Abstract
Control of bipedal posture is highly automatized but requires attentional investment, the amount of which varies between participants and with postural constraints, such as plantar-flexor muscle fatigue. Elevated attentional demands for standing with fatigued plantar flexors have been demonstrated using a stimulus–response reaction-time paradigm. Recently, a direct relation between the regularity of center-of-pressure (COP) fluctuations and the amount of attention invested in posture was proposed, according to which more regular COP fluctuations are expected with muscle fatigue than without. To study this prediction, we registered anterior–posterior COP fluctuations for bipedal stance with eyes closed prior to and after a plantar-flexor muscle fatiguing exercise protocol in 16 healthy young adults. We quantified the magnitude of COP fluctuations with conventional posturography and its regularity with sample entropy. The magnitude of COP fluctuations increased significantly with fatigued plantar flexors. In addition, more regular COP fluctuations were observed with fatigued plantar flexors, as evidenced by significantly lower sample entropy values. These findings corroborated our hypotheses. Moreover, COP regularity assisted in qualifying the change in sway magnitude with fatigue. Whereas increased sway is customary taken to reflect impaired postural control, we interpret it as a functional, but attention-demanding adaptation to the alteration of important posture-specific information.
Keywords: Postural control, Attentional investment, Center-of-pressure regularity, Muscle fatigue
Introduction
Bipedal standing is a largely automatized basic activity, i.e., it is controlled without placing a substantial cognitive burden or attentional demand on the controller. However, numerous investigations using dual-task paradigms made apparent that postural control is not entirely automatic and that the degree of attentional investment needed to execute this postural task varies with the performer’s health status, age, and expertise (see Fraizer and Mitra 2008; Woollacott and Shumway-Cook 2002, for reviews). Indeed, individuals with impaired postural control capacities (e.g., fall-prone elderly, stroke patients) are more affected by posture-cognition dual-tasking than controls (e.g., Brown et al. 2002; Huxhold et al. 2006; Lacour et al. 2008), whereas attentional effects in balancing experts (e.g., gymnasts, ballet dancers) have only been reported in more difficult postural configurations like standing on one leg (Stins et al. 2009; Vuillerme and Nougier 2004). Attentional investment in the control of posture not only varies between different groups of individuals, but also within individuals as a function of instructions or task constraints. Examples are instructed directed forms of attention such as adopting an internal or external focus of attention (McNevin and Wulf 2002; Vuillerme and Nafati 2007), induced postural threat situations such as standing at the edge of a cliff (Huffman et al. 2009; Stins et al. 2011), and manipulation of the size of the base of support (Roerdink et al. 2011; Vuillerme and Nougier 2004). Several of those investigations have used stimulus–response reaction times to operationalize changes in attentional investment.
In recent reports on the dynamical structure of center-of-pressure (COP) profiles during bipedal quiet standing, a direct relation between COP regularity and the amount of attention invested in postural control was proposed (e.g., Donker et al. 2007, 2008; Roerdink et al. 2006, 2011; Stins et al. 2009). This proposal was based on the empirical findings that posturograms were more regular for pathological groups than for controls (e.g., Cavanaugh et al. 2006; Donker et al. 2008; Roerdink et al. 2006, 2009; Schmit et al. 2006), less regular in balance experts than controls (Schmit et al. 2005; Stins et al. 2009), and less regular when attention was experimentally withdrawn from posture using secondary tasks (Cavanaugh et al. 2007; Donker et al. 2007; Madeleine et al. 2011; Roerdink et al. 2006; Stins et al. 2009), regardless of the employed method to compute COP regularity (e.g., sample entropy, approximate entropy, recurrence quantification analysis). Clearly, the interpretations of the COP regularity findings for these between- and within-subject comparisons are congruent with aforementioned interpretations of stimulus–response reaction-time results and culminated in the proposal of two associated continua, viz. a spectrum of COP regularity parallel to a spectrum of automaticity of postural control, according to which more regular posturograms are associated with increased attentional investments in postural control and vice versa (cf. Figure 4 of Roerdink et al. 2011).
As a further within-subject test-case of the proposed relation between COP regularity and the amount of attention invested in posture, this study was designed to investigate the effects of plantar-flexor muscle fatigue on COP regularity. Plantar-flexor muscles are the primary sagittal plane movers during bipedal stance (Winter et al. 1996). With plantar-flexor muscle fatigue, the control of bipedal posture has been shown to be modified in terms of an increased attentional investment, as evidenced by a stimulus–response reaction-time study reporting increased reaction times for controlling quiet stance with fatigued plantar flexors (Vuillerme et al. 2002) and the observed centrally mediated fatigue-induced adaptive changes of anticipatory postural adjustments (Strang and Berg 2007; Strang et al. 2008, 2009). Thus, following the proposed relation between the amount of attention invested in posture and COP regularity (e.g., Donker et al. 2007, 2008; Roerdink et al. 2006, 2011; Stins et al. 2009), more regular COP trajectories—operationalized in terms of sample entropy, a measure of time series regularity with lower values representing increased regularity (see “Methods” for more details)—were expected with fatigued than with non-fatigued plantar flexors. Moreover, with fatigued plantar flexors, COP excursions in the anterior–posterior (AP) direction have been found to increase during bipedal standing with eyes closed (cf. Ledin et al. 2004; Pinsault and Vuillerme 2008; Vuillerme and Demetz 2007; Vuillerme et al. 2006). Consistent with the notion that muscle fatigue influences various aspects of neuromuscular function (see for a review, Enoka and Duchateau 2008), the increased postural sway with fatigued plantar-flexor muscles has been attributed to stem from an alteration of ankle proprioceptive acuity (Vuillerme et al. 2007; Vuillerme and Boisgontier 2008) and to an inability to produce or sustain a required force output (Trappe et al. 2001). In this study, we thus also expect to observe the customary larger AP COP fluctuations with fatigued plantar flexors (cf. Ledin et al. 2004; Pinsault and Vuillerme 2008; Vuillerme and Demetz 2007; Vuillerme et al. 2006), quantified by two conventional posturographic measures (i.e., range and standard deviation; see “Methods”).
Methods
Participants
Sixteen young male university students (age: 22.4 ± 1.9 years; body weight: 70.7 ± 7.6 kg; height: 177.0 ± 6.8 cm; mean ± SD) volunteered in the experiment. None of the participants presented any history of injury, surgery, or pathology to either lower extremity that could affect their ability to carry out the experiment. They gave their informed consent to the experimental procedure, which was approved by the local Ethics Committee.
Experimental setup and procedure
Participants stood barefoot on the force platform (Equi+, model PF01, Aix-les-Bains, France) in a natural position (feet abducted at 30°, heels separated by 3 cm), their arms hanging loosely by their sides with eyes closed. Participants were instructed to sway as little as possible in two conditions, pre- and post-fatigue (one trial each). Trial duration was 32 s, and AP COP trajectories were registered with a sampling rate of 64 Hz. The pre-fatigue condition served as a control registration for quiet stance with non-fatigued plantar-flexor muscles, while, in the post-fatigue condition, the quiet standing registration was performed immediately after a designated fatiguing exercise protocol for the plantar flexors.
Fatiguing exercise protocol
The fatiguing exercise took place next to the force platform. Participants were asked to repeatedly raise their heels by standing on their toes with straight knees as many times as possible, following the beat of a metronome (40 beats/min). This task, requiring alternate lifts and drops of the center of mass in a vertical direction, fatigues the plantar-flexor muscles considerably. Participants were encouraged verbally to work to exhaustion, i.e., until they were no longer able to perform the exercise. Immediately after the cessation of the fatiguing exercise, the subjective exertion level was assessed through a category-ratio scale ranging from “nothing at all” (0) to “extremely strong” (10) (Borg CR-10 scale, Borg 1990), and the post-fatigue force-platform registration was performed.
Data analysis
Three dependent COP variables were computed. To be able to compare our results with previous research (e.g., Ledin et al. 2004; Pinsault and Vuillerme 2008; Vuillerme and Demetz 2007; Vuillerme et al. 2006), we quantified AP COP fluctuations in terms of their range and standard deviation (i.e., RCOP and SDCOP, respectively). In addition, we determined COP regularity by taking the sample entropy of AP COP time series (SEnCOP) using algorithms of Lake and colleagues (Lake et al. 2002). Specifically, sample entropy is quantified as the negative natural logarithm of the conditional probability (CP = A/B) of a data set, having repeated itself within a tolerance r for m points, will also repeat itself for m + 1 points, without allowing self-matches (see also Lake et al. 2002; Richman and Moorman 2000). Accordingly, B represents the total number of matches of length m while A represents the subset of B that also matches for m + 1. Sample entropy follows from –log (A/B), with a low sample entropy value arising from a high probability of repeated template sequences in the data. In other words, the lower the sample entropy, the greater the regularity of the time series. Parameter choice was optimized to m as large and r as small as possible using a statistical criterion that simultaneously penalizes CPs near 0 and near 1 (cf. Lake et al. 2002; see also Ramdani et al. 2009; Roerdink et al. 2006, 2011). Accordingly, the number of matches remains small enough to preserve discriminative power but large enough for reliable sample entropy estimation. Thus, after normalizing AP COP time series to unit variance, sample entropy was determined with the optimized parameters m = 3 and r = 0.03. Note that sample entropy has proven surplus value over other commonly used regularity statistics (e.g., approximate entropy, entropy in recurrence quantification analysis). The advantage of using sample entropy over approximate entropy is clear, as it is an improved version of the approximate entropy algorithm initially proposed by Pincus (1991; see also Richman and Moorman 2000). The use of sample entropy over recurrence quantification analysis is that, with the former, parameter settings can be objectively picked on the basis of a single criterion (as described earlier), whereas recurrence quantification analysis requires a selection of considerably more parameters (7 vs. 2), for which a single optimal parameter setting selection criterion is lacking.
Statistical analysis
Data obtained in the pre-fatigue and post-fatigue conditions were compared using paired samples t-tests; the level of significance was set at P < 0.05.
Results
The fatiguing exercise protocol worked sufficiently, as participants rated their perceived plantar-flexor muscle fatigue as 8.5 ± 0.7 on average, ranging from “very strong” (7) to “extremely strong” (10).
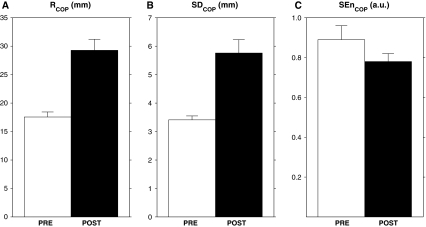
Paired samples t-tests revealed significant differences between pre- and post-fatigue values for all three dependent COP variables; average values and associated standard errors for pre- and post-fatigue conditions are depicted in Fig. 1. As expected, significant increments in RCOP (t (15) = 5.53, P < 0.001, panel a) and SDCOP (t (15) = 5.04, P < 0.001, panel b) were observed after the fatiguing protocol. Furthermore, more regular COP trajectories were observed with fatigued plantar flexors, as evidenced by significantly lower SEnCOP values in the post- than pre-fatigue condition (t (15) = 2.27, P < 0.05, Fig. 1c).
Fig. 1.
Average values and standard errors for (a) range (RCOP), (b) standard deviation (SDCOP), and (c) regularity (SEnCOP) of center-of-pressure (COP) trajectories for pre- and post-fatigue conditions (PRE, white bars; POST, black bars, respectively)
Discussion
Taken together, these findings corroborated our hypotheses. AP COP fluctuations were more pronounced with than without fatigued plantar flexors (Fig. 1a, b), thereby replicating results of previous reports on the effect of plantar-flexor muscle fatigue on conventional posturography (e.g., Ledin et al. 2004; Pinsault and Vuillerme 2008; Vuillerme and Demetz 2007; Vuillerme et al. 2006), including a study demonstrating increased stimulus–response reaction times with plantar-flexor muscle fatigue (Vuillerme et al. 2002). The novel finding of the present study was that AP COP fluctuations were more regular with than without fatigued plantar flexors, as reflected by lower SEnCOP values (Fig. 1c). According to the proposed relation between COP regularity and the amount of attention invested in posture (Donker et al. 2007, 2008; Roerdink et al. 2006, 2011; Stins et al. 2009), this finding suggests that participants invested a larger amount of attention in the regulation of posture with fatigued plantar flexors. This interpretation is consistent with the increased attentional demand—operationalized with a stimulus–response reaction-time task—previously reported in such situations (Vuillerme et al. 2002). The finding of more regular COP fluctuations with fatigued plantar flexors and its interpretation in terms of a less automatized postural control fits in the within-subject path of the two parallel continua, viz. automaticity of control and COP regularity, proposed by Roerdink et al. (2011, Figure 4). Although the association between COP regularity and attentional investment in postural control is empirically demonstrated for both between-subject (e.g., pathology, expertise) and within-subject (e.g., recovery, attentional instructions, task constraints, sensory deprivation, fatigue) factors, evidence for a causal relationship between the two is limited.
We now discuss the putative interrelationship between a compromised sensory-motor system as induced by fatigued plantar flexors on the one hand and the corresponding increased attentional investment (Vuillerme et al. 2002) and more regular COP fluctuations (this study) on the other hand. It has been postulated that the postural control system can adaptively reweigh the relative contribution of particular posture-specific sensory modalities, depending on the availability and reliability of the various sensory inputs in a given environmental context (e.g., Horak and Macpherson 1996; Nashner et al. 1982). For standing upright on a non-compliant surface, for example, visual information and somatosensory information from the legs provide the most discriminative information for the regulation of posture, whereas the contribution of the vestibular system is then only marginal because sway accelerations remain sub-threshold (cf. Fitzpatrick and McCloskey 1994). In the present study, posture-specific visual information was made unavailable because we instructed participants to stand still with their eyes closed while we severely affected the reliability of ankle proprioception with the fatiguing protocol (Vuillerme et al. 2007; Vuillerme and Boisgontier 2008), which resulted in an overall increased magnitude of AP COP fluctuations (Fig. 1a, b). Typically, this increased postural sway is taken to reflect a degraded postural control. This interpretation may, however, be challenged in the context of our manipulations considering that visual information was made unavailable, whereas proprioception became unreliable due to plantar-flexor muscle fatigue. Given that an altered neuromuscular state is known to evoke centrally mediated adaptive changes of anticipatory postural adjustments (Strang and Berg 2007; Strang et al. 2008, 2009), it may well be that the participants in the present study adaptively compensated for the alteration of important posture-specific sensory inputs by deliberately increasing the magnitude of sway in order to get the vestibular system up and running (see for a similar line of reasoning Strang et al. 2011). This adaptation is likely not an automatized postural response, given the increased attention demands observed for standing with fatigued plantar flexors (Vuillerme et al. 2002), and our observation that COP fluctuations were more regular with fatigued plantar flexors (cf. Fig. 1c), indicative of an increased attentional investment according to the proposed relation between COP regularity and attentional investment in posture (Donker et al. 2007, 2008; Roerdink et al. 2006, 2011; Stins et al. 2009). In other words, we suggest that standing quietly upright with fatigued plantar flexors may be manifested by a deliberate increase in sway magnitude to exploit the vestibular system, which is accompanied by an increased attentional investment to closely monitor and control posture.
Acknowledgments
The contributions of MR, PH, and NV were supported by Veni grant 451-09-024 of the Netherlands Organization for Scientific Research (NWO), the Czech Ministry of Education, Youth and Sports (grant no. RP 6198959221), and the Centre National de la Recherche Scientifique (France), respectively.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Borg G. Psychological scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16:55–58. doi: 10.5271/sjweh.1815. [DOI] [PubMed] [Google Scholar]
- Brown LA, Sleik RJ, Winder TR. Attentional demands for static postural control after stroke. Arch Phys Med Rehabil. 2002;83:1732–1735. doi: 10.1053/apmr.2002.36400. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–313. [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JT, Mercer VS, Stergiou N. Approximate entropy detects the effect of a secondary cognitive task on postural control in healthy young adults: a methodological report. J Neuroeng Rehabil. 2007;4:42. doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker SF, Roerdink M, Greven AJ, Beek PJ. Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp Brain Res. 2007;181:1–11. doi: 10.1007/s00221-007-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker SF, Ledebt A, Roerdink M, Savelsbergh GJP, Beek PJ. Children with cerebral palsy exhibit greater and more regular postural sway than typically developing children. Exp Brain Res. 2008;184:363–370. doi: 10.1007/s00221-007-1105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, McCloskey DR. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Phys. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraizer EV, Mitra S. Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait Posture. 2008;27:271–279. doi: 10.1016/j.gaitpost.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepard JT, editors. Handbook of physiology. Exercise: regulation and integration of multiple systems. Oxford: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- Huffman JL, Horslen BC, Carpenter MG, Adkin AL. Does increased postural threat lead to more conscious control of posture? Gait Posture. 2009;30:528–532. doi: 10.1016/j.gaitpost.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Huxhold O, Li S-C, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Lacour M, Bernard-Demanze L, Dumitrescu M. Posture control, aging, and attention resources: models and posture-analysis methods. Neurophysiol Clin. 2008;38:411–421. doi: 10.1016/j.neucli.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2002;283:789–797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- Ledin T, Fransson PA, Magnusson M. Effects of postural disturbances with fatigued triceps surae muscles or with 20% additional body weight. Gait Posture. 2004;19:184–193. doi: 10.1016/S0966-6362(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Madeleine P, Nielsen M, Arendt-Nielsen L. Characterization of postural control deficit in whiplash patients by means of linear and nonlinear analyses: a pilot study. J Electromyogr Kinesiol. 2011;21:291–297. doi: 10.1016/j.jelekin.2010.05.006. [DOI] [PubMed] [Google Scholar]
- McNevin NH, Wulf G. Attentional focus on supra-postural tasks affects postural control. Hum Mov Sci. 2002;21:187–202. doi: 10.1016/S0167-9457(02)00095-7. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Black FO, Wall C. Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2:536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM (1991) Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 88:2297–2301 [DOI] [PMC free article] [PubMed]
- Pinsault N, Vuillerme N. Differential postural effects of plantar-flexor muscle fatigue under normal, altered and improved vestibular and neck somatosensory conditions. Exp Brain Res. 2008;191:99–107. doi: 10.1007/s00221-008-1500-z. [DOI] [PubMed] [Google Scholar]
- Ramdani S, Seigle B, Lagarde J, Bouchara F, Bernard PL. On the use of sample entropy to analyze human postural sway data. Med Eng Phys. 2009;31:1023–1031. doi: 10.1016/j.medengphy.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Roerdink M, de Haart MD, Daffertshofer A, Donker SF, Geurts AC, Beek PJ. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp Brain Res. 2006;174:256–269. doi: 10.1007/s00221-006-0441-7. [DOI] [PubMed] [Google Scholar]
- Roerdink M, Geurts AC, de Haart M, Beek PJ. On the relative contribution of the paretic leg to the control of posture after stroke. Neurorehabil Neural Repair. 2009;23:267–274. doi: 10.1177/1545968308323928. [DOI] [PubMed] [Google Scholar]
- Roerdink M, Hlavackova P, Vuillerme N. Center-of-pressure regularity as a marker for attentional investment in postural control: a comparison between sitting and standing postures. Hum Mov Sci. 2011;30:203–212. doi: 10.1016/j.humov.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Schmit JM, Regis DI, Riley MA. Dynamic patterns of postural sway in ballet dancers and track athletes. Exp Brain Res. 2005;163:370–378. doi: 10.1007/s00221-004-2185-6. [DOI] [PubMed] [Google Scholar]
- Schmit JM, Riley MA, Dalvi A, Sahay A, Shear PK, Shockley KD, Pun RY. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2006;168:357–367. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- Stins JF, Michielsen ME, Roerdink M, Beek PJ. Sway regularity reflects attentional involvement in postural control: Effects of expertise, vision and cognition. Gait Posture. 2009;30:106–109. doi: 10.1016/j.gaitpost.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Stins JF, Roerdink M, Beek PJ. To freeze or not to freeze? Affective and cognitive perturbations have markedly different effects on postural control. Hum Mov Sci. 2011;30:190–202. doi: 10.1016/j.humov.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Strang AJ, Berg WP. Fatigue-induced adaptive changes of anticipatory postural adjustments. Exp Brain Res. 2007;178:49–61. doi: 10.1007/s00221-006-0710-5. [DOI] [PubMed] [Google Scholar]
- Strang AJ, Choi HJ, Berg WP. The effect of exhausting aerobic exercise on the timing of anticipatory postural adjustments. J Sports Med Phys Fitness. 2008;48:9–16. [PubMed] [Google Scholar]
- Strang AJ, Berg WP, Hieronymus M. Fatigue-induced early onset of anticipatory postural adjustments in non-fatigued muscles: support for a centrally mediated adaptation. Exp Brain Res. 2009;197:245–254. doi: 10.1007/s00221-009-1908-0. [DOI] [PubMed] [Google Scholar]
- Strang AJ, Haworth J, Hieronymus M, Walsh M, Smart LJ (2011) Structural changes in postural sway lend insight into effects of balance training, vision, and support surface on postural control in a healthy population. Eur J Appl Physiol. doi:10.1007/s00421-010-1770-6 [DOI] [PubMed]
- Trappe SW, Trappe TA, Lee GA, Costill DL. Calf muscle strength in humans. Int J Sports Med. 2001;22:186–191. doi: 10.1055/s-2001-16385. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Boisgontier M. Muscle fatigue degrades force sense at the ankle joint. Gait Posture. 2008;28:521–524. doi: 10.1016/j.gaitpost.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Demetz S. Do ankle foot orthoses modify postural control during bipedal quiet standing following a localized fatigue of the ankle muscles? Int J Sports Med. 2007;28:243–246. doi: 10.1055/s-2006-924292. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Nafati G. How attentional focus on body sway affects postural control during quiet standing. Psychol Res. 2007;71:192–200. doi: 10.1007/s00426-005-0018-2. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Nougier V. Attentional demands for regulating postural sway: the effect of expertise in gymnastics. Brain Res Bull. 2004;63:161–165. doi: 10.1016/j.brainresbull.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Forestier N, Nougier V. Attentional demands and postural sway: the effect of the calf muscles fatigue. Med Sci Sports Exerc. 2002;34:1607–1612. doi: 10.1097/00005768-200212000-00008. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Burdet C, Isableu B, Demetz S. The magnitude of the effect of calf muscles fatigue on postural control during bipedal quiet standing with vision depends on the eye-visual target distance. Gait Posture. 2006;24:169–172. doi: 10.1016/j.gaitpost.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Boisgontier M, Chenu O, Demongeot J, Payan Y. Tongue-placed tactile biofeedback suppresses the deleterious effects of muscle fatigue on joint position sense at the ankle. Exp Brain Res. 2007;183:235–240. doi: 10.1007/s00221-007-1039-4. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Franck JS, Powel C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet standing. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/S0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]