Abstract
Background
Bone quantity, quality, and turnover contribute to whole bone strength. Although bone mineral density, or bone quantity, is associated with increased fracture risk, less is known about bone quality. Various conditions, including disorders of mineral homeostasis, disorders in bone remodeling, collagen disorders, and drugs, affect bone quality.
Questions/purposes
The objectives of this review are to (1) identify the conditions and diseases that could adversely affect bone quality besides osteoporosis, and (2) evaluate how these conditions influence bone quality.
Methods
We searched PubMed using the keywords “causes” combined with “secondary osteoporosis” or “fragility fracture.” After identifying 20 disorders/conditions, we subsequently searched each condition to evaluate its effect on bone quality.
Results
Many disorders or conditions have an effect on bone metabolism, leading to fragility fractures. These disorders include abnormalities that disrupt mineral homeostasis, lead to an alteration of the mineralization process, and ultimately reduce bone strength. The balance between bone formation and resorption is also essential to prevent microdamage accumulation and maintain proper material and structural integrity of the bone. As a result, diseases that alter the bone turnover process lead to a reduction of bone strength. Because Type I collagen is the most abundant protein found in bone, defects in Type I collagen can result in alterations of material property, ultimately leading to fragility fractures. Additionally, some medications can adversely affect bone.
Conclusions
Recognizing these conditions and diseases and understanding their etiology and pathogenesis is crucial for patient care and maintaining overall bone health.
Introduction
Bone strength is a term used to describe the ability of bone to resist fracture [9]. Determining bone strength reflects the integration of three factors: quantity, quality, and turnover [34]. Bone mineral density (BMD), measured by dual-energy X-ray absorptiometry (DXA), reflects bone quantity. BMD is expressed as a T-score. The T-score is reported as the number of standard deviations a patient’s BMD value is above or below the reference value for a healthy 30-year-old adult. Although measurements from DXA are an important tool in measuring BMD and assessing the risk of fracture, less than 50% of variation in whole-bone strength is attributable to variations in BMD [22, 27, 67]. In fact, the majority of patients who experience fragility fractures have BMD T-scores above −2.5 [88, 93, 94]. Furthermore, in women with a T-score of −2.5, the probability of hip fracture is five times greater at age 80 than at age 50 [55]. Although the cause of hip fracture in the elderly remains multifactorial, advanced age poses a substantial risk. Additionally, DXA has a limited utility to diagnose secondary causes of bone loss. Therefore, the other two determinants of bone strength (quality and turnover) should be included when assessing fracture risk in each individual, rather than just BMD alone.
Bone quality is a function of the structural and material properties of bone. The structural properties include bone geometry (size and shape of the skeleton) and microarchitecture, whereas the material properties include the organization and composition of the mineral and collagen components of the extracellular matrix, as well as the extent of microdamage within the tissue [34]. In general, bone undergoes continuous renewal by the process of coupled bone resorption and formation, known as bone remodeling, or bone turnover. The balance between bone resorption and bone formation allows the bone to remove fatigue damage and replace it with new bone that reinforces the bone integrity. An imbalance between bone resorption and bone formation ultimately results in a net loss or gain of bone tissue. The process of bone turnover influences both BMD and bone quality and consequently affects bone strength [9, 34].
A comprehensive evaluation of bone strength, including quantity, quality, and turnover, is critical to identify individuals with increased risk of fracture. Although numerous reports address the relationship between primary osteoporosis and fracture risk, there is less emphasis on other conditions that can adversely affect bone quality, leading to fragility fracture.
Therefore, the objectives of this review are to (1) identify the conditions and diseases, besides osteoporosis, that could adversely affect bone quality, and (2) evaluate how these conditions, including disorders of mineral homeostasis, disorders in bone remodeling, collagen disorders and drugs, affect bone quality.
Methods
To identify the conditions or diseases that could adversely affect bone quality, we started a comprehensive literature search using the National Library of Medicine’s PubMed database. We searched “causes and ‘secondary osteoporosis’” or “causes and ‘fragility fracture’” which identified 519 publications for potential inclusion. When we applied additional limits of English language and studies on human subjects, the number of articles reduced to 362. We reviewed the titles of each paper and excluded them if they were related to primary or postmenopausal osteoporosis. This yielded 197 articles. Using this search technique, we identified 20 conditions that could adversely affect bone quality or bone strength.
In order to determine the effect of each condition on bone quality, we performed a further search of the PubMed database by using keywords “bone quality” or “bone strength” combined with each condition. At this step, we evaluated both basic science and clinical studies involving the effect of each condition or disease on bone quality. For clinical studies, we included all study designs, including case reports and case series, with a limit of English language. For basic science studies, we identified relevant in vivo and in vitro studies through a similar search technique. Three authors (AU, BJR, and MMK) independently reviewed all abstracts. Additionally, we also evaluated cited references to identify papers not included in the original research.
Conditions and Diseases Affecting Bone Quality Besides Osteoporosis
Many diseases and conditions affect bone quality besides osteoporosis. These include disorders of bone mineral homeostasis, imbalance of bone remodeling, collagen disorders and drugs affecting bone quality (Table 1). Awareness of the association between these conditions and bone quality is important in understanding the pathogenesis of fracture in groups at risk. It is also crucial to recognize these clinical conditions because some of them are more amenable to treatment than primary osteoporosis. Many of these conditions affect more than one of the components of bone strength. For example, glucocorticoids not only increase bone turnover, resulting in increased bone resorption, but also affect mineral homeostasis by reducing calcium absorption and causing secondary hyperparathyroidism [44, 106, 109]. For the purpose of discussion, we categorized conditions affecting bone quality based on their impact to the most affected component of bone quality.
Table 1.
Diseases affecting bone quality
| Diseases affecting bone quality | Pathophysiology |
|---|---|
| Disorders of bone mineral homeostasis | |
| Rickets and osteomalacia [31] | Impairment of mineral deposition |
| Hyperparathyroidism [38] | Excessive secretion of PTH, leading to increased osteoclastic bone resorption |
| Hypogonadism [33, 37] | Reduced sex hormones, resulting in increased osteoclastic bone resorption |
| Hyperthyroidism [85] | Increased bone resorption |
| Type I diabetes mellitus [83] | Suppression of bone turnover |
| Cushing’s disease [51, 58, 106] | See Glucocorticoids |
| Imbalance of bone remodeling | |
| Renal osteodystrophy [98] | Increased osteoclastic bone resorption in high turnover state or decreased bone formation in low turnover state |
| Paget’s disease [81] | Rapid bone turnover, leading to disorganized mosaic pattern of woven and lamellar bone |
| Disuse osteoporosis [35, 110] | Uncoupling between bone resorption and formation with increased in bone resorption |
| Sclerosing bone dysplasias (osteopetrosis) [18, 105] | Defects in osteoclastic bone resorption (retention of primary spongiosa) |
| Disorders of collagen | |
| Osteogenesis imperfecta [6] | Mutation of Type I collagen genes |
| Scurvy [78] | Impairment of hydroxylation process during collagen synthesis |
| Marfan syndrome [40] | Abnormalities of fibrillin-1 glycoprotein |
| Ehlers-Danlos syndromes [13] | Defects in lysyl hydroxylase and collagen type I, III, and V |
| Drugs | |
| Glucocorticoids [51, 58, 106] | Increased osteoclastic resorption, impaired maturation of osteoblasts and altered calcium metabolism |
| Chemotherapeutic agents [49, 79] | Hypogonadism or direct toxic effects on bone |
| Disease modifying antirheumatic drugs (DMARDs) [26] | Imbalance of bone turnover |
| Bisphosphonates [76, 96] | Suppression of osteoclastic activity, leading to microdamage accumulation |
The Effect of Disorders of Bone Mineral Homeostasis on Bone Quality
Rickets and osteomalacia are disorders of bone mineral metabolism characterized by an accumulation of unmineralized osteoid on bone trabeculae as a result of impaired mineral deposition (Fig. 1). Rickets occurs in childhood and affects not only the bone structure, but also the physeal cartilage. Conversely, osteomalacia occurs after cessation of skeletal growth. Bone mineralization depends on the presence of calcium, phosphate, and the enzyme alkaline phosphatase. Therefore, any condition reducing the availability of serum calcium, phosphate, or alkaline phosphatase results in rickets or osteomalacia [2]. Although there are a number of causes for rickets and osteomalacia, most forms share similar histologic changes as well as clinical and radiographic appearances [31].
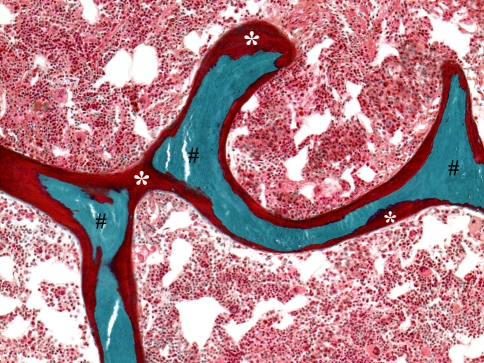
Fig. 1.
The photomicrograph of a patient diagnosed with osteomalacia shows interconnected trabeculae that contains central regions of mineralized bone (#) covered almost completely by an excessive amount of unmineralized osteoid matrix (*). The marrow contents consist of an unremarkable amount of hematopoietic elements and intermixed fat cells (Stain, Goldner trichrome; original magnification, ×10).
Nutritional rickets and osteomalacia resulting from inadequate vitamin D supplementation are the best-known forms of these disorders. Low levels of vitamin D result in decreased absorption of calcium across the intestinal tract, causing a decline in the concentration of serum calcium. Vitamin D deficiency may result from inadequate vitamin D absorption, vitamin D resistance, impaired vitamin D synthesis, or increased vitamin D degradation [31, 32] (Table 2). Although vitamin D deficiency is the primary etiology of rickets and osteomalacia, other less common causes of skeletal demineralization exist. X-linked hypophosphatemia is the most common inherited etiology for rickets [2]. The disease causes isolated renal phosphate wasting, resulting in hypophosphatemia and decreased bone mineralization. Because calcium and phosphate are essential minerals of the inorganic components of the bone matrix, a defect in this process leads to poor bone quality, resulting in increased risk of fragility fracture.
Table 2.
| Inadequate vitamin D absorption |
| Inadequate vitamin D intake from diet |
| Insufficient sunlight exposure |
| Malabsorption |
| Inflammatory bowel disease |
| Celiac disease |
| Bariatric surgery |
| Impaired vitamin D synthesis |
| 1 α-hydroxylase deficiency (Type 1-dependent rickets) |
| Chronic liver disease |
| Chronic renal failure |
| Vitamin D resistance |
| End organ insensitivity (Type 2-dependent rickets) |
| Drugs affecting vitamin D metabolism |
| Phenytoin |
| Phenobarbital |
| Carbamazepine |
| Primidone |
Hyperparathyroidism is the result of increased activity of parathyroid glands. This hyperactivity occurs either from an intrinsic abnormal change altering excretion of parathyroid hormone (primary or tertiary hyperparathyroidism) or an extrinsic abnormal change affecting calcium homeostasis stimulating production of parathyroid hormone (secondary hyperparathyroidism). Primary hyperparathyroidism is the third most common endocrine disorder, with the highest incidence in postmenopausal women [38]. Approximately 75% to 85% of patients with primary hyperparathyroidism have adenomas of a single gland [38]. The majority of patients with primary hyperparathyroidism have no obvious symptoms or signs of the disease, with the disorder detected by an incidental finding of hypercalcemia [8]. In symptomatic patients, the clinical presentation often relates to hypercalcemia rather than increased parathyroid hormone. These symptoms include lethargy, confusion, impaired mentation, depression, memory loss, and muscle weakness [70]. The skeletal changes are sometimes striking, with radiographs demonstrating osteopenia and subperiosteal resorption of the tufts and digits of the hands and feet (Fig. 2A) [92]. Fractures of long bones, clavicles, pelvis, and ribs are also common [70]. Since the disease usually occurs in postmenopausal women, the findings may be confused with primary osteoporosis.
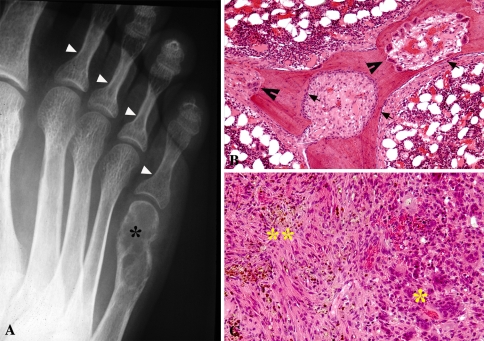
Fig. 2A–C.
Images of a patient diagnosed with hyperparathyroidism. (A) Radiograph of the right foot shows subperiosteal resorption of the second, third, fourth, and fifth proximal phalanges (white arrowheads) and brown tumor at the distal metaphysis of the fifth metatarsal (*). Note the bone cortices are thin on both sides but remain intact. Courtesy of Bernard Ghelman, MD. (B) The photomicrograph shows thickened trabecula undergoing tunneling resorption with a central defect undergoing reparative activity. Multinucleate osteoclasts are present in resorption bays (black arrowheads). The osteoblasts are present in single cell layers (black arrows) on the smooth surfaces inside the central tunnel and on the marrow surface of the bone (Stain, hematoxylin and eosin; original magnification, ×10). (C) The photomicrograph shows the histologic presentation of brown tumor resulting from secondary hyperparathyroidism. Typical features of brown tumor are seen with osteoclast-like giant cells and foci of microhemorrhage (asterisk) and fibroblastic stroma with hemosiderin (double asterisks) (Stain, hematoxylin and eosin; original magnification, ×20).
Many endocrine disorders are linked to the pathogenesis of secondary osteoporosis. These include hypogonadism, hyperthyroidism, Type I diabetes mellitus, and Cushing’s syndrome. One of the most frequent causes of endocrine-related fractures in men is hypogonadism [29]. Evidence shows that 66% of elderly men in a nursing home with hip fractures had hypogonadism [1]. In hypogonadal states, both cortical and trabecular bone are affected by increased osteoclastic resorption and decreased osteoblastic activity [33, 101]. Decreased calcium absorption is also seen in hypogonadism, which, for men, can be reversed with testosterone [37]. Hyperthyroidism is reported to cause increases in ionized and total serum calcium in up to 50% of affected patients. In general, the hypercalcemia is mild. It is believed to result from increases in bone resorption, leading to secondary osteoporosis [85, 97]. Therefore, it is crucial to monitor thyroid hormone levels in patients with postmenopausal osteoporosis or during treatment of thyroid dysfunction. Type I diabetes mellitus may also play a role in osteoporotic fractures. Although increased fracture risk in Type I diabetes is associated with low BMD and reduced bone turnover [64, 83], the true mechanism of bone loss remains inconclusive. The effect of excess glucocorticoids on bone metabolism is discussed later in this article.
The Effect of Disorders in Bone Remodeling on Bone Quality
Renal osteodystrophy is a pathologic bone condition in which the primary cause of the disorder is chronic renal failure. The pathophysiology of renal osteodystrophy is subdivided into two groups based on bone turnover: high turnover and low turnover [12, 90, 98]. The high turnover state is the classic form of this disease and occurs in the course of chronic renal failure. This form of renal osteodystrophy is associated with high PTH. In the presence of elevated PTH levels, bone turnover remains high and increases the activity of both osteoblasts and osteoclasts. Conversely, the low turnover state is associated with normal to low serum PTH and can be developed following therapeutic interventions for a high turnover state. The pathogenesis of low turnover renal osteodystrophy is complex and includes a large number of factors, such as aluminum-based phosphate binder, excess use of active vitamin D sterols, and peritoneal dialysis [95]. It is also believed that changes in a variety of growth factors and cytokines could directly impact the bone formation rate [39].
The skeletal manifestations in patients with renal osteodystrophy show changes consistent with rickets, osteomalacia, and hyperparathyroidism. The elevation of both serum calcium and phosphate levels leads to extraskeletal calcification. This ectopic calcification or ossification usually occurs in conjunctivae, blood vessels, skin, and periarticular areas [70]. In its most severe form, hyperparathyroid bone disease may predominate and manifest as subperiosteal or subchondral erosions. Brown tumor, which is a lytic area with a marked decrease in cortical structure, may also lead to pathologic fracture (Fig. 2) [50].
Paget’s disease of bone (also known as osteitis deformans) is a localized disorder of bone remodeling. The disease process is initiated by increases in bone resorption with subsequent compensatory increases in new bone formation [81]. As a result of the rapid bone turnover rate, the affected bone loses its control of the bony structure, resulting in a disorganized mosaic pattern of woven and lamellar bone (Fig. 3). The early phase is dominated by increased bone resorption by activated osteoclasts, resulting in a lytic lesion. To respond to the increased bone resorption, osteoblasts are recruited to the affected area. During this mixed phase, because of the nature of rapid turnover, the newly deposited collagen fibers are laid down in a disorganized pattern, creating a more primitive woven bone. This results in an irregularity of contour of the new trabeculae and cortices. Over time, the hypervascularity and hypercellularity process ceases, leaving a sclerotic, enlarged, mosaic pattern. This is a sclerotic phase or a so-called “burned out Paget’s disease.” Paget’s disease is often asymptomatic but can be associated with bone pain and other complications, such as deafness and nerve compression syndromes. Skeletal complications attributable to Paget’s disease include bowing deformities of long bones (7.6%), fracture of pagetic bone (9.7%), and osteosarcoma (0.4%) [107].
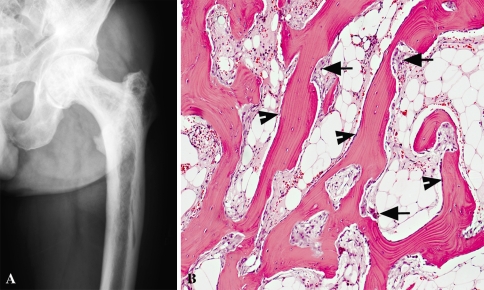
Fig. 3A–B.
Images of a patient diagnosed with Paget’s disease. (A) AP radiograph of the left proximal femur shows increased width of the femoral shaft, markedly thickened cortices, coarse but disorganized trabeculae, and small lytic areas within the medullary canal. (B) The photomicrograph illustrates the active phase of Paget’s disease with numerous trabeculae undergoing resorption by osteoclasts (arrows) and a thin layer of surface-related osteoblasts (arrowheads). Subsequent osteoblastic activity results in cement lines and the “mosaic” pattern apparent in sclerotic phases (Stain, hematoxylin and eosin; original magnification, ×10).
Bone loss associated with skeletal unloading, also known as disuse osteoporosis, is a critical issue for bedridden patients or patients with a spinal cord injury. The diminution of mechanical stimuli to bone is considered a powerful contributor to bone demineralization [63, 89]. Complete unloading of the limbs, such as in patients with spinal cord injury, leads to bone loss approximately five to 20 times greater than losses from metabolic etiologies [73]. When weight-bearing and muscular contractions diminish or cease, the loss of mechanical loading yields an imbalance between osteoclastic and osteoblastic activity. The histomorphometric study in patients with prolonged immobilization showed an increase in the number of osteoclasts and an enlargement of resorption cavities [103]. Additionally, biochemical analysis in this group of patients demonstrated that bone resorption markers are substantially increased, whereas bone formation markers are only slightly elevated or remain in a normal reference range [35, 110]. These findings suggest that bone resorption outpaces bone formation, leading to disuse osteoporosis.
Sclerosing bone dysplasia is a rare genetic disorder characterized by the creation of abnormally dense (sclerosis) and overgrown bone (hyperostosis). This is caused by defective remodeling processes where the rate of formation of bone or cartilage exceeds the rate of resorption. When there is defective resorption, as is the case with osteopetrosis, the osteoclasts fail to remove bone, resulting in a marked increase in bone density, microdamage accumulation, loss of bone heterogeneity, and abnormalities of osseous structure (Fig. 4) [18, 71, 105]. Despite the presence of increased bone density on imaging studies, the bones of these patients are fragile and fractures are common.
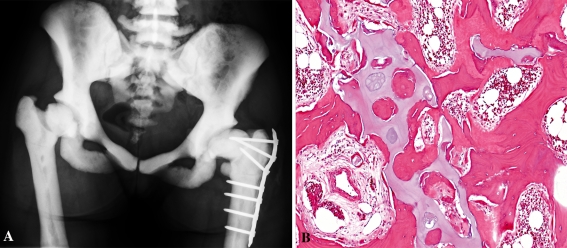
Fig. 4A–B.
Images of a patient diagnosed with osteopetrosis. (A) An AP radiograph of the pelvis shows uniform increased bone density. The trabecular pattern is difficult to identify in the bones, hence the name “marble-bone disease.” The medullary spaces are mostly obliterated. (B) The photomicrograph illustrates thickened and dystrophic trabeculae (pink) containing irregularly shaped central cores and fragments of residual cartilage (blue). Defective osteoclasts lead to abnormal bone remodeling, which precludes repair of local bone damage, resulting in production of abnormal trabecular architecture (Stain, hematoxylin and eosin; original magnification, ×10).
There are three traditional forms of osteopetrosis: malignant autosomal-recessive, intermediate autosomal-recessive, and benign autosomal-dominant. The malignant form typically presents within the first year of life. Bony overgrowth of the marrow spaces results in decreased hematopoiesis, leading to myelophthisic anemia and subsequent hepatosplenomegaly [56]. If untreated, most patients do not survive past their first decade [57]. The intermediate form of osteopetrosis shares many features of the malignant counterpart; however, it is less severe and has a delayed onset. Most patients with the intermediate form survive into adulthood; however, fractures are commonly seen within the first decade. Patients may have hematologic abnormalities, as well as clinical symptoms, from the compression of cranial nerves. The benign autosomal-dominant form, adult osteopetrosis, is the most common type of osteopetrosis. It typically presents with pathologic fractures that can occur at any age. Although most patients are diagnosed after their first fractures, 40% of patients will remain asymptomatic [61].
The Effect of Collagen Disorders on Bone Quality
Osteogenesis imperfecta (OI) is an inheritable disease of Type I collagen. Type I collagen is composed of three polypeptide chains. These chains are synthesized by two genes: COL1A1 and COL1A2, encoding for the pro-α1 (I) and pro-α2 (I), respectively. The carboxy terminal propeptides of each chain combine to form Type I procollagen molecules [100]. The formation of the triple helix starts at the carboxy terminal end and then propagates to the amino terminal end of the molecule. Any mutations of Type I collagen genes result in either inadequate collagen production or abnormal collagen structure, leading to OI [17]. Although more than 800 mutations in Type I collagen genes have been reported, the majority of patients with OI are caused by autosomal dominant defects in the genes that encode Type I collagen, COL1A1 or COL1A2 [72].
Although several clinical subtypes of OI have recently been identified based on the clinical, biochemical, and molecular nature of the disorder [4, 6], many clinicians still commonly use the Silence classification with the description of four types of OI [91]. This classification was largely based on clinical and radiological subgrouping before the identification of a molecular defect of Type I collagen [6, 91]. Recent classifications have included eight types of OI [6], with recent findings suggesting abnormal collagen folding may contribute to further subtypes of OI (Type IX) [4]. In general, the clinical presentations vary considerably, ranging from a severe perinatal lethal form to more mild presentations. Severe and mild forms share the cardinal feature of bone fragility, characterized by fractures after minimal or no trauma. Hearing loss in early adulthood is the result of damage to the ossicles in the middle ear [59]. Short stature and bone deformity are also common features of this disorder. Additionally, several clinical presentations of OI are commonly seen in other connective tissue diseases. These include hypermobile joints, blue sclera, and brittle opalescent teeth (dentinogenesis imperfecta).
Scurvy is an acquired collagen disorder resulting from a nutritional deficiency of ascorbic acid (vitamin C). Although it was once common in sailors whose diets lacked adequate vitamin C while on long voyages, it is now seen mostly in patients with limited dietary intake of citrus fruits. Vitamin C is essential for the hydroxylation of proline and lysine during collagen synthesis. This hydroxylation process is important for stabilizing collagen structure by crosslinking the triple helix of collagen [78]. Therefore, a deficiency of vitamin C results in primitive collagen formation throughout the body. Patients with scurvy may present with spots on the skin, spongy gums, and bleeding from the mucous membranes [80]. The skeletal presentations include thin cortices and marrow cavities with few trabeculae; thus, fractures are common in these patients [80]. Additionally, one of the remarkable features of scurvy is subperiosteal hemorrhage, which results in marked swelling and pain in the affected extremity. This leads to pseudoparalysis and contractures of the limbs [19]. According to the Framingham Osteoporosis Study [86], over a 15- to 17-year followup of 958 elderly men and women, subjects in the highest category of supplemental vitamin C intake had fewer hip and nonvertebral fractures compared with nonsupplement users. Therefore, vitamin C may have a protective effect on bone health in older adults.
Musculoskeletal manifestations resulting from connective tissue disorders are related to abnormal processes in the structural support of tissues. Marfan syndrome is an autosomal-dominant disorder that affects fibrillin-1, a glycoprotein that provides structural support in elastin [40]. The abnormalities in fibrillin-1 result in abnormalities of the cardiovascular, ocular, and musculoskeletal system. Common skeletal findings include idiopathic scoliosis, lumbosacral dural ectasia, acetabular protrusion, and ligamentous laxity [41]. Patients with Marfan syndrome are also reported to have reduced BMD [40, 66]. The decrease in BMD is equally present in both genders and is more pronounced at predominantly cortical sites [66]. Ehlers-Danlos syndrome is a heterogeneous group of inherited connective tissue disorders divided into six major categories, with defects in lysyl hydroxylase and collagen Types I, III, and V [13]. The characteristics of this syndrome are skin hyperextensibility, hypermobile joints, and delayed wound healing. Although the changes in bone mineral content of patients with Ehlers-Danlos syndrome are still unclear [16], some studies indicate there is an alteration in bone metabolism, resulting in structural changes within the collagen fibrils, leading to an increased frequency of vertebral fractures [7, 20].
The Effect of Drugs on Bone Quality
Evidence shows daily doses of corticosteroids for less than 6 months can cause rapid bone loss [106], although the mechanism of bone loss from corticosteroids has not been fully elucidated. It is known that after several days of corticosteroid therapy, osteoclast and osteoblast activity and maturation are affected [5, 58, 106]. This results in less osteoblast to form bone. Current data suggest osteocytes, terminally differentiated osteoblasts, are also affected. In several studies, moderate to high doses of glucocorticoids caused apoptosis of osteocytes [54]. The loss of osteocytes, the most abundant cell in bone, has important implications on the microarchitecture of bone and bone’s propensity to fracture. Although osteoblast maturation is altered and slowed, it appears that osteoclast maturation and activity is increased due to elevated levels of glucocorticoids [58]. This results in increased bone resorption. Additionally, glucocorticoids directly affect bone metabolism by decreasing calcium absorption in the intestines and calcium reabsorption in the kidneys, instead promoting excretion [51]. This leads to an increase in PTH secretion and a net increase in bone loss as PTH tries to maintain calcium homeostasis.
Disease-modifying antirheumatic drugs (DMARDs) are either used independently or in combination with steroids and nonsteroidal anti-inflammatory drugs to treat rheumatologic diseases. Each of these drugs has a specific mechanism of action and their own side effect profile applying to bone. For example, methotrexate (MTX) is a folic acid antagonist used to treat both rheumatologic disease and neoplastic disease. At the high doses used to treat cancers, MTX can cause a dose-dependent decrease in osteoblast proliferation, resulting in osteoporosis, stress fractures, and bone pain. At low doses, MTX is generally prescribed for rheumatologic diseases and is not believed to adversely affect bone metabolism [26]. Cyclosporine A is an important drug in transplant medicine and rheumatology. At high levels, cyclosporine uncouples bone resorption and bone formation, leading to a net bone loss as resorption exceeds formation. However, much like MTX, at the low doses used for rheumatic disease, there is little evidence to show that cyclosporine affects bone mass adversely [44].
The bone loss that occurs secondary to chemotherapy tends to be more rapid and severe than either age-related or postmenopausal bone loss, in some cases accelerating it by as much as 10 times [28, 42, 49]. The mechanism by which chemotherapy induces bone loss appears to be primarily through the creation of hypogonadism in patients, specifically those with prostate and breast cancer. However, there is also evidence that chemotherapeutics affect bone maturation and, in some cases, may have direct toxic effects on bone [79].
Patients with prostate cancer treated with gonadotropin-releasing hormone antagonists produce decreased levels of androgens and estrogens. Studies have linked the duration of antiandrogen therapy in men to the risk of fracture with greater numbers of treatments associated with increased rates of fracture [68]. Similarly, new data in women with breast cancer have linked the use of aromatase inhibitors to increased bone loss [10]. Aromatase inhibitors are much more effective than estrogen receptor antagonists and agonists and tamoxifen in the treatment of estrogen receptor-positive early-stage breast cancers. However, in trials of these chemotherapeutic agents, women on aromatase inhibitors had greater losses in BMD at the hip and spine, as well as increased rates of fracture [10].
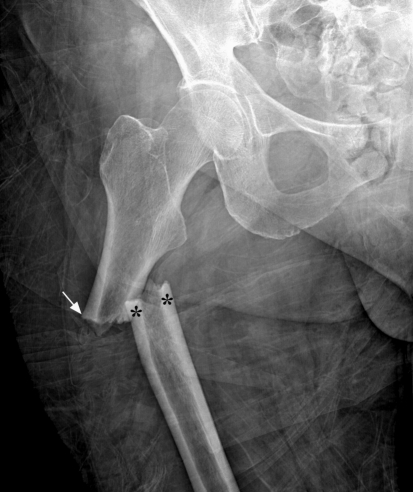
Bisphosphonates have been widely used for the treatment of osteoporosis. Recently, a number of studies reported a specific pattern of fracture in the subtrochanteric region or the upper diaphysis of the femur (Fig. 5); these fractures have been designated as bisphosphonate-related atypical fractures [43, 60, 65]. The mechanism and pathophysiology of these fractures remain unclear; however, it is believed that prolonged bisphosphonate therapy leads to oversuppression of bone turnover, resulting in microdamage accumulation and decreased mechanical properties of bone [76, 96]. Although there is no study showing causality or definitive association between long-term bisphosphonates treatment and these atypical fractures, clinicians should be aware of this potential problem and provide close evaluation when presented with thigh or hip pain in patients on long-term bisphosphonates.
Fig. 5.
Radiograph of a patient diagnosed with subtrochanteric femoral fracture attributed to long-term bisphosphonate exposure. Fracture after prolonged treatment with alendronate is characterized by (1) simple or transverse fracture, (2) beaking of the cortex on one side (white arrow), (3) hypertrophied diaphyseal cortices (asterisks), and (4) result from minimal or no trauma.
Some medications adversely affect bone quality by altering calcium and vitamin D metabolism. Phenobarbital, phenytoin, carbamazepine, and primidone are well-known inducers of hepatic cytochrome P450 enzymes (Table 2) [21, 30]. The observed reduction in serum vitamin D levels with these agents is believed to arise from their enhancing the hepatic breakdown of vitamin D into inactive metabolites [25, 84]. Proton pump inhibitors (PPIs) are commonly used drugs for gastrointestinal disorders. There is an emerging concern about chronic PPI therapy and the risk of fragility fractures [45, 102, 108]. One potential mechanism by which PPIs may affect fracture incidence is by impairing intestinal calcium absorption [46, 47, 75]. Without an acidic environment in the stomach and upper small bowel, calcium may be retained in the food matrix, preventing absorption [82]. Impaired calcium absorption leads to secondary hyperparathyroidism. Therefore, in patients who take these medications, supplementation with adequate calcium and vitamin D should be considered.
Discussion
Although primary osteoporosis remains the most common cause of fragility fractures, other disorders also cause fragility fractures. Several studies show secondary causes of osteoporosis are found in 20% to 30% of postmenopausal women, and can account for more than 50% of men who are diagnosed with osteoporosis [15, 36, 77, 87]. Common disorders that affect bone strength may be misdiagnosed as primary osteoporosis and potentially lead to a delay for appropriate treatment. Therefore, recognizing these diseases and understanding their etiology and pathogenesis is important for patient care and maintaining overall bone health. The objectives of this review were to (1) identify the conditions and diseases that could adversely affect bone quality besides osteoporosis, and (2) evaluate how these conditions, including disorders of mineral homeostasis, disorders of bone remodeling, collagen disorders and drugs, affect bone quality.
There are several limitations to our study design. First, our study was a selective review, and not a rigorous systematic review of the literature. We did not include other search engines, such as Cochrane Central Register of Controlled Trials or EMBASE database. Additionally, we limited our search to only English language articles. Thus, our search strategy may have missed eligible scientific articles within this field of study. Second, for some rare diseases, such as Ehlers-Danlos syndrome, there is limited information on how these diseases affect bone quality. All studies in our literature search for this particular disease were small case reports or case series. Third, we did not assess study quality. Fourth, this review article focused on conditions that affect bone quality and can lead to generalized bone loss. Thus, some diseases, such as complex regional pain syndrome, which results in local osteoporosis, were not covered in this review. Additionally, the role of diet, including alcohol, caffeine, and phytic acid, on bone quality is complex; therefore, further review in this particular issue is warranted.
Metabolically active bone provides a space for hematopoiesis, and is a reservoir for minerals. Mineral homeostasis controls mineralization of the bone, which is one of the major determinants in bone quality [34]. As a result, any disease or condition that interferes with mineral homeostasis will adversely affect bone quality, leading to fragility fractures. Since calcium and vitamin D are critically important for bone mineralization, all patients should be supplemented with adequate calcium and vitamin D intake. A dosing regimen of 1000 to 1500 mg of daily calcium is commonly used. Patients with vitamin D deficiency should be rapidly corrected with pharmacologic doses of vitamin D. After correcting for low serum vitamin D levels, adequate vitamin D should be given to sustain 25-hydroxyvitamin D levels above 32 ng/ml [48, 52, 53]. In general, 50,000 IU of vitamin D2 (ergocalciferol) can be given one to two times per week for 8 weeks, followed by a maintenance dose of vitamin D3 of 1000 to 2000 IU/day [69, 104]. Toxicity is rare even if a daily dosage of 10,000 IU vitamin D3 is given for up to 4 months [3].
Bone remodeling is a complex process that is regulated by both local and systemic factors. Derangement of this process can interfere with the delicate balance between bone resorption and bone formation, resulting in an alteration of quantity and quality of the skeleton [9]. Diseases with high turnover state, such as renal osteodystrophy or disuse osteoporosis, are characterized by increased activity of the osteoclasts [12, 35, 110]. Therefore, the bone remodeling process is shifted toward bone resorption, resulting in an imbalance of bone turnover that causes fragility fracture. Conversely, in a setting where there is a defect in osteoclast function, such as in osteopetrosis, this could lead to a lack of bone heterogeneity, microdamage accumulation, and ultimately fragility fracture [18, 105].
Collagen is the most abundant protein found in bone. Type I collagen comprises approximately 90% to 95% of the organic matrix. Individual collagen helices are crosslinked within and between other collagen helices, thereby increasing their strength. Crosslinked collagen is a structural template for mineralization [62]. Both collagen and mineral contribute to the material properties of bone. Bone tissue strength and stiffness depend heavily on mineral content across a wide range of anatomic locations [11, 23, 24]. Equally important, collagen plays a critical role in bone structural integrity, because it primarily provides the tensile strength [14, 74, 99]. Generally, bones fail in tension and thus collagen provides the main structural framework to prevent this failure process. Therefore, the disruption of collagen structure or its function such as OI results in bone fragility.
Although a number of medications can be of great therapeutic benefit in many diseases, they are not without side effects. Corticosteroids, anti-rheumatic drugs, cancer chemotherapeutic agents or even with osteoporosis medications can adversely affect bone [26, 58, 76, 106]. These skeletal side effects should be considered when assessing a patient’s fracture risk. For individuals taking such medications, regular monitoring to evaluate for bone loss should be incorporated. Additionally, a further treatment plan should be used to prevent fragility fractures.
Bone quantity, quality, and turnover are all important in determining bone strength. Any disease that compromises bone quality can lead to a decrease in bone strength and, ultimately, fragility fractures. As a result, clinicians should always be aware of secondary causes that affect bone quality in patients presenting with fragility fracture; particularly, diseases amenable to treatment, such as rickets and osteomalacia. In a setting where there is no specific treatment available for the disease, it is still crucial to identify diseases and conditions affecting bone quality to prevent future fracture.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Abbasi AA, Rudman D, Wilson CR, Drinka PJ, Basu SN, Mattson DE, Richardson TJ. Observations on nursing home residents with a history of hip fracture. Am J Med Sci. 1995;310:229–234. [PubMed] [Google Scholar]
- 2.Alman BA, Goldberg MJ. Metabolic and endocrine abnormalities. In: Lovell WW, Winter RB, Morrissy RT, Weinstein SL, editors. Lovell and Winter’s Pediatric Orthopaedics. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 167–203. [Google Scholar]
- 3.Amir E, Simmons CE, Freedman OC, Dranitsaris G, Cole DE, Vieth R, Ooi WS, Clemons M. A phase 2 trial exploring the effects of high-dose (10, 000 IU/day) vitamin D(3) in breast cancer patients with bone metastases. Cancer. 2010;116:284–291. doi: 10.1002/cncr.24749. [DOI] [PubMed] [Google Scholar]
- 4.Barnes AM, Carter EM, Cabral WA, Weis M, Chang W, Makareeva E, Leikin S, Rotimi CN, Eyre DR, Raggio CL, Marini JC. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;6:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 6.Basel D, Steiner RD. Osteogenesis imperfecta: recent findings shed new light on this once well-understood condition. Genet Med. 2009;6:375–385. doi: 10.1097/GIM.0b013e3181a1ff7b. [DOI] [PubMed] [Google Scholar]
- 7.Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51:444–453. [PubMed] [Google Scholar]
- 8.Bilezikian JP, Potts JT Jr. Asymptomatic primary hyperparathyroidism: new issues and new questions—bridging the past with the future. J Bone Miner Res. 2002:N57–67. [PubMed]
- 9.Bouxsein ML. Determinants of skeletal fragility. Best Pract Res Clin Rheumatol. 2005;6:897–911. doi: 10.1016/j.berh.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Brown SA, Guise TA. Cancer treatment-related bone disease. Crit Rev Eukaryot Gene Expr. 2009;19:47–60. doi: 10.1615/critreveukargeneexpr.v19.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg Am. 1975;57:956–961. [PubMed] [Google Scholar]
- 12.Bushinsky DA. Bone disease in moderate renal failure: cause, nature and prevention. Annu Rev Med. 1997:167–176. [DOI] [PubMed]
- 13.Callewaert B, Malfait F, Loeys B, Paepe A. Ehlers-Danlos syndromes and Marfan syndrome. Best Pract Res Clin Rheumatol. 2008;1:165–189. doi: 10.1016/j.berh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Calve S, Lytle IF, Grosh K, Brown DL, Arruda EM. Implantation increases tensile strength and collagen content of self-assembled tendon constructs. J Appl Physiol. 2010;108:875–881. doi: 10.1152/japplphysiol.00921.2009. [DOI] [PubMed] [Google Scholar]
- 15.Caplan GA, Scane AC, Francis RM. Pathogenesis of vertebral crush fractures in women. J R Soc Med. 1994;87:200–202. [PMC free article] [PubMed] [Google Scholar]
- 16.Carbone L, Tylavsky FA, Bush AJ, Koo W, Orwoll E, Cheng S. Bone density in Ehlers-Danlos syndrome. Osteoporos Int. 2000;5:388–392. doi: 10.1007/s001980070104. [DOI] [PubMed] [Google Scholar]
- 17.Cheung MS, Glorieux FH. Osteogenesis imperfecta: update on presentation and management. Rev Endocr Metab Disord. 2008;2:153–160. doi: 10.1007/s11154-008-9074-4. [DOI] [PubMed] [Google Scholar]
- 18.Cleiren E, Benichou O, Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, Vernejoul MC, Hul W. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet. 2001;25:2861–2867. doi: 10.1093/hmg/10.25.2861. [DOI] [PubMed] [Google Scholar]
- 19.Clemetson CA. Barlow’s disease. Med Hypotheses. 2002;1:52–56. doi: 10.1016/s0306-9877(02)00114-7. [DOI] [PubMed] [Google Scholar]
- 20.Coelho PC, Santos RA, Gomes JA. Osteoporosis and Ehlers-Danlos syndrome. Ann Rheum Dis. 1994;3:212–213. doi: 10.1136/ard.53.3.212-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conney AH. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967;19:317–366. [PubMed] [Google Scholar]
- 22.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;4:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 23.Currey JD. The relationship between the stiffness and the mineral content of bone. J Biomech. 1969;4:477–480. doi: 10.1016/0021-9290(69)90023-2. [DOI] [PubMed] [Google Scholar]
- 24.Currey JD. Strain rate and mineral content in fracture models of bone. J Orthop Res. 1988;1:32–38. doi: 10.1002/jor.1100060105. [DOI] [PubMed] [Google Scholar]
- 25.Dent CE, Richens A, Rowe DJ, Stamp TC. Osteomalacia with long-term anticonvulsant therapy in epilepsy. Br Med J. 1970;5727:69–72. doi: 10.1136/bmj.4.5727.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Munno O, Delle Sedie A, Rossini M, Adami S. Disease-modifying antirheumatic drugs and bone mass in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:137–144. [PubMed] [Google Scholar]
- 27.Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;5:423–432. doi: 10.1007/s00223-002-2104-4. [DOI] [PubMed] [Google Scholar]
- 28.Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. ATAC Trialists’ group. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the anastrozole, tamoxifen, alone or in combination (ATAC) trial (18233230) J Bone Miner Res. 2006;8:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 29.Ebeling PR. Clinical practice. osteoporosis in men. N Engl J Med. 2008;14:1474–1482. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 30.Eichelbaum M, Ekbom K, Bertilsson L, Ringberger VA, Rane A. Plasma kinetics of carbamazepine and its epoxide metabolite in man after single and multiple doses. Eur J Clin Pharmacol. 1975;5:337–341. doi: 10.1007/BF00562659. [DOI] [PubMed] [Google Scholar]
- 31.Einhorn TA. Metabolic bone disease. In: Einhorn TA, Buckwalter JA, O’Keefe RJ, American Academy of Orthopaedic Surgeons, eds. Orthopaedic Basic Science: Foundations of Clinical Practice. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007:415–426.
- 32.Epstein S, Schneider AE. Drug and hormone effect on vitamin D metabolism. In: Feldman D, Glorieux FH, Pike JW, editors. Vitamin D. 2. San Diego: Elseiver Academic Press; 2005. p. 1291. [Google Scholar]
- 33.Erben RG, Eberle J, Stahr K, Goldberg M. Androgen deficiency induces high turnover osteopenia in aged male rats: a sequential histomorphometric study. J Bone Miner Res. 2000;6:1085–1098. doi: 10.1359/jbmr.2000.15.6.1085. [DOI] [PubMed] [Google Scholar]
- 34.Felsenberg D, Boonen S. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther. 2005;1:1–11. doi: 10.1016/j.clinthera.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Fiore CE, Pennisi P, Ciffo F, Scebba C, Amico A, Di Fazzio S. Immobilization-dependent bone collagen breakdown appears to increase with time: evidence for a lack of new bone equilibrium in response to reduced load during prolonged bed rest. Horm Metab Res. 1999;1:31–36. doi: 10.1055/s-2007-978693. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick LA. Secondary causes of osteoporosis. Mayo Clin Proc. 2002;5:453–468. doi: 10.4065/77.5.453. [DOI] [PubMed] [Google Scholar]
- 37.Francis RM, Peacock M, Aaron JE, Selby PL, Taylor GA, Thompson J, Marshall DH, Horsman A. Osteoporosis in hypogonadal men: role of decreased plasma 1, 25-dihydroxyvitamin D, calcium malabsorption, and low bone formation. Bone. 1986;4:261–268. doi: 10.1016/8756-3282(86)90205-x. [DOI] [PubMed] [Google Scholar]
- 38.Fraser WD. Hyperparathyroidism. Lancet. 2009;9684:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 39.Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res. 2003;7:1317–1325. doi: 10.1359/jbmr.2003.18.7.1317. [DOI] [PubMed] [Google Scholar]
- 40.Giampietro PF, Peterson MG, Schneider R, Davis JG, Burke SW, Boachie-Adjei O, Mueller CM, Raggio CL. Bone mineral density determinations by dual-energy x-ray absorptiometry in the management of patients with Marfan syndrome—some factors which affect the measurement. HSS J. 2007;1:89–92. doi: 10.1007/s11420-006-9030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giampietro PF, Raggio C, Davis JG. Marfan syndrome: orthopedic and genetic review. Curr Opin Pediatr. 2002;1:35–41. doi: 10.1097/00008480-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlbock M, Jakesz R. Austrian Breast and Colorectal Cancer Study Group (ABCSG). Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 43.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 44.Goodman SB, Jiranek W, Petrow E, Yasko AW. The effects of medications on bone. J Am Acad Orthop Surg. 2007;15:450–460. doi: 10.5435/00124635-200708000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, Chen Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the women’s health initiative. Arch Intern Med. 2010;9:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graziani G, Como G, Badalamenti S, Finazzi S, Malesci A, Gallieni M, Brancaccio D, Ponticelli C. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrol Dial Transplant. 1995;10:1376–1380. [PubMed] [Google Scholar]
- 47.Hardy P, Sechet A, Hottelart C, Oprisiu R, Abighanem O, Said S, Rasombololona M, Brazier M, Moriniere P, Achard JM, Pruna A, Fournier A. Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artif Organs. 1998;7:569–573. doi: 10.1046/j.1525-1594.1998.06200.x. [DOI] [PubMed] [Google Scholar]
- 48.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 49.Higano CS. Understanding treatments for bone loss and bone metastases in patients with prostate cancer: a practical review and guide for the clinician. Urol Clin North Am. 2004;2:331–352. doi: 10.1016/j.ucl.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Hmamouchi I, Renard E, Thomas E, Missounga L, Blotman F, Cyteval C. Fracture from brown tumor due to parathyroid adenoma secondary to vitamin D deficiency. J Radiol. 2008;9:1109–1112. doi: 10.1016/s0221-0363(08)73918-0. [DOI] [PubMed] [Google Scholar]
- 51.Hodgson SF. Corticosteroid-induced osteoporosis. Endocrinol Metab Clin North Am. 1990;19:95–111. [PubMed] [Google Scholar]
- 52.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 53.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;5:515–516. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 54.Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res. 2007;10:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 55.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. National Osteoporosis Guideline Group. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008;10:1395–1408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan FS, August CS, Dalinka MK, Karp J, Fallon MD, Haddad JG. Bone densitometry observations of osteopetrosis in response to bone marrow transplantation. Clin Orthop Relat Res. 1993;294:79–84. [PubMed] [Google Scholar]
- 57.Key LL, Jr, Rodriguiz RM, Willi SM, Wright NM, Hatcher HC, Eyre DR, Cure JK, Griffin PP, Ries WL. Long–term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med. 1995;24:1594–1599. doi: 10.1056/NEJM199506153322402. [DOI] [PubMed] [Google Scholar]
- 58.Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;8:2152–2160. doi: 10.1172/JCI28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuurila K, Grenman R, Johansson R, Kaitila I. Hearing loss in children with osteogenesis imperfecta. Eur J Pediatr. 2000;7:515–519. doi: 10.1007/s004310051322. [DOI] [PubMed] [Google Scholar]
- 60.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;2:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Landa J, Margolis N, Di Cesare P. Orthopaedic management of the patient with osteopetrosis. J Am Acad Orthop Surg. 2007;15:654–662. doi: 10.5435/00124635-200711000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Landis WJ, Hodgens KJ, Song MJ, Arena J, Kiyonaga S, Marko M, Owen C, McEwen BF. Mineralization of collagen may occur on fibril surfaces: evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J Struct Biol. 1996;1:24–35. doi: 10.1006/jsbi.1996.0066. [DOI] [PubMed] [Google Scholar]
- 63.Lanyon LE. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18(Suppl):37S–43S. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 64.Leidig-Bruckner G, Ziegler R. Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S493–S514. doi: 10.1055/s-2001-18605. [DOI] [PubMed] [Google Scholar]
- 65.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;8:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Parc JM, Plantin P, Jondeau G, Goldschild M, Albert M, Boileau C. Bone mineral density in sixty adult patients with Marfan syndrome. Osteoporos Int. 1999;6:475–479. doi: 10.1007/s001980050257. [DOI] [PubMed] [Google Scholar]
- 67.Lochmuller EM, Zeller JB, Kaiser D, Eckstein F, Landgraf J, Putz R, Steldinger R. Correlation of femoral and lumbar DXA and calcaneal ultrasound, measured in situ with intact soft tissues, with the in vitro failure loads of the proximal femur. Osteoporos Int. 1998;8:591–598. doi: 10.1007/s001980050104. [DOI] [PubMed] [Google Scholar]
- 68.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 69.Malabanan AO, Holick MF. Vitamin D and bone health in postmenopausal women. J Womens Health (Larchmt) 2003;2:151–156. doi: 10.1089/154099903321576547. [DOI] [PubMed] [Google Scholar]
- 70.Mankin HJ. Metabolic bone disease. Instr Course Lect. 1995;44:3–29. [PubMed] [Google Scholar]
- 71.Mankin HJ. Osteopetrosis. In: Mankin HJ, ed. Pathophysiology of Orthopaedic Diseases. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006:123–129.
- 72.Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Korkko J, Prockop DJ, Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila-Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;3:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazess RB, Whedon GD. Immobilization and bone. Calcif Tissue Int. 1983;3:265–267. doi: 10.1007/BF02405043. [DOI] [PubMed] [Google Scholar]
- 74.Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2 +) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum. 2010;62:1097–1107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;7:778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 77.Peris P, Guanabens N, Monegal A, Suris X, Alvarez L, Martinez de Osaba MJ, Hernandez MV, Munoz-Gomez J. Aetiology and presenting symptoms in male osteoporosis. Br J Rheumatol. 1995;10:936–941. doi: 10.1093/rheumatology/34.10.936. [DOI] [PubMed] [Google Scholar]
- 78.Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54(Suppl):1135S–1140S. doi: 10.1093/ajcn/54.6.1135s. [DOI] [PubMed] [Google Scholar]
- 79.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol. 2000;18:1570–1593. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 80.Rajakumar K. Infantile scurvy: a historical perspective. Pediatrics. 2001;4:E76. doi: 10.1542/peds.108.4.e76. [DOI] [PubMed] [Google Scholar]
- 81.Ralston SH, Langston AL, Reid IR. Pathogenesis and management of Paget’s disease of bone. Lancet. 2008;9633:155–163. doi: 10.1016/S0140-6736(08)61035-1. [DOI] [PubMed] [Google Scholar]
- 82.Recker RR. Calcium absorption and achlorhydria. N Engl J Med. 1985;313:70–73. doi: 10.1056/NEJM198507113130202. [DOI] [PubMed] [Google Scholar]
- 83.Reddy GK, Stehno-Bittel L, Hamade S, Enwemeka CS. The biomechanical integrity of bone in experimental diabetes. Diabetes Res Clin Pract. 2001;1:1–8. doi: 10.1016/s0168-8227(01)00273-x. [DOI] [PubMed] [Google Scholar]
- 84.Richens A, Rowe DJ. Disturbance of calcium metabolism by anticonvulsant drugs. Br Med J. 1970;5727:73–76. doi: 10.1136/bmj.4.5727.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosen CJ, Adler RA. Longitudinal changes in lumbar bone density among thyrotoxic patients after attainment of euthyroidism. J Clin Endocrinol Metab. 1992;6:1531–1534. doi: 10.1210/jcem.75.6.1464660. [DOI] [PubMed] [Google Scholar]
- 86.Sahni S, Hannan MT, Gagnon D, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture–a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int. 2009;11:1853–1861. doi: 10.1007/s00198-009-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scane AC, Sutcliffe AM, Francis RM. Osteoporosis in men. Baillieres Clin Rheumatol. 1993;3:589–601. doi: 10.1016/s0950-3579(05)80081-0. [DOI] [PubMed] [Google Scholar]
- 88.Schuit SC, Klift M, Weel AE, Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, Leeuwen JP, Pols HA. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;1:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Schultheis L. The mechanical control system of bone in weightless spaceflight and in aging. Exp Gerontol. 1991;26:203–214. doi: 10.1016/0531-5565(91)90012-b. [DOI] [PubMed] [Google Scholar]
- 90.Sherrard DJ, Hercz G, Pei Y, Segre G. The aplastic form of renal osteodystrophy. Nephrol Dial Transplant. 1996;11(Suppl 3):29–31. doi: 10.1093/ndt/11.supp3.29. [DOI] [PubMed] [Google Scholar]
- 91.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;2:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silverberg SJ, Shane E, la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;3:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 93.Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;10:1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 94.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;10:1813–1819. doi: 10.1359/JBMR.050609. [DOI] [PubMed] [Google Scholar]
- 95.Spasovski G. Low turn-over bone disease in patients with chronic renal disease. Med Pregl. 2007;60(Suppl 2):21–24. [PubMed] [Google Scholar]
- 96.Stepan JJ, Burr DB, Pavo I, Sipos A, Michalska D, Li J, Fahrleitner-Pammer A, Petto H, Westmore M, Michalsky D, Sato M, Dobnig H. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone. 2007;3:378–385. doi: 10.1016/j.bone.2007.04.198. [DOI] [PubMed] [Google Scholar]
- 97.Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clin Cornerstone. 2000;6:11–21. doi: 10.1016/s1098-3597(00)90002-4. [DOI] [PubMed] [Google Scholar]
- 98.Tejwani NC, Schachter AK, Immerman I, Achan P. Renal osteodystrophy. J Am Acad Orthop Surg. 2006;14:303–311. doi: 10.5435/00124635-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Turner CH. Bone strength: current concepts. Ann N Y Acad Sci. 2006;1068:429–446. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- 100.Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 101.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;3:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 102.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;2:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 103.Vico L, Chappard D, Alexandre C, Palle S, Minaire P, Riffat G, Morukov B, Rakhmanov S. Effects of a 120 day period of bed-rest on bone mass and bone cell activities in man: attempts at countermeasure. Bone Miner. 1987;2:383–394. [PubMed] [Google Scholar]
- 104.Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22(Suppl 2):V64–V68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 105.Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ. Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res. 2003;8:1513–1518. doi: 10.1359/jbmr.2003.18.8.1513. [DOI] [PubMed] [Google Scholar]
- 106.Weng MY, Lane NE. Medication-induced osteoporosis. Curr Osteoporos Rep. 2007;4:139–145. doi: 10.1007/s11914-007-0008-y. [DOI] [PubMed] [Google Scholar]
- 107.Wermers RA, Tiegs RD, Atkinson EJ, Achenbach SJ, Melton LJ., 3rd Morbidity and mortality associated with Paget’s disease of bone: a population-based study. J Bone Miner Res. 2008;6:819–825. doi: 10.1359/JBMR.080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;24:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 109.Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;6:1674–1686. doi: 10.1002/art.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, Kraenzlin M, Zach G, Lippuner K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;3:180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]