Abstract
Background
Treatment with anti-estrogens or aromatase inhibitors is commonly used for patients with estrogen receptor-positive (ER+) breast cancers; however resistant disease develops almost inevitably, requiring a choice of secondary therapy. One possibility is to use inhibitors of the PI3K/mTOR pathway and several candidate drugs are in development. We examined the in vitro effects of two inhibitors of the PI3K/mTOR pathway on resistant MCF-7 cells.
Results
The derived sub-lines showed increased resistance to tamoxifen but none exhibited concomitantly increased sensitivity to the PI3K inhibitors. NVP-BEZ235 and GSK2126458 acted mainly by induction of cell cycle arrest, particularly in G1-phase, rather than by induction of apoptosis. The lines varied considerably in their utilization of the AKT, p70S6K and ERK pathways. NVP-BEZ235 and GSK2126458 inhibited AKT signaling but NVP-BEZ235 showed greater effects than GSK2126458 on p70S6K and rpS6 signaling with effects resembling those of rapamycin.
Methods
We cultured MCF-7 cells for prolonged periods either in the presence of the anti-estrogen tamoxifen (three sub-lines) or in estrogen free medium (two sub-lines) to mimic the effects of clinical treatment. We then analyzed the effects of two dual PI3K/mTOR phosphoinositide-3-kinase inhibitors, NVP-BEZ235 and GSK2126458, on the growth and signaling pathways of these MCF-7 sub-lines. The functional status of the PI3K, mTOR and ERK pathways was analyzed by measuring phosphorylation of AKT, p70S6K, rpS6 and ERK.
Conclusion
Increased resistance to tamoxifen in these MCF-7 sub-lines is not associated with hypersensitivity to PI3K inhibitors. While both drugs inhibited AKT signaling, NVP-BEZ235 resembled rapamycin in inhibiting the mTOR pathway.
Key words: breast cancer, PI3K, mTOR, BEZ235, GSK2126458, estrogen receptor, MCF-7
Introduction
Breast cancer is the major malignancy in women and one of the main therapeutic principles, after surgery, is to block the action of the hormone estrogen. However, patients treated with anti-estrogens such as tamoxifen or aromatase inhibitors such as letrozole, often develop resistant disease that is sometimes more aggressive than the original. Cell culture models provide a method to investigate the onset of such resistance and we have previously developed a series of sub-lines of the MCF-7 breast cancer cell line by culturing them for a prolonged period either in the presence of increasing concentrations of tamoxifen, or in the absence of estrogen (using serum absorbed with charcoal), mimicking the emergence of clinical resistance to tamoxifen or to aromatase inhibitors, respectively.1 Our previous work, together with that of other groups2,3 suggests that these sub-lines correspond to pre-existing minor populations in the parental population that develop under restrictive conditions. Thus, human breast cancers may generally contain pre-existing minor tamoxifen-resistant populations that expand during treatment. The series of MCF-7 sub-lines developed may therefore be useful in the testing of new treatment strategies.
Previous research has shown a high degree of cross-talk between the estrogen receptor (ER) pathway and the growth factor receptor (GFR) pathways.4 Phosphoinositide-3-kinase (PI3K) is a critical mediator of GFR signaling and the PI3K signaling pathway is one of the most mutationally altered pathways in breast cancer.5 Patients with tumors exhibiting aberrant PI3K/Akt/mTOR signaling may benefit from therapy targeting specific components of this pathway and some PI3K/Akt/mTOR inhibitors have been reported to be efficacious in breast cancers.6 NVP-BEZ235 (BEZ235)7 and GSK2126458 (GSK212)8 are highly selective and potent small molecule inhibitors that target both multiple class I PI3K isoforms and mTOR kinase activity7,8 and have been considered as potential second line therapies for breast cancer.9,10 BEZ235 is currently being tested in phase I/II clinical trials in breast cancer patients with advanced disease (www.clinicaltrials.gov/ct2/show/NCT00620594), while GSK212 is being evaluated in a phase I trial in patients with solid tumors or lymphoma (www.clinicaltrials.gov/ct2/show/NCT00972686).
Cell lines harboring PIK3CA mutations have been shown to be more sensitive to a selective class I PI3K inhibitor11 and luminal breast cancer cells preferentially respond to PI3K inhibitors.6 As PIK3CA mutations have been found in 18–40% of human breast cancer, it was hypothesized that these mutation could be responsible for the deregulation in the signaling pathway and consequently these patients would be most suitable for PI3K/mTOR pathway inhibition.12 The luminal-epithelial like MCF-7 cell line, a recognized model for estrogen receptor positive breast cancer, harbors a PI3KCA helical E545K mutation (www.sanger.ac.uk/genetics/CGP/cosmic/).13 Our panel of MCF-7 and its sub-lines, developed to model clinical tamoxifen-resistant and estrogen-independent breast cancer, respectively, showed phenotypic changes indicating that they arose from minor subpopulations of the original MCF-7 cell line. Rapamycin resistance was a feature of the MCF-7 sub-lines developed under estrogen deprivation and was associated with loss of active phospho-HER2 and acquisition of PAX2 expression.1 Consequently, we wished to determine whether cell lines expressing aberrant PI3K signaling would be sensitive to PI3K inhibitors treatment in our MCF-7 cell line models. Here, we compare the sensitivity to BEZ235 and GSK212 of MCF-7 parental and tamoxifen-resistant sub-lines, and also investigate the effects of these two drugs on the cellular utilization of the PI3K/Akt, mTOR and ERK pathways.
Results
Cytotoxic effects of BEZ235 and GSK212 on of MCF-7 sub-lines.
The effects of BEZ235 and GSK212 on the growth of MCF-7 parental and TamR7 cells were determined by sulforhodamine B assay (Fig. 1A and Sup. Fig. S1A and B). At the highest drug concentrations tested, both BEZ235 and GSK212 treatment induced cell death in the two cell lines, as shown by the reduction of cell number below that present at the treatment start. We also measured cleavage of poly (ADP-ribose) polymerase (PARP),14 as a marker for the induction of apoptosis. At the highest drug concentrations tested (1,000 nM BEZ235 and 50 nM GSK212), cleavage of PARP was significantly induced in the MCF-7 parental and TamR7 sub-line (Fig. 1B and C). Observation of PARP cleavage in MCF-7 parental and TamR7 correlated with their decrease in cell density in response to BEZ235 or GSK212.
Figure 1.
Effects of BEZ235 and GSK212 in MCF-7 parental and its derived sub-lines in proliferation and apoptosis. MCF-7 parental and its sub-lines were exposed to indicated concentration of BEZ235 and GSK212 (A) for 3 days, and cell proliferation was measured by sulforhodamine B assay. Bars represent percent changes in cell density after 72 h compared with initial amount present at the treatment start and expressed as the mean ± standard error from three experiments. Immunoblotting for cleaved PARP (cPARP) in MCF-7 cell lines treated with different concentration of BEZ235 (B) or GSK212 (C) for 72 h. Actin was used as a loading control. Bands were normalized to total protein and bars represent changes in fold compared with untreated cells and expressed as the mean ± standard deviation from three experiments. Representative blots are shown above bar graph. *Significant difference from treatment control (p < 0.05).
Mechanism of growth inhibitory action of BEZ235 and GSK212.
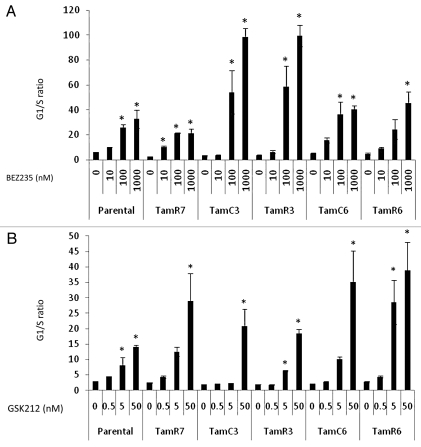
As measured by flow cytometry, both drugs significantly induced G1-phase arrest in each of the sub-lines (Fig. 2A and B). However, G1-phase arrest did not correlate to growth response for both of the drugs tested.
Figure 2.
G1/S cell cycle arrest in MCF-7 cell lines treated with indicated concentration of BEZ235 (A) or GSK212 (B) for 24 h analyzed by flow cytometry. Results were shown as the mean ± standard deviation from two experiments. *Significant difference from treatment control (p < 0.05).
Effects of BEZ235 and GSK212 on Akt, rpS6 and ERK phosphorylation.
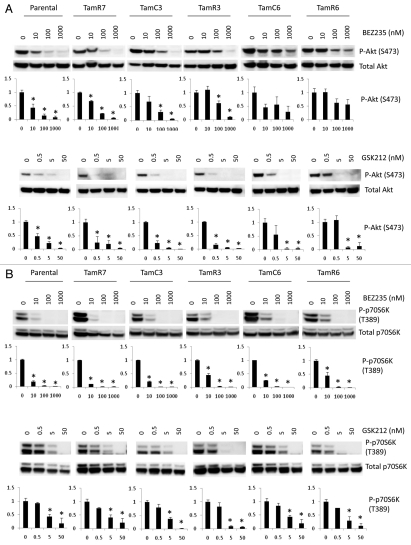
The downstream cellular responses to BEZ235 and GSK212 were assessed by measuring phosphorylation of Akt, p70S6K, rpS6 and ERK (Fig. 3). BEZ235 significantly inhibited Akt phosphorylation in a concentration-dependent manner in MCF-7 parental, TamR7, TamC3 and TamR3 cells. No significant change in phosphorylation of Akt was observed in TamC6 and TamR6 cells (Fig. 3A). Though GSK212 significantly inhibited Akt phosphorylation in all six cell lines (Fig. 3B), TamC6 and TamR6 showed lower responses to GSK212 as compared to MCF-7 parental cells. The downstream signals in phospho-p70S6K and phospho-rpS6 were significantly suppressed in all sub-lines tested, irrespective of their differential growth response to BEZ235 or GSK212 (Fig. 3C). While p70S6K is a known modulator of the PI3K pathway's feedback loop,15 no correlation between p70S6K phosphorylation and active Akt levels was observed as both BEZ235 and GSK212 are dual PI3K/mTOR inhibitors.
Figure 3.
Immunoblotting for PI3K pathway signaling proteins in MCF-7 cell lines treated with different concentrations of BEZ235 or GSK212 for 24 h. BEZ235 and GSK212 inhibit signaling through the PI3K/mTOR pathway and its downstream effectors Akt (A), p70S6K (B), rpS6 (C) irrespective of the cellular growth response to the inhibitors. Effect on ERK activation was assessed (D). Immunoblotting used antibodies for p-Akt(S473), p-p70S6K(T389), p-rpS6 (S235/236), p-ERK(T202/Y204) and their respective proteins (which were used as loading controls). Bands were normalized to total protein and bars represent changes in fold compared with untreated cells and expressed as the mean ± standard deviation from three experiments. Representative blots are shown above bar graph. *Significant difference from treatment control (p < 0.05).
Inhibition of mTOR signaling can lead to increased activation of ERK, presumably via a p70S6K/PI3K/RAS feedback loop.16–18 We therefore investigated the effects of BEZ235 and GSK212 on the ERK pathway but no significant change in ERK activation was observed (Fig. 3D).
Effects of inhibition of PI3K/mTOR signaling on ER expression.
Since ER-dependent signaling via the PI3K pathway has been shown to be related to Akt activation,4,19 we determined whether inhibition of Akt phosphorylation by BEZ235 or GSK212 was associated with changes in expression of ER protein. In the presence of inhibitors the TamC3 sub-line showed a significant increase of ER protein expression in response to BEZ235, whereas GSK212 induced a significant increase of ER protein in TamC3, TamR3 and TamC6 cells as compared to the increase in MCF-7 parental cell line (Fig. 4).
Figure 4.
Immunoblotting for p-Akt(S473) and ER antibodies. MCF-7 sub-lines were treated with the indicated concentrations of BEZ235 or GSK212 overnight. Actin is shown as a loading control. Bands were normalized to actin and bars represent changes in fold compared with untreated cells and expressed as the mean ± standard deviation from three experiments. Representative blots are shown above bar graph. *Significant difference from treatment control (p < 0.05).
Discussion
The main finding of this study is that the tamoxifen-resistant lines emerging following prolonged culture of MCF-7 cells in the presence of tamoxifen or in the absence of estrogen do not show significant increased sensitivity to PI3K/mTOR inhibitors. While one sub-line, (TamR7) resembled the parental line in its sensitivity to the PI3K/mTOR inhibitors, four other sub-lines (TamC3, TamR3, TamC6 and TamR6) were significantly more resistant. The MCF-7 line is ER-positive and it is interesting that all of the derived tamoxifen-resistant sub-lines expressed ER, generally at levels higher than that of the parent line. This supports the hypothesis that the tamoxifen resistance of the sub-lines is associated with increased ER expression and consequent maintenance of ER signaling pathways, as reported by others.20–22 Another feature of the results is that ER expression is modulated by exposure to PI3K/mTOR inhibitors (Fig. 4), emphasizing the high degree of cross-talk that exists in these cellular signaling pathways. However, ER expression levels do not correlate to PI3K pathway utilization in MCF-7 parental and the tamoxifen resistant sub-lines.1 It has been reported that the luminal B molecular subtype MCF-7 has low PI3K expression pattern.4 Our MCF-7 line has low levels of phospho-Akt (Ser473), supporting of the suggestion that PI3K signaling in cell lines with helical domain mutation in PIK3CA is mediated via SGK3 rather than AKT activity.13 However, other sub-lines TamC6 and TamR6 showed increased level of phospho-Akt and may be utilizing a different pathway. Such differences in pathway utilization may be important in designing therapeutic strategies. The relationship between sensitivity to estrogen and sensitivity to PI3K/mTOR inhibitors is another area that could be explored with these MCF-7 sub-lines. It has been suggested that targeting the PI3K pathway may reverse the loss of ER signaling and restore endocrine treatment sensitivity.4 However, we did not observe increased sensitivity to tamoxifen in combination treatment with the PI3K/mTOR inhibitors in the sub-lines (unpublished data). MCF-7 has been shown to be one of the most sensitive of a number of breast cancer cell lines to BEZ235,23 and this would be expected because of the presence of a PI3KCA mutation. The IC50 values for GSK212, as well as the drug concentrations required to inhibit the PI3K pathway, are in general considerably lower than those for BEZ235. The correlation between BEZ235 and GSK212 IC50 values (Sup. Fig. S2) supports the hypothesis that both are acting on the AKT pathway. On the other hand, examination of the effects of the two drugs on signaling pathways shows BEZ235 to be relatively more active than GSK212 in the inhibition of p70S6K phosphorylation, with patterns that are very similar to that of rapamycin.1 A possible explanation of these results is that inhibition of the AKT pathway has a larger effect than inhibition of the mTOR pathway on cell growth. Our previous studies have shown that the growth of the parental line and the TamR7 sub-line are substantially inhibited by rapamycin while growth of TamC6 and TamR6 is largely unaffected despite strong inhibition of phosphorylation of p70S6K and rpS6.1 However, inhibition of the Akt pathway by inhibitors did not translate to anti-proliferation in TamC3, TamR3, TamC6 and TamR6 in this study.
Analysis of the cellular responses of MCF-7 and its sub-lines to BEZ235 and GSK212 shows that the predominant effect of the drugs is inhibition of the transition from G1-phase to S-phase (Fig. 2) rather than the induction of apoptosis. Apoptosis was observed only in the parental line and one sub-line following exposure to drugs at concentrations that are well above those required to inhibit individual signaling pathways (Fig. 2). Other studies have shown that individual breast cancer cell lines vary in the ability of BEZ235 to induce apoptosis with some cell lines more susceptible than others.23,24 A recent study reported a significant increase in apoptosis induced by BEZ235 in MCF-7 and MCF-7/LTED (long term estrogen deprived) cells but not HCC-1428 and HCC-1428/LTED cells. Studies of the effect of ZSTK474, another PI3K inhibitor, on PC3 prostate cancer cells indicated that cell cycle arrest was the dominant cellular response to this class of agents. The protein p27KIP1, an inhibitor of cyclin-dependent kinase-2, was induced by ZSTK474 and may be responsible to the arrest of cells in G1-phase.25
Increases in phospho-Akt in some cells are due to an inhibitory feedback mechanism between the mTOR effector p70S6K and the insulin receptor substrate-PI3K upstream of Akt.26 Our previous results are consistent with reports that inhibition of mTOR signaling by rapamycin increases Akt phosphorylation in MCF-7 cells.15,27 Here, we observed that the PI3K/mTOR inhibitors BEZ235 and GSK212 efficiently inhibited PI3K/mTOR signaling and resulted in PI3K (Akt) and mTORC1 (p70S6K and rpS6) downstream effectors de-phosphorylation, which is in agreement with reports by others.24 Inhibition of mTOR signaling can lead to increased activation of ERK presumably via a p70S6K/PI3K/RAS feedback loop.16–18 PI3K and MAPK signaling pathways have reciprocal pathway activation induced by inhibitor mediated release of negative feedback loops.28,29 Although all cell lines tested presented higher activated ERK levels (phospho-ERK) in response to inhibitors, no significant change in ERK activation was observed. In conclusion, the results with the sub-lines of MCF-7, if extrapolated to human cancer, present a picture where tumors are heterogeneous and composed of many different phenotypes. Each phenotype may have its own phosphorylation pattern of cross-talk that determines the relative expression of components of the AKT, ERK and mTOR pathways, such that it is not possible to use the results of one cell line to predict cross talk in another. Exposure of this heterogeneous population of cells to a therapeutic agent such as tamoxifen causes growth inhibition of some component phenotypes but not others, leading to the evolution of an altered distribution of phenotypes towards tamoxifen resistance. Similarly, exposure to a PI3K/mTOR inhibitor would also lead to the evolution of a new distribution of phenotypes. The results from this study indicate that at least under in vitro conditions, the sensitivity to tamoxifen or to PI3K/mTOR inhibitors cannot easily be predicted by analysis of phosphorylation patterns of component proteins of the AKT, ERK and mTOR pathways. And the majority of the sub-lines also developed resistance to PI3K/mTOR inhibitors, resembling their response to rapamycin.1
Materials and Methods
Cell culture.
All growth media contained insulin/transferrin/selenium supplement, added according to the manufacturer's instructions (Roche), as well as penicillin/streptomycin (100 U/ml and 100 µg/ml, respectively). The human breast cancer cell line MCF-7 (parental cell line) was purchased from the American Type Culture Collection (ATCC) and grown in α-MEM containing 5% fetal bovine serum (FBS). The TamR7 cell line was established by culturing MCF-7 cells in the above medium but in the presence of progressively increasing concentrations of tamoxifen (10−7 to 3 × 10−6 M in ethanol) and then maintaining them for >15 months in 3 × 10−6 M tamoxifen.1 The TamR3 and TamR6 cell lines were generated by growth of MCF-7 cells in phenol-red-free RPMI containing 10% charcoal-stripped fetal bovine serum (Invitrogen, Auckland, NZ), over a period of 3 months to progressively increasing concentrations of tamoxifen (10−9 to 10−6 M in ethanol) and then maintaining them for >15 months in 10−6 M tamoxifen. The TamC3 and TamC6 cell lines were generated by exposure of MCF-7 cells for >16 months to the above growth medium but lacking tamoxifen. All experiments were carried out on cells grown in their respective growth media but without tamoxifen.
Reagents.
Propidium iodide and tamoxifen were purchased from Sigma (Auckland, NZ). BEZ23530,31 and GSK212,8 was synthesized according to published protocols.
Protein gel blotting.
Parental MCF-7 cells and the variants TamC3, TamC6, TamR3, TamR6 and TamR7 were grown to log-phase, washed twice with ice-cold PBS and lysed in SDS lysis. buffer according to the manufacturer's protocol (Cell Signaling Technology, Danvers, MA). Protein concentration was quantified using the BCA protein assay reagent bicinchoninic acid (Sigma). Cell lysates containing 20 µg of protein were separated by SDS-PAGE gel electrophoresis and transferred to PVDF membranes (Millipore). Membranes were immunoblotted with antibodies against phospho-Akt (S473), total Akt, phospho-70S6K (T389), total p70S6K, phospho-rpS6 (S235/236), total rpS6, phospho-ERK (T202/Y204), total ERK, (all from Cell Signaling Technology), ER (Chemicon, Millipore, Billerica, MA) and actin (Millipore), using SuperSignal West Pico (Thermo Scientific, Waltham, MA) or ECL advance (GE Healthcare, Auckland, NZ). Antibody reactivity was visualized using the chemiluminescence detection system by Fujifilm Las-3000.
Cell proliferation assay.
Cell proliferation was measured using a thymidine incorporation assay in which 3,000 cells were seeded in 96-well plates in the presence of varying concentrations of inhibitors for 3 days. Briefly, 0.04 µCi of 3H-thymidine was added to each well and incubated for 5 h, after which the cells were harvested onto glass fiber filters using an automated TomTec harvester. Filters were incubated with Betaplate Scint and thymidine incorporation counted in Trilux/Betaplate counter. Cell proliferation was determined by the percentage of cells showing incorporation of 3H-thymidine into DNA. The sulforhodamine B colorimetric assay,32 which is based on the measurement of cellular protein content, was used to measure cell density. All experiments were done using triplicate wells and repeated at least three times.
Flow cytometry.
Cells (1 × 106 cells) were grown in 3.5 cm Petri dishes and incubated with inhibitors for 24 h. They were harvested, washed with 1% FCS/PBS, resuspended in 200 µl of PBS, fixed in 2 ml of ice-cold 100% ethanol and stored overnight at −20°C. The cells were washed and resuspended in 1 ml of 3% FCS/PBS containing RNase (1 µg/ml) and propidium iodide (PI) (10 µg/ml) for 30 min at room temperature. DNA content was determined using forward scatter (FSC) intensity by PI staining based on a total 30,000 acquired events by FACScan cytometry.
Statistical analysis.
Data were analyzed using a one-way ANOVA coupled with multiple comparisons versus treatment control applying the Holm-Sidak method correction, where p < 0.05 denotes a statistically significant difference.
Acknowledgments
We thank Dr. Marion Blumenstein and Dr. Frederik Pruijn for their helpful assistance and discussions on statistical analysis. Funding for this work was obtained from New Zealand Breast Cancer Foundation, New Zealand Lottery Grant, The Genesis Oncology Trust, and this work is also supported by Auckland Cancer Society Research Centre.
Abbreviations
- ER+
estrogen receptor-positive
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- PI3K
phosphoinositide-3-kinase
- α-MEM
alpha-modified Eagle's medium
- PBS
phosphate-buffered saline
- FBS
fetal bovine serum
- rpS6
ribosomal protein S6
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- PI
propidium iodide
- ERK
extracellular signal-regulated kinase (MAP kinase)
Supplementary Material
References
- 1.Leung E, Kannan N, Krissansen GW, Findlay MP, Baguley BC. MCF-7 breast cancer cells selected for tamoxifen resistance acquire new phenotypes differing in DNA content, phospho-HER2 and PAX2 expression, and rapamycin sensitivity. Cancer Biol Ther. 2010;9:717–724. doi: 10.4161/cbt.9.9.11432. [DOI] [PubMed] [Google Scholar]
- 2.Coser KR, Wittner BS, Rosenthal NF, Collins SC, Melas A, Smith SL, et al. Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor. Proc Natl Acad Sci USA. 2009;106:14536–14541. doi: 10.1073/pnas.0907560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nugoli M, Chuchana P, Vendrell J, Orsetti B, Ursule L, Nguyen C, et al. Genetic variability in MCF-7 sub-lines: evidence of rapid genomic and RNA expression profile modifications. BMC Cancer. 2003;3:13. doi: 10.1186/1471-2407-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, et al. Proteomic and transcrip-tomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowder RJ, Ellis MJ. Treating breast cancer through novel inhibitors of the phosphatidylinositol-3′-kinase pathway. Breast Cancer Res. 2005;7:212–214. doi: 10.1186/bcr1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greshock J, Bachman KE, Degenhardt YY, Jing J, Wen YH, Eastman S, et al. Molecular target class is predictive of in vitro response profile. Cancer Res. 2010;70:3677–3686. doi: 10.1158/0008-5472.CAN-09-3788. [DOI] [PubMed] [Google Scholar]
- 7.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 8.Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, et al. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med Chem Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J, Infante JR, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28:3005. [Google Scholar]
- 10.Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, et al. Identification of GSK2126458, a highly potent inhibitor of phosphoinositide-3-kinase (PI3K) and the mammalian target of rapamycin (mTOR) Mol Cancer Ther. 2009:62. [Google Scholar]
- 11.O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol-3′-kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 12.Loi S, Haibe-Kains B, Majjaj S, Lallemand F, Durbecq V, Larsimont D, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci USA. 2010;107:10208–10213. doi: 10.1073/pnas.0907011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 2001;8:588–594. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 2010;16:530–540. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- 19.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H. Upregulation of estrogen receptor by tamoxifen in human breast cancer. Cancer. 1993;71:1266–1272. doi: 10.1002/1097-0142(19930215)71:4<1266::aid-cncr2820710416>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 22.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3-kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 24.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci USA. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dan S, Yoshimi H, Okamura M, Mukai Y, Yamori T. Inhibition of PI3K by ZSTK474 suppressed tumor growth not via apoptosis but G0/G1 arrest. Biochem Biophys Res Commun. 2009;379:104–109. doi: 10.1016/j.bbrc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by upregulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol-3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 27.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 28.Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cell Signal. 2010;22:1369–1378. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Echeverria C, Stauffer F, Furet P. Preparation of imidazo[4,5-c]quinolin-2-ones and -thiones as lipid, PI3 and/or DNA protein kinase inhibitors with therapeutic uses. >PCT Int Appl WO 2006122806 A2. 2006 [Google Scholar]
- 31.Stowasser F, Baenziger M, Garad SD. Preparation of salts and crystalline forms of 2-methyl-2-[4-(3-methyl-2-oxo-8-quinolin-3-yl-2, -dihydroimidazo[4,5-c] quinolin-1-yl)-phenyl]propionitrile and its use as a drug. PCT Int Appl WO 2008064093A2. 2008 [Google Scholar]
- 32.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.