and gamma inert liners were implanted for a longer period of time than both the annealed and the remelted liners (Table 2). There was no difference in body mass index detected across the groups (Table 2). The patients with nonionized liners were younger than those implanted with gamma inert, annealed, and the remelted liners (Table 2). Preliminary statistical analysis revealed there was no difference among the four remelted groups in oxidative properties (Table 3); therefore, they were treated as one group (“remelted”) for statistical purposes.
Table 1.
Processing details of the seven different cohorts of acetabular liners [1]
| Cohort | Manufacturer | Resin | Highly crosslinked? | Thermal treatment | Total irradiation dose (kGy) | Terminal sterilization | Analysis category |
|---|---|---|---|---|---|---|---|
| GP | DePuy Orthopedics | GUR 1050 | N | None | 0 | Nonionized (gas plasma) | Nonionized |
| EtO | Smith & Nephew | GUR 4150 | N | None | 0 | Nonionized (EtO) | Nonionized |
| Gamma inert | Zimmer, DePuy Orthopedics | GUR 1050 | N | None | 25–40 | Gamma in inert environment | Gamma inert |
| Crossfire™ | Stryker Orthopedics | GUR 1050 | Y | Annealed | 105 | Gamma in nitrogen | Annealed |
| Durasul™ | Zimmer | GUR 1050 | Y | Remelted | 95 | EtO gas | Remelted |
| Marathon™ | DePuy Orthopedics | GUR 1050 GUR 4150 (n = 1) | Y | Remelted then annealed | 50 | Gas plasma | Remelted |
| XLPE™ | Smith & Nephew | GUR 1050 | Y | Remelted | 100 | EtO gas | Remelted |
| Longevity™ | Zimmer | GUR 1050 | Y | Remelted | 100 | Gas plasma | Remelted |
N = no; Y = yes; EtO = ethylene oxide; GP = gas plasma.
Table 2.
Clinical details of the seven different cohorts of acetabular liner
| Cohort | Number | Implantation time (years) 95% CI Range | Percent Traced | Shelf life (years) 95% CI Range | Gender (percent female) | Age at insertion (years) 95% CI Range | Body mass index (kg/m2) 95% CI Range | UCLA maximum score (average [range]) |
|---|---|---|---|---|---|---|---|---|
| GP | 8 | 6.4 ± 3.7 3.3–9.5 1.1–10.5 |
100% | 0.8 ± 1.0 −0.1–1.6 0.0–3.1 |
50% | 58 ± 16 45–71 40–79 |
32 ± 7 26–38 23–46 |
6 (3–10) |
| EtO | 16 | 8.7 ± 3.0 7.0–10.3 1.4–12.8 |
75% | 2.1 ± 3.3 0.0–4.2 0.1–11.3 |
63% | 43 ± 17 34–53 13–72 |
33 ± 7 29–37 24–45 |
7 (2–10) |
| Gamma inert | 43 | 5.8 ± 3.7 4.7–7.0 0.0–13.8 |
72% | 1.1 ± 1.7 0.4–1.7 0.0–9.3 |
61% | 59 ± 15 54–63 27–82 |
30 ± 7 27–32 16–58 |
6 (1–9) |
| Crossfire™ | 80 | 3.5 ± 2.6 2.9–4.0 0.0–10.3 |
78% | 0.5 ± 0.7 0.3–0.7 0.0–3.4 |
54% | 62 ± 12 60–65 29–81 |
28 ± 6 27–30 16–47 |
5 (2–10) |
| Durasul™ | 7 | 0.7 ± 0.7 0.1–1.3 0.1–2.0 |
14% | 1.3 (n = 1) | 29% | 63 ± 17 42–84 46–86 |
34 ± 12 19–49 20–52 |
4 (3–6) |
| Marathon™ | 25 | 2.0 ± 2.1 1.2–2.9 0.1–8.7 |
76% | 0.8 ± 1.0 0.3–1.3 0.1–3.6 |
60% | 57 ± 16 50–64 24–92 |
30 ± 8 26–33 19–50 |
6 (3–10) |
| XLPE™ | 15 | 2.7 ± 3.5 0.6–4.7 0.1–11.4 |
47% | 2.2 ± 1.6 0.7–3.8 0.4–4.8 |
73% | 55 ± 13 48–62 37–77 |
29 ± 5 26–32 25–46 |
5 (2–10) |
| Longevity™ | 113 | 1.9 ± 1.9 1.5–2.2 0.0–8.0 |
66% | 0.9 ± 1.1 0.6 ± 1.1 0.0–4.1 |
55% | 61 ± 13 58–63 33–89 |
28 ± 6 27–29 13–46 |
5 (1–10) |
| Total nonionized | 24 | 7.9 ± 3.4 6.4–9.3 1.1–12.8 |
83% | 1.6 ± 2.7 0.3–2.8 0.0–11.3 |
58% | 49 ± 18 41–56 13–79 |
33 ± 7 29–36 23–46 |
7 (2–10) |
| Total remelted | 160 | 1.9 ± 2.1 1.6–2.2 0.0–11.4 |
63% | 1.0 ± 1.1 0.7–1.2 0.0–4.8 |
56% | 60 ± 14 57–62 24–92 |
29 ± 6 28–30 13–52 |
5 (1–10) |
CI = confidence interval; GP = gas plasma; EtO = ethylene oxide.
Table 3.
Oxidation and oxidation potential of the acetabular liners
| Cohort | Oxidation index (average ± SD) 95% CI Range |
Hydroperoxide index (average ± SD) 95% CI Range |
Superior surface mechanical properties (average ± SD) 95% CI Range |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bearing | Backside | Rim | Bearing | Backside | Rim | Peak load (N) | Ultimate load (N) | Ultimate displacement (mm) | Work to failure (mJ) | |
| GP | 0.1 ± 0.1 0.1–0.2 0.1–0.2 |
0.1 ± 0.1 0.1–0.2 0.1–0.3 |
0.2 ± 0.1 0.1–0.3 0.1–0.5 |
0.2 ± 0.0 0.1–0.2 0.1–0.2 |
0.1 ± 0.0 0.1–0.2 0.1–0.2 |
0.1 ± 0.0 0.1–0.2 0.1–0.2 |
70.6 ± 4.1 67.2–74.0 63.8–75.5 |
73.1 ± 2.1 71.4–74.8 70.2–76.5 |
4.8 ± 0.4 4.5–5.2 4.4–5.6 |
271 ± 28 248–294 239–319 |
| EtO | 0.1 ± 0.0 0.1–0.1 0.0–0.1 |
0.1 ± 0.1 0.1–0.1 0.0–0.3 |
0.1 ± 0.0 0.0–0.1 0.0–0.1 |
0.1 ± 0.0 0.1–0.1 0.0–0.2 |
0.1 ± 0.0 0.1–0.1 0.1–0.1 |
0.1 ± 0.0 0.1–0.1 0.0–0.1 |
69.8 ± 4.6 67.2–72.3 65.2–78.8 |
69.5 ± 3.1 67.7–71.2 64.2–78.0 |
4.7 ± 0.2 4.6–4.8 4.4–5.0 |
259 ± 13 252–267 235–291 |
| Gamma inert | 0.4 ± 0.4 0.2–0.5 0.0–2.1 |
0.4 ± 0.6 0.2–0.6 0.0–3.0 |
1.1 ± 1.6 0.6–1.6 0.0–6.2 |
0.2 ± 0.1 0.2–0.3 0.1–0.6 |
0.2 ± 0.1 0.2–0.3 0.1–0.6 |
0.4 ± 0.2 0.3–0.4 0.1–0.8 |
69.3 ± 3.3 68.2–70.3 63.0–79.6 |
78.9 ± 10.6 75.6–82.2 47.4–103.7 |
4.5 ± 0.4 4.4–4.6 3.8–5.6 |
257 ± 35 246–268 202–377 |
| Crossfire™ | 0.5 ± 0.4 0.4–0.6 0.0–2.1 |
0.4 ± 0.2 0.3–0.4 0.1–1.3 |
3.7 ± 3.1 3.0–4.4 0.2–8.8 |
0.3 ± 0.2 0.3–0.3 0.1–1.0 |
0.3 ± 0.2 0.3–0.4 0.1–1.0 |
0.5 ± 0.2 0.5–0.6 0.1–1.4 |
70.9 ± 4.7 69.9–72.0 49.4–79.3 |
92.1 ± 9.7 89.9–94.2 61.3–107.5 |
3.7 ± 0.4 3.6–3.8 2.5–4.6 |
220 ± 36 212–229 127–308 |
| Durasul™ | 0.1 ± 0.0 0.1–0.1 0.1–0.1 |
0.1 ± 0.1 0.1–0.2 0.1–0.2 |
0.1 ± 0.0 0.1–0.1 0.0–0.2 |
0.1 ± 0.1 0.1–0.2 0.1–0.3 |
0.1 ± 0.1 0.1–0.2 0.1–0.3 |
0.1 ± 0.0 0.1–0.1 0.1–0.2 |
58.0 ± 2.3 55.9–60.2 56.2–62.5 |
67.5 ± 4.0 63.8–71.2 63.3–75.0 |
3.8 ± 0.1 3.7–3.9 3.5–4.0 |
175 ± 8 167–183 162–190 |
| Marathon™ | 0.1 ± 0.1 0.1–0.1 0.0–0.2 |
0.1 ± 0.1 0.1–0.1 0.0–0.3 |
0.1 ± 0.1 0.1–0.2 0.0–0.6 |
0.1 ± 0.1 0.1–0.1 0.1–0.3 |
0.1 ± 0.0 0.1–0.1 0.0–0.2 |
0.1 ± 0.1 0.1–0.2 0.1–0.5 |
64.8 ± 6.1 62.2–67.5 57.8–78.1 |
85.2 ± 5.6 82.8–87.6 76.7–97.3 |
4.3 ± 0.3 4.2–4.5 3.8–5.0 |
242 ± 29 230–255 204–304 |
| XLPE™ | 0.1 ± 0.1 0.1–0.1 0.0–0.2 |
0.1 ± 0.0 0.1–0.1 0.0–0.2 |
0.1 ± 0.0 0.0–0.1 0.0–0.1 |
0.1 ± 0.1 0.1–0.2 0.1–0.3 |
0.1 ± 0.0 0.1–0.1 0.1–0.2 |
0.1 ± 0.0 0.1–0.1 0.1–0.2 |
62.8 ± 3.2 61.0–64.6 59.5–72.5 |
85.5 ± 8.7 80.5–90.5 66.4–103.9 |
4.0 ± 0.5 3.7–4.3 3.3–4.9 |
221 ± 50 192–250 145–327 |
| Longevity™ | 0.1 ± 0.1 0.1–0.1 0.0–0.5 |
0.1 ± 0.1 0.1–0.1 0.0–0.3 |
0.1 ± 0.1 0.1–0.1 0.0–0.5 |
0.1 ± 0.1 0.1–0.1 0.0–0.4 |
0.1 ± 0.1 0.1–0.1 0.0–0.3 |
0.1 ± 0.1 0.1–0.1 0.0–0.4 |
62.4 ± 4.3 61.6–63.2 52.8–73.7 |
83.4 ± 10.6 81.3–85.4 56.8–117.1 |
4.3 ± 0.9 4.1–4.4 2.3–6.5 |
239 ± 80 224–254 93–454 |
| Total nonionized | 0.1 ± 0.1 0.1–0.1 0.0–0.2 |
0.1 ± 0.1 0.1–0.1 0.0–0.3 |
0.1 ± 0.1 0.1–0.1 0.0–0.5 |
0.1 ± 0.0 0.1–0.1 0.0–0.2 |
0.1 ± 0.0 0.1–0.1 0.1–0.2 |
0.1 ± 0.0 0.1–0.1 0.0–0.2 |
70.0 ± 4.4 68.2–71.9 63.8–78.8 |
70.7 ± 3.3 69.3–72.2 64.2–78.0 |
4.7 ± 0.3 4.6–4.9 4.4–5.6 |
263 ± 20 255–272 235–319 |
| Total remelted | 0.1 ± 0.1 0.1–0.1 0.0–0.5 |
0.1 ± 0.1 0.1–0.1 0.0–0.3 |
0.1 ± 0.1 0.1–0.1 0.0–0.6 |
0.1 ± 0.1 0.1–0.1 0.0–0.4 |
0.1 ± 0.0 0.1–0.1 0.0–0.3 |
0.1 ± 0.1 0.1–0.1 0.0–0.5 |
62.6 ± 4.6 61.9–63.4 52.8–78.1 |
83.1 ± 10.2 81.5–84.8 56.8–117.1 |
4.2 ± 0.8 4.1–4.4 2.3–6.5 |
235 ± 71 223–246 93–454 |
CI = confidence interval; GP = gas plasma; EtO = ethylene oxide.
Fourier transmission infrared spectroscopy (FTIR) was conducted on sections from the retrieved components to evaluate oxidation. Thin sections (200 μm) were obtained from the superior/inferior axis of the acetabular liners using a microtome (Fig. 1C). To eliminate confounding of the FTIR oxidation analysis resulting from adsorbed lipids, microtomed sections were boiled in heptane for 6 hours for lipid extraction [15]. FTIR assessment of oxidation was conducted on the extracted polyethylene sections in accordance with ASTM F2102 [1]. The sections were scanned through the thickness in 0.1-mm deep increments from the surface using a FTIR spectrometer with a microscope attachment. At each location, FTIR spectra resulted from averaging 32 independent scans with a resolution of 4 cm−1. The maximum oxidation index (OI) was calculated from the infrared spectra as the area ratio between the carbonyl peak centered at 1715 cm−1 and the reference band at 1370 cm−1. Previous studies have suggested an ASTM OI exceeding 3 is the critical level, above which the mechanical properties are reduced and severely compromised [8, 17, 19]. An OI of less than 1 on the ASTM scale is associated with low oxidation and negligible impact on mechanical behavior. Regions of interest from the sections of the acetabular liners included the rim and backside surfaces in both the superior and inferior regions of the component as well as the superior region of the bearing surface.
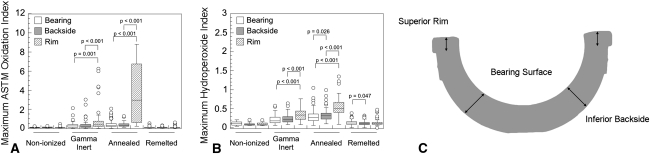
Fig. 1.
Box plots depicting the regional variation in oxidation index (A) and hydroperoxide index (B). Note the lower levels of oxidation in the remelted and nonionized liners. A schematic (C) illustrates the sampling locations used for analysis.
After oxidation analysis, hydroperoxide content was also evaluated by exposing microtomed sections to nitric oxide for at least 16 hours, thereby converting hydroperoxides to nitrates that are easily identifiable using FTIR [6]. After exposure, the sections were scanned using the same protocol as used for oxidation analysis. The hydroperoxide index was calculated by dividing the area under the curve between 1600 cm−1 and 1670 cm−1 by the area under the curve between 1330 cm−1 and 1396 cm−1 as described previously [17, 26]. All FTIR data were collected using a Thermo Nicolet 6700 FTIR spectroscope with a Continuum FTIR microscope attachment (Thermo Fisher Scientific, Waltham, MA).
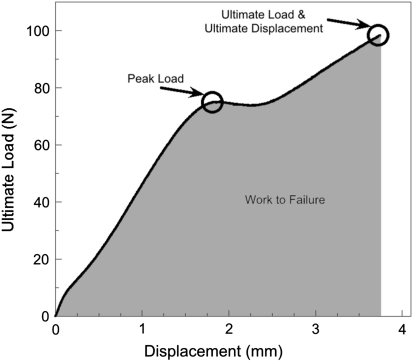
For mechanical testing, the small punch test was used as a result of the fact that conventional mechanical tests on retrieved components are impossible as a result of the unique geometry and limited volumes of material. Four cores were obtained from each liner (two from the superior portion and two from the inferior portion of the cup). From each core, two samples, one from near the surface (0.0–0.5 mm) and one subsurface (2.0–2.5 mm), were machined into miniature disk specimens measuring 0.5 mm thick and 6.4 mm in diameter. Small punch testing was conducted in accordance with ASTM 2183 [2]. Four metrics can be extracted from the small punch test, namely the peak load, ultimate load, ultimate displacement, and the work to failure [2] (Appendix 1). These parameters provide metrics of yielding, ultimate strength, ductility, and toughness of the material under multiaxial loading conditions [2]. Ultimate load was chosen as the primary test metric for evaluation, because ultimate load is reportedly most sensitive to oxidative degradation and is a metric of the ultimate strength of the material [20, 23].
Appendix 1.
Schematic of a typical load displacement curve from a small punch test illustrating the 4 metrics obtained during the test.
The distributions of oxidation, hydroperoxide indices, and ultimate load were typically nonnormal (Shapiro-Wilk test). Therefore, differences in oxidation, oxidation potential, and ultimate load between the cohorts were assessed by the Kruskal-Wallis analysis of variance (ANOVA) with a post hoc Dunn test. Regional variations within cohorts were analyzed using the Friedman’s ANOVA with a post hoc Dunn test. Correlation between the metrics and implantation time was assessed using the Spearman’s ranked correlation test. For all statistical tests, PASW Statistics (Version 18.0.0; IBM, Chicago, IL) was used.
Results
Annealed liners had higher oxidation indices than the gamma inert, nonionizing, and remelted liners at both the backside surface (p = 0.048, < 0.001, and < 0.0001, respectively; Table 3) and at the rim (p = 0.025, < 0.001, and < 0.001, respectively). At the bearing surface, annealed and gamma inert liners were indistinguishable (p = 0.489) and had higher oxidation indices than nonionized (p < 0.001 and p < 0.001, respectively; Table 3) and remelted (p < 0.001 and p < 0.001, respectively; Table 3) liners. The hydroperoxide index was similar between the annealed and gamma inert liners at all locations (p = 0.087, 0.130, and 0.138 at the bearing, backside, and rim surfaces, respectively; Table 3) and was greater than the hydroperoxide index of the nonionized (p < 0.001 at all locations; Table 3) and remelted (p < 0.001 at all locations; Table 3) liners. Oxidation indices also varied depending on the region sampled for the annealed and gamma inert liners. Oxidation indices were higher at the rim for both gamma inert and annealed cohorts as compared with the bearing surface (p = 0.001 and p < 0.001, respectively) (Fig. 1) and the backside surface (p < 0.001 and p < 0.001, respectively). Variation in oxidation indices among the bearing, backside, and rim surfaces was not detected in the nonionized (p = 0.197) or remelted (p = 0.357) liners. Oxidation correlated with implantation time only at the rim for annealed liners (ρ = 0.69, p < 0.001) and at the bearing surface of the remelted liners (ρ = 0.205, p = 0.01).
Despite the low hydroperoxide indices, we were able to detect low levels of oxidation (oxidation index > 0.1) in both the nonionized and remelted retrievals. The oxidation index at the bearing surface of the remelted liners correlated with implantation time (ρ = 0.205, p = 0.01) but not at the backside (ρ = −0.022, p = 0.782) or rim (ρ = 0.019, p = 0.816) surfaces. We could not detect variations in oxidation among the bearing, backside, and rim locations in either the nonionized (p = 0.197) or the remelted (p = 0.357) liners.
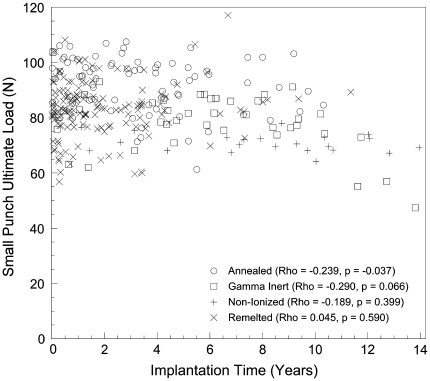
The ultimate load at the superior articulating surface was negatively correlated with implantation time in the annealed liners (ρ = −0.239; p = 0.037; Fig. 2), but this correlation was not seen in the gamma inert (ρ = −0.290; p = 0.066), nonionized (ρ = −0.189; p = 0.399), or remelted (ρ = 0.045; p = 0.590) liners. In the annealed and gamma inert components, the ultimate load at the inferior articulating surface also negatively correlated with implantation time (ρ = −0.341 and −0.311; p = 0.003 and 0.048, respectively). The annealed components had the highest ultimate load at the superior surface (p < 0.001) and subsurface (p < 0.001) and the inferior subsurface (p < 0.001). There were no differences in the ultimate load between the superior and inferior portions of the liners near the surface or subsurface within any of the groups except in the annealed group (p = 0.006).
Fig. 2.
Plots illustrating the progression of the ultimate load with implantation time.
Discussion
Oxidative degradation of polyethylene continues to be a concern in THA. Thermal treatments (annealing and remelting) have been implemented to reduce oxidation; however, the efficacy of these methods with regard to reducing in vivo oxidative degradation remains to be seen. We investigated the oxidative and mechanical behavior of a large consecutive series of retrieved acetabular liners. We asked whether (1) annealed and gamma inert sterilized polyethylene acetabular components would exhibit greater oxidation and oxidation potential than the remelted and nonionized components; (2) as a result of the lack of free radicals, both the remelted and gas sterilized components would exhibit undetectable oxidation; and (3) mechanical properties would be maintained at the articulating surface of the polyethylene hip bearings regardless of formulation.
We acknowledge limitations of our study. First, retrieval studies typically rely on components associated with clinical failure. However, we know of no mechanism whereby the oxidation of clinically failed components would be substantially different from well-functioning components. Second, as a result of the staggered release of the various technologies, the implantation time varied between cohorts. We believe this limitation does not substantially detract from the findings from this study, because each cohort was analyzed independently to determine whether changes in oxidation and mechanical behavior occurred with implantation time. Third, mechanical specimens were only possible within the bearing region of the liners. As a result of the geometry of the liners, specimens could not be obtained from the rims of the components where oxidation is typically the highest and we would expect to see the greatest extent of mechanical degradation. However, the small punch test allowed us to characterize the mechanical behavior at the intended bearing surface.
Our data suggest contemporary gamma inert and annealed liners are associated with the highest levels of rim oxidation and hydroperoxide content and are consistent with previous studies suggesting the femoral head may play a role in mitigating oxidation at the bearing surface [20, 21, 30]. Moderate oxidation (average OI > 1) was evident at the rim in annealed and gamma inert liners, but over half of the retrieved annealed liners exhibited severe rim oxidation (average OI > 3). Previous studies have suggested an ASTM OI exceeding 3 is the critical level at which the mechanical properties are reduced severely enough that the material’s ability to withstand repeated loading is diminished [8, 17, 19]. The rims of acetabular components do not necessarily undergo repetitive mechanical loading throughout the duration of implantation. On the other hand, several recent studies [7, 21, 29] have demonstrated evidence of impingement (34%–56% of retrieved liners) at the rim of both historical and contemporary liner designs. With repeated loading at the rim, it remains unknown whether elevated rim oxidation may play a role in the long-term clinical performance of annealed and gamma inert liners.
Although we found that both remelted and nonionized retrievals had low levels of oxidation, we found remelted liners do not remain oxidatively stable during implantation. Rather, we observed a slight increase in both oxidation and hydroperoxide indices over time at the bearing surface. This increase was not seen at either the backside or the rim, suggesting a mechanism involving mechanical loading. Previous studies of retrieved and unimplanted [5, 30] remelted liners have shown little to no oxidation; however, the retrieval results were based on a comparatively short implantation time. Although we could detect an increase in OI over time, the magnitude was below 1 (even at implantation times close to a decade); this level is not anticipated to negatively influence the mechanical behavior [7, 17]. Nevertheless, the subtle changes in oxidation and oxidation potential at the bearing surface of the remelted components provide motivation for further long-term investigations of these materials as well as the potential degradation mechanism. We found no evidence of comparable degradation changes in any of the regions for the uncrosslinked, nonionizing sterilization components. It may be that, as a result of the greater wear rate of uncrosslinked polyethylene, the bearing surface of uncrosslinked hip bearings may be eroded faster than the in vivo oxidation process detected with remelted liners.
Overall, the small punch ultimate load of the superior bearing surface of the liners appeared to be maintained (because they were not negatively correlated with implantation time) with the exception of the annealed liners. In the annealed components, we observed impairment in the small punch ultimate load at the superior bearing surface over time by approximately 10% to 15% at 10 years. A previous study of long-term implants showed historical acetabular liners that were gamma sterilized in air and shelf aged in air had greater reduction in ultimate load by up to 90% after implantation for 11.5 years on average [19]. We also observed stronger negative correlations of ultimate load with implantation time at the inferior bearing surface of the annealed and gamma inert liners, which may be explained by lack of contact with the femoral head and greater access to oxygen at this location.
Within the first decade of implantation, the clinical outlook for first-generation highly crosslinked polyethylene remains promising given the oxidation and mechanical properties reported in this study. Although the uncrosslinked EtO sterilized liners showed undetectable oxidation or oxidation potential, such materials are currently less desirable for hip bearings as a result of their inferior wear resistance [25] and were included in this study primarily as an unirradiated control. Ongoing research continues to be warranted for first-generation highly crosslinked polyethylene bearings to monitor the implications of elevated oxidation at the rim of annealed liners as well as to better understand the subtle changes in oxidation at the bearing surface of remelted liners that occur in vivo.
Acknowledgments
We thank Javad Parvizi, MD, William Hozack, MD, Matthew Austin, MD, and Peter Sharkey, MD, Rothman Institute at Thomas Jefferson University; Bernard Stulberg, MD, Cleveland Clinic, Center for Joint Reconstruction; Gregg Klein, MD, Harlan Levine, MD, and Mark Hartzband, MD, Hartzband Center for Hip and Knee Replacement and Hackensack University Medical Center; Jonathan Garino, MD, Craig Israelite, MD, and Charles Nelson, MD, University of Pennsylvania School of Medicine; and Matthew Kraay, MD, and Victor Goldberg, MD, Case Western Reserve University, University Hospitals Case Medical Center, for their collaboration and contributions to the retrieval program. We also thank Francisco Medel, PhD, University of Zaragoza; Madeline Olsen, Drexel University; and Rebecca Moore, Case Western Reserve University, for their assistance with retrieval analysis.
Appendix
Footnotes
Institutional funding has been received from the National Institutes of Health (NIAMS) R01 AR47904; Stryker Orthopaedics, Inc; Zimmer, Inc; and through the Wilbert J. Austin Professor of Engineering Chair (CMR).
Each author certifies that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Implant Research Center, Drexel University, Philadelphia, PA, USA.
Contributor Information
Daniel MacDonald, Email: danwmac@gmail.com.
Steven M. Kurtz, Email: skurtz@drexel.edu.
References
- 1.ASTMF2102-06-e1. Standard Guide for Evaluating the Extent of Oxidation in Ultra-high Molecular Weight Polyethylene Fabricated Forms Intended for Surgical Implants. West Conshohocken, PA: American Society for Testing and Materials; 2006.
- 2.ASTMF2183-02. Standard Test Method for Small Punch Testing of Ultra-high Molecular Weight Polyethylene Used in Surgical Implants. West Conshocken, PA: American Society for Testing and Materials; 2008.
- 3.Brach Del Prever EM, Bistolfi A, Bracco P, Costa L. UHMWPE for arthroplasty: past or future? J Orthop Traumatol. 2009;10:1–8. doi: 10.1007/s10195-008-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bragdon CR, Barrett S, Martell JM, Greene ME, Malchau H, Harris WH. Steady-state penetration rates of electron beam-irradiated, highly cross-linked polyethylene at an average 45-month follow-up. J Arthroplasty. 2006;21:935–943. doi: 10.1016/j.arth.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Collier JP, Currier BH, Kennedy FE, Currier JH, Timmins GS, Jackson SK, Brewer RL. Comparison of cross-linked polyethylene materials for orthopaedic applications. Clin Orthop Relat Res. 2003;414:289–304. doi: 10.1097/01.blo.0000073343.50837.03. [DOI] [PubMed] [Google Scholar]
- 6.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. Oxidation in orthopaedic UHMWPE sterilized by gamma-radiation and ethylene oxide. Biomaterials. 1998;19:659–668. doi: 10.1016/S0142-9612(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 7.Currier BH, Currier JH, Mayor MB, Lyford KA, Collier JP, Citters DW. Evaluation of oxidation and fatigue damage of retrieved crossfire polyethylene acetabular cups. J Bone Joint Surg Am. 2007;89:2023–2029. doi: 10.2106/JBJS.F.00336. [DOI] [PubMed] [Google Scholar]
- 8.Currier BH, Currier JH, Mayor MB, Lyford KA, Citters DW, Collier JP. In vivo oxidation of gamma-barrier-sterilized ultra-high-molecular-weight polyethylene bearings. J Arthroplasty. 2007;22:721–731. doi: 10.1016/j.arth.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.D’Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;429:6–16. doi: 10.1097/01.blo.0000150314.70919.e3. [DOI] [PubMed] [Google Scholar]
- 11.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 12.Engh CA, Jr, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Jr, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21:17–25. doi: 10.1016/j.arth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Heisel C, Silva M, Schmalzried TP. In vivo wear of bilateral total hip replacements: conventional versus crosslinked polyethylene. Arch Orthop Trauma Surg. 2005;125:555–557. doi: 10.1007/s00402-005-0041-1. [DOI] [PubMed] [Google Scholar]
- 14.Hopper RH, Jr, Young AM, Orishimo KF, McAuley JP. Correlation between early and late wear rates in total hip arthroplasty with application to the performance of marathon cross-linked polyethylene liners. J Arthroplasty. 2003;18:60–67. doi: 10.1016/S0883-5403(03)00294-8. [DOI] [PubMed] [Google Scholar]
- 15.James SP, Blazka S, Merrill EW, Jasty M, Lee KR, Bragdon CR, Harris WH. Challenge to the concept that UHMWPE acetabular components oxidize in-vivo. Biomaterials. 1993;14:643–647. doi: 10.1016/0142-9612(93)90062-7. [DOI] [PubMed] [Google Scholar]
- 16.Krushell RJ, Fingeroth RJ, Cushing MC. Early femoral head penetration of a highly cross-linked polyethylene liner vs a conventional polyethylene liner: a case-controlled study. J Arthroplasty. 2005;20:73–76. doi: 10.1016/j.arth.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz SM. UHMWPE Biomaterials Handbook: Ultra-high molecular weight polyethylene in total joint replacement and medical devices. 2. Burlington, MA: Academic Press; 2009. [Google Scholar]
- 18.Kurtz SM, Austin A, Azzam K, Sharkey P, MacDonald D, Medel FJ, Hozack W. Mechanical properties, oxidation, and clinical performance of retrieved highly cross-linked Crossfire liners after intermediate-term implantation. J Arthroplasty. 2010;25:614–623. doi: 10.1016/j.arth.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtz SM, Hozack W, Marcolongo M, Turner J, Rimnac C, Edidin A. Degradation of mechanical properties of UHMWPE acetabular liners following long-term implantation. J Arthroplasty. 2003;18:68–78. doi: 10.1016/S0883-5403(03)00292-4. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz SM, Hozack W, Turner J, Purtill J, MacDonald D, Sharkey P, Parvizi J, Manley M, Rothman R. Mechanical properties of retrieved highly cross-linked crossfire liners after short-term implantation. J Arthroplasty. 2005;20:840–849. doi: 10.1016/j.arth.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtz SM, Hozack WJ, Purtill JJ, Marcolongo M, Kraay MJ, Goldberg VM, Sharkey PF, Parvizi J, Rimnac CM, Edidin AA. 2006 Otto Aufranc Award paper. Significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin Orthop Relat Res. 2006;453:47–57. doi: 10.1097/01.blo.0000246547.18187.0b. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20:1659–1688. doi: 10.1016/S0142-9612(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz SM, Rimnac CM, Hozack WJ, Turner J, Marcolongo M, Goldberg VM, Kraay MJ, Edidin AA. In vivo degradation of polyethylene liners after gamma sterilization in air. J Bone Joint Surg Am. 2005;87:815–823. doi: 10.2106/JBJS.D.02111. [DOI] [PubMed] [Google Scholar]
- 24.Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20:880–886. doi: 10.1016/j.arth.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 25.McKellop HA, Shen FW, Campbell P, Ota T. Effect of molecular weight, calcium stearate, and sterilization methods on the wear of ultra high molecular weight polyethylene acetabular cups in a hip joint simulator. J Orthop Res. 1999;17:329–339. doi: 10.1002/jor.1100170306. [DOI] [PubMed] [Google Scholar]
- 26.Medel FJ, Kurtz SM, Hozack WJ, Parvizi J, Purtill JJ, Sharkey PF, MacDonald D, Kraay MJ, Goldberg V, Rimnac CM. Gamma inert sterilization: a solution to polyethylene oxidation? J Bone Joint Surg Am. 2009;91:839–849. doi: 10.2106/JBJS.H.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 2001;16:149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 28.Rohrl SM, Li MG, Nilsson KG, Nivbrant B. Very low wear of non-remelted highly cross-linked polyethylene cups: an RSA study lasting up to 6 years. Acta Orthop. 2007;78:739–745. doi: 10.1080/17453670710014509. [DOI] [PubMed] [Google Scholar]
- 29.Shon WY, Baldini T, Peterson MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty a study of retrieved acetabular components. J Arthroplasty. 2005;20:427–435. doi: 10.1016/j.arth.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 30.Wannomae KK, Bhattacharyya S, Freiberg A, Estok D, Harris WH, Muratoglu O. In vivo oxidation of retrieved cross-linked ultra-high-molecular-weight polyethylene acetabular components with residual free radicals. J Arthroplasty. 2006;21:1005–1011. doi: 10.1016/j.arth.2005.07.019. [DOI] [PubMed] [Google Scholar]