Abstract
Extraintestinal manifestations of inflammatory bowel disease are prevalent in both ulcerative colitis and Crohn's disease. The most common manifestations involve the musculoskeletal and dermatologic systems. Other manifestations involve the hepatopan-creatobiliary system (eg, primary sclerosing cholangitis) as well as the ocular, renal, and pulmonary systems. A multidisciplinary team approach is often needed for effective management, and emergency situations require prompt evaluation.
Keywords: Inflammatory bowel disease, extraintestinal manifestations, arthritis, primary sclerosing cholangitis, dermatology
Extraintestinal manifestations (EIMs) of inflammatory bowel disease (IBD) are common in both ulcerative colitis (UC) and Crohn's disease (CD). These manifestations can involve nearly any organ system—including the musculoskeletal, dermatologic, hepatopancreatobiliary, ocular, renal, and pulmonary systems—and can cause a significant challenge to physicians managing IBD patients. Most IBD patients with EIMs have colonic inflammation, although some patients develop EIMs prior to the onset of colonic symptoms. In this paper, we review the major EIMs of IBD and strategies for their management. An overview of these manifestations is presented in Table 1.
Table 1.
Extraintestinal Manifestations of Inflammatory Bowel Disease
| Sites | Extraintestinal manifestations |
|---|---|
| Musculoskeletal system |
|
| Dermatologic and oral systems |
|
| Hepatopancreatobiliary system |
|
| Ocular system |
|
| Metabolic system |
|
| Renal system |
|
Prevalence
EIMs are seen in 25–40% of IBD patients.1 Inflammatory manifestations of the skin, eyes, liver, and joints are considered primary manifestations. If secondary effects of disease activity are also considered, nearly 100% of IBD patients have an abnormality outside of the gastrointestinal tract lumen.2 Twenty-five percent of IBD patients have more than 1 EIM. The development of 1 EIM appears to increase the risk of developing a second EIM.3 Few studies have specifically examined how frequently an EIM is a patient's presenting symptom or is present at the time of diagnosis versus occurring later in the disease course. In a retrospective study of 448 IBD patients, Aghazadeh and associates showed that 31.4% of UC patients and 40.4% of CD patients had 1 of the 5 major manifestations; a smaller percentage of patients had more than 1 major EIM.4 Limited data have shown that approximately one third of patients will develop symptomatic primary sclerosing cholangitis (PSC) prior to a diagnosis of IBD. Based on several small studies, 10–30% of patients with arthritis related to IBD will have arthritic symptoms prior to IBD diagnosis.
Musculoskeletal Manifestations
Musculoskeletal pain occurs in 9–53% of IBD patients and is considered the most common EIM.1,2,4 The differential diagnosis of this condition includes articular, periarticular, and muscular involvement; osteoporosis and related fractures; and fibromyalgia.
Arthritis can affect the spine, sacroiliac joint, peripheral joints, or a combination of these sites. Classically, inflammatory arthritis is defined by pain, an increase in local temperature, and joint swelling with or without effusion, leading to decreased joint mobility. Associated peri-articular features include tendonitis, clubbing, periostitis, and granulomatous lesions of the joint and bone. Inflammatory arthritis can be differentiated from osteoarthritis by morning stiffness and improvement with ambulation.
As previously mentioned, peripheral or axial articular involvement can precede, be synchronous with, or develop following the diagnosis of IBD, often by as many as 10 years. IBD-related arthropathy is part of a subset of diseases broadly termed “seronegative spondy-loarthropathies.” In addition to IBD-related disease, this category includes psoriatic arthritis, reactive arthritis, and idiopathic ankylosing spondylitis (AS). Arthritis occurs equally in males and females and is generally more common in patients with colonic disease than those with small-bowel disease. In addition, arthritis is more common in CD with colonic involvement than UC and is more common in UC with pancolitis than isolated left-sided disease. Subclinical colonic inflammation has been documented in approximately two thirds of patients with spondyloarthropathies.3
Occurring in 5–20% of IBD patients, peripheral arthritis classically involves large joints and is asymmetric.5 Peripheral arthritis is also classically rheumatoid factor–negative (and, hence, seronegative), nondeforming, and nonerosive, although erosive lesions mimicking rheumatoid arthritis have been described.
Type 1 peripheral arthritis is pauciarticular—involving fewer than 5 joints—and is strongly associated with IBD activity and other EIMs. The knee is the most commonly affected site. Occurring in approximately 3.6% of UC patients and 6% of CD patients, fares are self-limiting, with attacks lasting 5–10 weeks.6 Flares usually parallel the severity of bowel symptoms.
Type 2 peripheral arthritis is polyarticular, independent of disease activity, and associated with fares that can last months or years. The metacarpophalangeal joint is the most commonly involved site. Less commonly involved sites include the knees, ankles, shoulders, proximal interphalangeal joint, and metatarsophalangeal joint. Type 2 peripheral arthritis is not usually associated with other EIMs, with the exception of uveitis. The severity of this arthritis appears to be independent of active bowel disease.
Spondylitis can occur in 1–26% of patients with IBD. Males are more frequently affected than females. Typical presentations include back or buttock pain, which worsens in the morning or after rest and is relieved with exercise. Spinal pain is often felt moving from the lumbar spine to the cervical spine. Buttock pain often alternates with chest wall pain. A physical examination may reveal limited spinal flexion and reduced chest expansion. AS occurs in 3–12% of patients with IBD. Nearly all IBD patients who are positive for human leukocyte antigen B27 will develop AS. Axial involvement is independent of gut pathology.
Asymptomatic sacroiliitis is increasingly being recognized in the general population, particularly due to improvements in the sensitivity of magnetic resonance imaging (MRI). A Spanish prospective study looked at 62 IBD patients without axial symptoms who underwent MRIs; sacroiliitis was radiographically detected in 24% of patients who were asymptomatic.7 Approximately two thirds of patients had evidence of CD consistent with previously reported distributions.
Treatment of peripheral arthritis is aimed at treating the underlying bowel disease as well as achieving symptom relief. There has been concern over the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective cyclooxygenase-2 (COX-2) inhibitors in IBD patients due to their established propensity for worsening IBD symptoms. Mahadevan and colleagues conducted a retrospective chart review of 27 patients with CD or UC receiving celecoxib (Celebrex, Pfizer) or rofecoxib (Vioxx, Merck); 22 patients showed no change in IBD activity, and 14 patients showed improvement in their arthritic symptoms.8 An open-label trial of rofecoxib in 32 patients showed no worsening of IBD symptoms during a 20-day trial; a 60% improvement was seen in patients with either arthropathy or arthralgias.9
Sulfasalazine has also been studied for symptom relief and is usually used if NSAIDs do not ameliorate symptoms. A meta-analysis of 5 placebo-controlled trials showed that sulfasalazine—at an initial dose of 500 mg BID titrated to a maximal dose of 1,500 mg TID—was better than placebo at reducing morning stiffness and improving quality of life.10 Mesalamine has been used only anecdotally; no good placebo-controlled trials have been conducted yet. If these drugs do not improve symptoms, a tumor necrosis factor (TNF)-α inhibitor trial can be used in CD patients at the doses used for rheumatoid arthritis; in UC patients, a TNF-α inhibitor or a short course of steroids can be used.
Spinal and axial involvement are treated similarly to other spondyloarthropathies. These patients should always be referred to a physical therapist for back exercises, which may prevent back and neck deformities. NSAIDs and COX-2 inhibitors are used for symptom relief. Due to the efficacy of anti–TNF-α therapy for AS, infliximab (Remicade, Centocor) has been studied for treatment of IBD-related disease. Herfarth and coworkers conducted an open-label trial of infliximab in patients with active CD and axial symptoms; 61% of patients showed improvement in arthritis or arthralgias, and 46% were free of symptoms.11 Etanercept (Enbrel, Immunex) has reportedly provided improvement in musculoskeletal symptoms, but this drug has no effect on intestinal manifestations. Partial or total proctocolectomy can induce remission of peripheral arthritis in UC patients, but these surgeries have no effect on axial involvement. In contrast, colonic resection in CD does not appear to affect the course of arthritis.
There are multiple risk factors for osteoporosis in IBD patients. Beyond age-related risk factors that are present in the general population, IBD-specific risk factors include corticosteroid therapy, reduced physical activity, inflammatory-mediated bone resorption (increased levels of interleukin [IL]-1, IL-6, TNF-α), calcium and magnesium dietary malabsorption, vitamin D deficiency, poor dietary calcium intake (related to lactose intolerance), decreased serum albumin levels, and ileal resorption. The overall risk of fracture in IBD patients is 1 per 100 patient-years—40% higher than in the general population—and this risk increases with age.12 Unlike the risk in the general population, male and female patients with IBD may have similar risks of fracture. Likewise, CD and UC are associated with similar fracture risks.
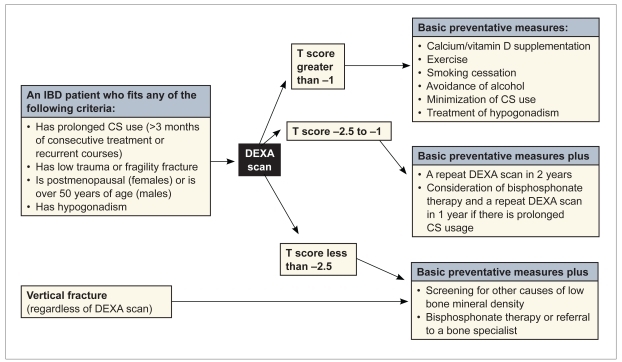
We recommend a dual-energy X-ray absorptiometry scan for any IBD patient who has undergone prolonged use of steroids (defined as >3 months of consecutive treatment or recurrent courses), postmenopausal females, males over 50 years of age, males with hypogonadism, or any patient with evidence of a fragility fracture. Figure 1 outlines the American Gastroenterological Association's recommendations for management of osteoporosis in IBD patients. We also recommend rheumatologic evaluation for comanagement of any IBD patient who has concurrent arthritis or evidence of osteoporosis.
Figure 1.
The American Gastroenterological Association's recommendations for management of osteoporosis in IBD patients.
- CS
- corticosteroid
- DEXA
- dual-energy X-ray absorptiometry
- IBD
- inflammatory bowel disease.
Modified from Bernstein CN, et al.40
Dermatologic Manifestations
Major dermatologic manifestations have been reported in 2–34% of IBD patients.13 A broad spectrum of skin diseases may occur, and patients may develop multiple dermatologic manifestations concurrently during the natural course of their disease.14 Erythema nodosum (EN) and pyoderma gangrenosum (PG) are the most common cutaneous manifestations.13 Other skin lesions include psoriasis, oral aphthous stomatitis, and Sweet syndrome. A dermatologic evaluation is mandatory for any of these disorders to help confirm the diagnosis and assist in management.
A prospective study conducted by Yüksel and associates looked at the prevalence of cutaneous manifestations in 352 patients with IBD over 4.5 years.15 Thirty-four patients presented with either EN or PG, yielding prevalences of 7.4% and 2.3%, respectively. Psoriasis was found in 11 patients, aphthous stomatitis in 132 patients, and genital ulcers in 15 patients.
EN is the most common cutaneous lesion and appears as a series of deep, tender nodules most commonly found overlying the shins. Although overlying skin can appear to be bruised or erythematosus, EN lesions are frequently palpable and are not easily visible. Inflammation occurs in subcutaneous fat (panniculitis) and can develop wherever subcutaneous fat is present; the anterior tibial area is the most common site, but the arms and trunk can also be affected. Lesions are typically 1–5 cm in diameter. EN has also been associated with numerous infections, including tuberculosis (TB), coccidioidomycosis, histoplasmosis, blastomycosis, Yersinia, Behçet syndrome, and sarcoidosis.16–19 In addition, EN has been associated with the use of medications such as sulfonamides, iodides, bromides, and estrogens. EN can occur simultaneously in multiple locations, and studies have suggested that the prevalence of recurrent disease is approximately 20%.20
In the past, EN was thought to be 3–6 times more common in women than in men; however, the difference in prevalence between genders did not reach statistical significance in a recent Turkish study.13,15 This condition is more common in CD than in UC.21 The occurrence of lesions parallels intestinal disease activity, and lesions frequently resolve when bowel disease subsides; thus, treatment is usually aimed at the underlying bowel disease. At times, EN can precede bowel exacerbations and can require treatment with oral steroids. Based on these findings, patients with idiopathic EN should be evaluated for IBD.
PG begins with a pustule or erythematous papule or nodule that quickly breaks down to form an ulcer with violaceous undermined borders. Covered with pus or necrotic debris, ulcers contain fistulous tracts that open into characteristic crater-like holes that leave a typical pattern of cribriform scarring when healed. These ulcers can be solitary or multiple, unilateral or bilateral, and can range in size from several centimeters to an entire limb. Although the legs are most commonly affected, PG ulcers can appear on any part of the body, most notably the abdominal wall adjacent to a postsurgical stoma.22
A characteristic feature of PG is pathergy, which is an exaggerated physiologic response to minor trauma. Pathergy is seen in only 2 conditions: PG and Behçet syndrome. PG can develop after any type of trauma, with postsurgical wounds on one end of the spectrum and injuries as small as needle sticks from venipunctures on the other end.
PG has been reported in 1–10% of UC patients and 0.5–20% of CD patients.22 In a recent Turkish study, PG was observed in 6 of 234 UC patients and 2 of 118 CD patients.15 There was no significant difference between genders, which supports previous reports.23 There have been conflicting data regarding the distribution of PG among CD and UC; the Turkish study showed no statistically significant difference between the 2 groups.
Controversy exists as to whether PG parallels disease activity. Some reports have shown that approximately half of cases parallel intestinal disease.24 In the recent Turkish study, 6 of 8 patients with PG had active intestinal disease.15 Therapy can be concentrated on underlying intestinal disease in these cases, although high-dose steroids usually need to be part of the regimen. Localized disease can be treated with an intralesional corticosteroid injection. Other immunosuppressive agents such as cyclophosphamide and azathioprine have been used alone or in conjunction with steroids for treatment.22 Cyclosporine has been used effectively at low doses in patients resistant to steroid therapy.25 Patients will often show a rapid response, with crusting of lesions in less than 24 hours. Infliximab use in these patients is currently under investigation.
Psoriasis appears to be more common in CD patients than in the general population.26 Psoriatic lesions have a high concentration of TNF-α, similar to lesions seen in CD, suggesting some immunologic overlap. In the Turkish study, 11 of 352 patients had psoriasis.15 There was no statistically significant difference in the occurrence of psoriasis between CD and UC. Psoriasis occurred an average of 16.8 years before the initial presentation of intestinal disease. In a study of 15 patients with psoriasis or psoriatic arthritis (but not active bowel disease), 9 patients had microscopic changes in biopsies of macroscopically normal colons.27 This finding supports a common pathogenetic link among psoriasis, psoriatic arthritis, and IBD.
Aphthous stomatitis consists of oral ulcers that are common in both UC and CD. Clinically, aphthous stomatitis is indistinguishable from canker sores, which can be seen with herpes simplex virus type 1 infection or idiopathic oral ulcers. Aphthous stomatitis typically occurs on the buccal mucosa and lips. Although a biopsy is rarely required, histology can reveal noncaseating granulomas in CD similar to those seen in the colon. Treatment is symptomatic, with topical anesthetics such as viscous xylocaine. Topical antibiotics can be used for bacterial superinfection, although they are rarely required.
Other skin disorders that are less common and thus beyond the scope of this paper include Sweet syndrome (acute inflammatory dermatitis), metastatic CD (ulcerating nodules with noncaseating granulomas on biopsy that can occur in virtually any part of the body), and eczematous lesions (sometimes seen as a complication of anti–TNF-α therapy).
Hepatopancreatobiliary Manifestations
Hepatopancreatobiliary manifestations of IBD include PSC, cholelithiasis, portal vein thrombosis, drug-induced hepatotoxicity, and drug-induced pancreatitis. One of the most serious complications of IBD is PSC, a chronic, progressive disorder of unknown etiology that manifests as inflammation, stricturing, and fibrosis of medium and large intra- and extrahepatic bile ducts.28 PSC has an established strong association with IBD, particularly UC. At least 75% of PSC patients have coexisting UC. Another 5–10% of PSC patients have CD. However, only 5% of UC patients and 2% of CD patients develop PSC. Patients with UC and extensive colonic involvement with or without backwash ileitis are more likely to develop PSC than patients with only left colon involvement. PSC is most common in patients 30–59 years of age, and there is a 2:1 male prevalence for the disorder. The clinical course of PSC bears no relationship to underlying bowel disease, and PSC can develop either years before or after the development of bowel symptoms.
The development of cholestasis in any IBD patient should prompt an evaluation for PSC. Jaundice may arise at any time due to biliary strictures, and it typically subsides spontaneously. Laboratory abnormalities show an elevated alkaline phosphatase level; in contrast, aspartate aminotransferase and alanine aminotransferase levels are typically normal. Albumin levels and prothrombin time are typically normal until the development of advanced disease and cirrhosis. Autoantibodies can be helpful for diagnosis. Approximately 33% of patients have an elevated antinuclear antibody level, and almost 80% of patients will have a positive antineutrophil cytoplasmic antibody level. Finally, visualization of the biliary tree is mandatory for diagnosis. Typical findings include multi-focal strictures and irregularity of both intra- and extra-hepatic bile ducts, leading to the classic “bead on a string” appearance. Endoscopic retrograde cholangiopancreatography (ERCP) can cause cholangitis in PSC patients due to inadequate biliary drainage; thus, this procedure is reserved for stenting of high-grade strictures. Magnetic resonance cholangiopancreatography is now the test of choice for diagnosis.
PSC is the greatest risk factor for developing cholangiocarcinoma, occurring in approximately 12–15% of patients undergoing liver transplantation for PSC. Diagnosis of cholangiocarcinoma can be extremely difficult due to the similarities between the cholangiographic appearances of PSC and cholangiocarcinoma. Colorectal cancer risk is also increased in PSC patients with or without concurrent IBD. No medical therapies have been effective at preventing the progression of PSC. Ursodeoxycholic acid, which was once recommended for these patients, has shown no effect in 2 randomized trials; in addition, at least 1 study has shown an increase in mortality associated with this treatment.
We recommend a multidisciplinary approach to managing PSC patients. Any patient with IBD and elevated liver function tests should warrant comanagment with a hepatologist, as orthotopic liver transplantation is the only curative treatment. A surgical consultation should also be obtained if there is any possibility of cholangiocarcinoma or colorectal cancer.
Cholelithiasis is common in IBD patients, particularly CD patients with ileal disease. Interruption of the enterohepatic circulation secondary to ileal disease and impaired bile salt absorption results in an increased incidence of 13–34% compared to the general population.29 Portal vein thrombosis is a rare complication in the nonsurgical setting that has been seen in association with coagulation abnormalities secondary to chronic bowel inflammation.
Finally, pancreatitis is a common side effect of 6-mercaptopurine or azathioprine therapy; this side effect is seen less commonly with 5-aminosalicylic acid or corticosteroid therapy. Pancreatitis may also be seen with gallstones, CD of the duodenum, or CD-associated granulomatous inflammation of the pancreas. Several medications have been reported to cause drug-induced hepatotoxicity, including thiopurines, methotrexate, sulfasalazine, cyclosporine, and biologic agents. Concomitant use of alcohol can increase this risk. Testing for hepatitis B virus infection, hepatitis C virus infection, and TB infection must always be performed prior to the initiation of biologic agents.
Ocular Manifestations
Ocular manifestations occur in 0.3–5% of all IBD patients.30 Patients with colitis or ileocolitis are affected more frequently than patients with isolated small-bowel disease. An immune complex hypersensitivity reaction to a colonic antigen has been postulated as an explanation for this difference in incidence.31 Ocular complications often present concurrently with other EIMs, particularly peripheral arthritis and EN. Less commonly, ocular complications can be seen with axial involvement.
The most common ocular manifestation is episcleritis, or inflammation of the blood-rich episclera. Flares characteristically parallel intestinal activity and resolve with treatment of the intestinal disease.32 Episcleritis should be suspected in patients with an active fare who present with acute redness in 1 or both eyes, irritation, and burning. Tenderness to palpation is common; a change in vision is not. Treatment is tailored to the underlying bowel disease. The application of cool compresses or topical steroids is occasionally required.
Scleritis is a more severe disorder, as it can impair vision. The clinical presentation of this condition may be similar to episcleritis; the conditions can be differentiated by the frequently pink or violet natural-light appearance of the sclera.32 Scleritis requires more aggressive treatment with systemic steroids or immunosuppressants; the condition also requires immediate evaluation by an ophthalmologist. Control of the underlying bowel disease is important in order to prevent recurrence.
Uveitis is less common and is often associated with joint and dermatologic manifestations. Tis condition is also associated with both peripheral and axial arthritis. Clinical suspicion of uveitis should be high in any patient with eye complaints and other EIMs. Uveitis is 4 times more common in women and is often chronic in nature.33 Patients present with eye pain, visual blurring, photophobia, and headaches. Classically, the eye exhibits a ciliary flush, in which the redness is most intense in the center and radiates outward with diminishing intensity. An immediate evaluation via a slit lamp examination is mandatory. Examination findings include corneal clouding and conjunctival injection. The occurrence of uveitis often does not parallel intestinal activity. Prompt treatment with topical and systemic steroids is mandatory in order to prevent permanent loss of vision. Cycloplegics can help to alleviate associated spasms. Infliximab treatment can be effective in patients with refractory disease.34
Of note, conjunctivitis continues to be the most common cause of red, itchy eyes in the general population. Thus, conjunctivitis is also prevalent among IBD patients, and clinicians must be aware that this condition may mimic more serious ocular manifestations.
Several IBD treatments may cause ocular pathology.32 Steroids can lead to cataracts and open-angle glaucoma. Anticholinergics can cause pupil dilatation. Cyclosporine has been linked to optic neuropathy, ophthalmoplegia, and nystagmus.
Renal and Pulmonary Manifestations
Nephrolithiasis, obstructive uropathy, and fistulization of the urinary tract are relatively common EIMs, occurring in 6–23% of patients with IBD.35 Secondary amyloidosis is a rare systemic complication that involves the kidneys. Patients can present with proteinuria, renal failure, and uremia. Studies have shown a 3-fold increased risk in males and a 10-fold increased risk in CD patients (over UC patients).36 In the majority of patients, other EIMs develop concurrently. A diagnosis can be made with a liver, rectal, or renal biopsy. Expedient renal transplantation in recent years has improved survival, although the survival rate after 15 years remains only 60%.37
Finally, subclinical disturbances in lung function are common in IBD patients.37 Clinically significant disease is extremely rare. Chronic bronchitis, subglottic stenosis, bronchiectasis, and bronchiolitis have all been reported in association with IBD.38,39 These conditions can occur in nonsmokers and do not parallel bowel disease. Sulfasalazine and mesalamine can induce interstitial lung disease on rare occasions.
Conclusion
EIMs are very common in both UC and CD patients. Generally, we recommend early involvement of specialists in the management of involved organ systems. Most EIMs parallel disease activity and will respond to treatment of underlying bowel disease; however, some diseases, such as PSC, warrant lifelong monitoring of extraintestinal systems. Clinicians must promptly evaluate complications that can cause emergencies, such as uveitis and cholangitis.
Contributor Information
Jonathan S. Levine, Dr. Levine is an Associate Physician in the Division of Gastroenterology, Hepatology, and Endoscopy at Brigham and Women's Hospital and an Instructor of Medicine at Harvard Medical School, both in Boston, Massachusetts..
Robert Burakoff, Dr. Burakoff is Clinical Chief of the Division of Gastroenterology, Hepatology, and Endoscopy at Brigham and Women's Hospital and an Associate Professor at Harvard Medical School..
References
- 1.Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- 2.Isaacs KL. How prevalent are extraintestinal manifestations at the initial diagnosis of IBD? Inflamm Bowel Dis. 2008;14(suppl 2):S198–S199. doi: 10.1002/ibd.20597. [DOI] [PubMed] [Google Scholar]
- 3.Monsén U, Sorstad J, Hellers G, Johansson C. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol. 1990;85:711–716. [PubMed] [Google Scholar]
- 4.Aghazadeh R, Zali MR, Bahari A, Amin K, Ghahghaie F, Firouzi F. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005;20:1691–1695. doi: 10.1111/j.1440-1746.2005.03905.x. [DOI] [PubMed] [Google Scholar]
- 5.Schorr-Lesnick B, Brandt LJ. Selected rheumatologic and dermatologic manifestations of inflammatory bowel disease. Am J Gastroenterol. 1988;83:216–223. [PubMed] [Google Scholar]
- 6.Scarpa R, del Puente A, D'Arienzo A, et al. The arthritis of ulcerative colitis: clinical and genetic aspects. J Rheumatol. 1992;19:373–377. [PubMed] [Google Scholar]
- 7.Queiro R, Maiz O, Intxausti J, et al. Subclinical sacroiliitis in inflammatory bowel disease: a clinical and follow-up study. Clin Rheumatol. 2000;19:445–449. doi: 10.1007/s100670070003. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan U, Loftus EV, Jr, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002;97:910–914. doi: 10.1111/j.1572-0241.2002.05608.x. [DOI] [PubMed] [Google Scholar]
- 9.Reinisch W, Miehsler W, Dejaco C, et al. An open-label trial of the selective cyclo-oxygenase-2 inhibitor, rofecoxib, in inflammatory bowel disease–associated peripheral arthritis and arthralgia. Aliment Pharmacol Ter. 2003;17:1371–1380. doi: 10.1046/j.1365-2036.2003.01596.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferraz MB, Tugwell P, Goldsmith CH, Atra E. Meta-analysis of sulfasalazine in ankylosing spondylitis. J Rheumatol. 1990;17:1482–1486. [PubMed] [Google Scholar]
- 11.Herfarth H, Obermeier F, Andus T, et al. Improvement of arthritis and arthralgia after treatment with infliximab (Remicade) in a German prospective, open-label, multicenter trial in refractory Crohn's disease. Am J Gastroenterol. 2002;97:2688–2690. doi: 10.1111/j.1572-0241.2002.06064.x. [DOI] [PubMed] [Google Scholar]
- 12.American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:791–794. doi: 10.1053/gast.2003.50107. [DOI] [PubMed] [Google Scholar]
- 13.Tavarela Veloso F. Review article: skin complications associated with inflammatory bowel disease. Aliment Pharmacol Ter. 2004;20(suppl 4):50–53. doi: 10.1111/j.1365-2036.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- 14.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34. doi: 10.1097/00004836-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Yüksel I, Başar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:546–550. doi: 10.1002/ibd.20807. [DOI] [PubMed] [Google Scholar]
- 16.Lofgren S. Erythema nodosum: studies on etiology and pathogenesis in 185 adults cases. Acta Med Scand. 1946;124(suppl 174):1–197. [Google Scholar]
- 17.Saslaw S, Beman FM. Erythema nodosum as a manifestation of histoplasmosis. JAMA. 1959;170:1178–1179. doi: 10.1001/jama.1959.63010100004012b. [DOI] [PubMed] [Google Scholar]
- 18.Hannuksela M. Human yersiniosis: a common cause of erythematous skin eruptions. Int J Dermatol. 1977;16:665–666. doi: 10.1111/j.1365-4362.1977.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 19.Soderstrom RM, Krull EA. Erythema nodosum. A review. Cutis. 1978;21:806–810. [PubMed] [Google Scholar]
- 20.Freeman HJ. Erythema nodosum and pyoderma gangrenosum in 50 patients with Crohn's disease. Can J Gastroenterol. 2005;19:603–606. doi: 10.1155/2005/323914. [DOI] [PubMed] [Google Scholar]
- 21.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Lebwohl M, Lebwohl O. Cutaneous manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:142–148. doi: 10.1002/ibd.3780040209. [DOI] [PubMed] [Google Scholar]
- 23.Trost LB, McDonnell JK. Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J. 2005;81:580–585. doi: 10.1136/pgmj.2004.031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton JR, Teague RH, Low-Beer TS, Read AE. Pyoderma gangrenosum and ulcerative colitis. Gut. 1980;21:247–248. doi: 10.1136/gut.21.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matis WL, Ellis CN, Grifths CE, Lazarus GS. Treatment of pyoderma gangrenosum with cyclosporine. Arch Dermatol. 1992;128:1060–1064. [PubMed] [Google Scholar]
- 26.Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn's disease. J Am Acad Dermatol. 2003;48:805–821. quiz 822–824. doi: 10.1067/mjd.2003.540. [DOI] [PubMed] [Google Scholar]
- 27.Scarpa R, Manguso F, D'Arienzo A, et al. Microscopic inflammatory changes in colon of patients with both active psoriasis and psoriatic arthritis without bowel symptoms. J Rheumatol. 2000;27:1241–1246. [PubMed] [Google Scholar]
- 28.Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332:924–933. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 29.Lapidus A, Bångstad M, Aström M, Muhrbeck O. The prevalence of gallstone disease in a defined cohort of patients with Crohn's disease. Am J Gastroenterol. 1999;94:1261–1266. doi: 10.1111/j.1572-0241.1999.01076.x. [DOI] [PubMed] [Google Scholar]
- 30.Petrelli EA, McKinley M, Troncale FJ. Ocular manifestations of inflammatory bowel disease. Ann Ophthalmol. 1982;14:356–360. [PubMed] [Google Scholar]
- 31.Das KM. Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig Dis Sci. 1999;44:1–13. doi: 10.1023/a:1026629528233. [DOI] [PubMed] [Google Scholar]
- 32.Mintz R, Feller ER, Bahr RL, Shah SA. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:135–139. doi: 10.1097/00054725-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Lyons JL, Rosenbaum J T. Uveitis associated with inflammatory bowel disease compared with uveitis associated with spondyloarthropathy. Arch Ophthalmol. 1997;115:61–64. doi: 10.1001/archopht.1997.01100150063010. [DOI] [PubMed] [Google Scholar]
- 34.Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005;123:903–912. doi: 10.1001/archopht.123.7.903. [DOI] [PubMed] [Google Scholar]
- 35.Banner M P. Genitourinary complications of inflammatory bowel disease. Radiol Clin North Am. 1987;25:199–209. [PubMed] [Google Scholar]
- 36.Wester AL, Vatn MH, Fausa O. Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm Bowel Dis. 2001;7:295–300. doi: 10.1097/00054725-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Herrlinger KR, Noftz MK, Dalhoff K, Ludwig D, Stange EF, Fellermann K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol. 2002;97:377–381. doi: 10.1111/j.1572-0241.2002.05473.x. [DOI] [PubMed] [Google Scholar]
- 38.Camus P, Piard F, Ashcroft T, Gal AA, Colby TV. The lung in inflammatory bowel disease. Medicine (Baltimore) 1993;72:151–183. [PubMed] [Google Scholar]
- 39.Spira A, Grossman R, Balter M. Large airway disease associated with inflammatory bowel disease. Chest. 1998;113:1723–1726. doi: 10.1378/chest.113.6.1723. [DOI] [PubMed] [Google Scholar]
- 40.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]