CLC anion transport proteins function as Cl− channels and Cl−/H+ exchangers and are found in all major groups of life including archaebacteria. Early electrophysiological studies suggested that CLC anion channels have two pores that are opened and closed independently by a “fast” gating process operating on a millisecond timescale, and a “common” or “slow” gate that opens and closes both pores simultaneously with a timescale of seconds (Fig. 1A). Subsequent biochemical and molecular experiments suggested that CLC channels/transporters are homodomeric proteins.1–3
Figure 1.
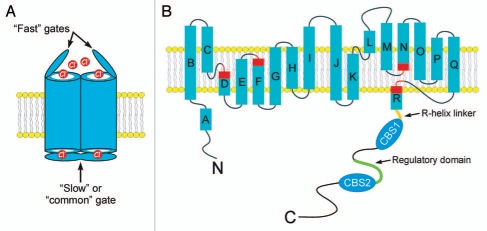
(A) Cartoon showing a CLC homodimer with “fast” and “slow” or “common”gates. (B) Predicted membrane topology of a CLC monomer. Rectangles indicate α-helices. Regions of helices highlighted in red function in channel gating and to coordinate Cl− within the ion conducting pore. Note that the R-helix protrudes into the cytoplasm. In eukaryotes, the R-helix is connected to a large cytoplasmic C-terminus. A model of the CLH-3b C-terminus is shown. The C-terminus contains two cystathionine-β-synthase or CBS domains. The R-helix is connected to CBS1 via a 20 amino acid linker shown in orange. The linker between CBS1 and CBS2 is 176 amino long and contains a 101 amino acid regulatory domain shown in green. GCK-3 binds to the first four amino acids of this domain and mediates phosphorylation of two serine residues 70 and 75 amino acids downstream.
A major break through in the field occurred when Rod MacKinnon and coworkers crystallized a bacterial CLC protein. The crystal structure confirmed the homodimeric CLC architecture. Each CLC monomer consists of 18 α-helical domains designated “A-R”. Helices B-R span or are embedded in the membrane4,5 (Fig. 1B). It is widely accepted that the overall membrane structure of bacterial CLC proteins is conserved throughout the CLC superfamily (reviewed in refs. 4 and 6–9).
Membrane helices D, F, N and R comprise the CLC channel selectivity filter and “fast” gate (Fig. 1B). A glutamate residue on helix F projects into the extracellular-facing portion of the ion conduction pathway and functions as the voltage-, Cl−- and H+-activated fast gate.4,5 The structural basis of slow gating remains unclear, but may be mediated10–15 or modulated16 by the cytoplasmic C-terminus.
Eukaryotic CLC proteins are distinguished from most of their bacterial counterparts by possessing extensive cytoplasmic C-termini containing two cystathionine-β-synthase (CBS) domains termed CBS1 and CBS2 (reviewed in refs. 10 and 17–19) (Fig. 1B). The CBS domain is a ubiquitous, 50–60 amino acid protein motif with a highly conserved secondary structure consisting of an N-terminal β-strand (β1) followed by an α-helix (α1), two β-strands (β2 and β3) and an α-helix (α2).20,21 The functions of CBS domains are poorly understood, but mutations in this motif give rise to several inherited diseases indicating that they play critical roles in protein structure and activity.20,21 Mutations in CLC CBS domains disrupt gating properties and give rise to muscle, kidney and bone disease.3,20
Biochemical and X-ray crystallography studies on isolated C-termini of eukaryotic CLCs demonstrate that they too form dimers.17–19,22 CBS motifs are typically present in proteins as two or four copies. Pairs of CBS motifs associate to form a dimeric structure known as a Bateman module. Bateman modules in turn interact to form quaternary protein structures.20,21 The Bateman modules of two CLC monomers dimerize to form the overall quaternary structure of the CLC cytoplasmic C-terminus.17–19
The structural bases of CLC gating have been studied extensively. What is far less well understood is how CLC transport proteins are regulated by cell signaling pathways. Several pharmacological and molecular studies suggest that CLC-1, CLC-2 and CLC-3 may be regulated by protein phosphorylation events.23–30 Adenine nucleotide binding to intracellular domains has been shown to play possible regulatory roles in CLC-1,31 CLC-2,32 and CLC-5.33
Most regulatory studies of various CLC transport proteins have been carried out using heterologous expression systems. Thus the physiological contexts under which such regulation occurs and the signaling mechanisms that mediate regulation are unclear. We have exploited the molecular, genetic and physiological tractability of the nematode C. elegans to characterize CLC anion channel function and regulation. The C. elegans clh-3 gene encodes two CLC anion channel splice variants, CLH-3a and CLH-3b. CLH-3b is expressed in the worm oocyte where it is activated by meiotic cell cycle progression and cell swelling.34 Activation requires protein dephosphorylation mediated by the type I protein phosphatases GSP-1 and GSP-2.35 The Ste20 kinase GCK-3 binds to CLH-3b and inhibits its activity by mediating phosphorylation of two serine residues.36,37 GCK-3 activity is regulated during cell volume and meiotic cell cycle changes by the MAP kinases MEK-2 and MPK-1.38 GCK-3 is an ortholog of the mammalian STK39/SPAK kinase. SPAK and the closely related kinase OSR1 regulate the activity of the volume sensitive K-Cl and Na-K-2Cl cotransporters and play central roles in systemic salt and water homeostasis.39,40
GCK-3 binds to a domain located between CBS1 and CBS2.37 This CBS linker is present in all CLC proteins and has a highly variable sequence. X-ray crystallography17–19,41 and NMR studies42 suggest that the linker is largely unstructured. The CBS linker in CLH-3b is 176 amino acids long. The last 101 amino acids of the linker are unique to the CLH-3b splice variant and are termed the “regulatory domain” (Fig. 1B). The first four amino acids of the regulatory domain constitute a SPAK interaction motif and is the site of GCK-3 binding.37 Inhibition of CLH-3b by GCK-3 requires phosphorylation of two serine residues located 70 and 75 amino acids downstream from this binding site.36
Phosphorylation of CLH-3b reduces current amplitude and induces striking changes in channel voltage sensitivity. Indirect evidence suggests that phosphorylation modulates voltage-dependent fast gating.36,37 As noted above, fast gating is mediated by an extracellular-facing glutamate residue on helix F.4,5
An outstanding question in the CLC field is whether conformational changes in intracellular domains alter channel/transporter function during cell signaling events. The membrane R-helix forms part of the CLC ion conducting pore and selectivity filter.5 A short stretch of cytoplasmic amino acids, termed the R-helix linker, connect helix R to the intracellular C-terminus (Fig. 1B). By virtue of its direct connection to the C-terminus, Dutzler et al.5 proposed that the R-helix could provide a pathway by which intracellular domain conformational changes regulate CLC activity.
Interestingly, despite significant differences in primary sequence, part of the R-helix linkers of ClC-0, ClC-5 and ClC-Ka have similar and well ordered crystal structures.17–19,41 The conservation of this structure suggests that the linker might play a role in CLC regulation by functioning to communicate intracellular conformational changes to membrane domains. Our recent studies support this idea. The CLH-3a and CLH-3b splice variants have strikingly different biophysical properties.43 Splice variation occurs exclusively in intracellular domains including α2 of CBS2. Interchanging α2 between CLH-3a and CLH-3b interchanges their gating properties.44
Crystal structures of CLC-0, CLC-Ka and CLC-5 cytoplasmic C-termini17–19 indicate that part of the cytoplasmic R-helix linker lies close to α2 of CBS2 suggesting that these domains may interact. Using a homology model of the CLH-3b C-terminus, we identified apposing and potentially interacting amino acids in α2 and the R-helix linker. Disrupting these putative interactions with single point mutations is sufficient to convert the gating properties and extracellular cysteine reactivity of CLH-3b to those resembling the CLH-3a splice variant.44
Our studies suggested that interaction of CBS2 and the R-helix linker plays a critical role in regulating CLC structure and function. This idea is now supported compellingly by a crystal structure. In yet another major advance for the field, MacKinnon's lab succeeded recently in crystallizing an intact eukaryotic CLC protein from the thermophilic red alga Cyanidioschyzon merolae.41 The crystal structure demonstrates that CBS2 abuts the intramembrane domain while CBS1 faces into the cytoplasm. The interface between CBS2 and membrane spanning helices is extensive with a shape complimentarity index, which is a measure of how well two protein domains fit together, similar to the interface between an antibody and antigen. This suggests that the interaction is highly specific and therefore functionally significant.
The R-helix linker passes over and makes multiple close contacts with CBS2 including α2. CBS2 also interacts with the D-helix, which like helix R forms part of the ion conducting pore4,5 (Fig. 2). This close interaction between intracellular domains and the CLC pore demonstrates clearly how CBS conformational changes could regulate CLC channel/transporter activity.
Figure 2.
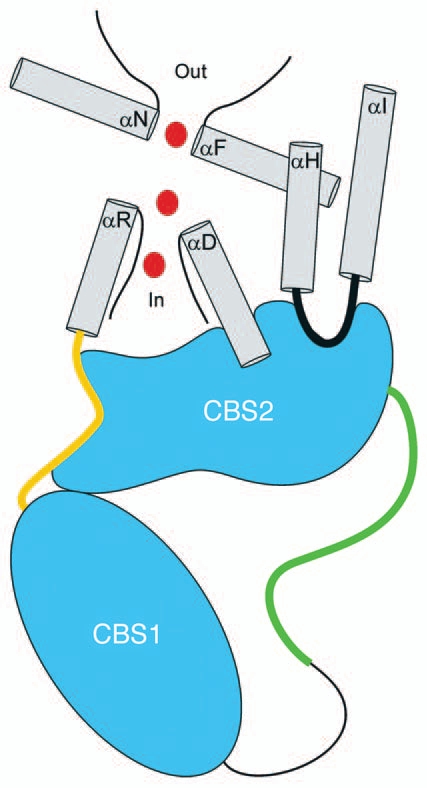
Cartoon showing interaction of CBS2 with intramembrane domains. CBS1 and CBS2 are located in the cytoplasm and dimerize to form a so-called Bateman module. A linker connects the two CBS domains. This linker is 176 amino acids long in CLH-3b and includes a 101 amino acid regulatory domain (shown in green), which is where GCK-3 binds and regulatory phosphorylation occurs. Gray helices form the membrane domain. CBS2 interacts extensively with the R-helix linker shown in orange and helix D. Phenylalanine and serine residues on helices R and D, respectively, function in Cl− coordination and selectivity within the ion conducting pore. A glutamate residue on helix F functions as the fast gate. The interface between CBS2 and membrane domains shows a high degree of shape complimentarity suggesting that the interaction is specific and functionally significant. Chloride ions moving through the pore are shown in red. An intracellular loop linking helices H and I also interacts with CBS2. Helices H and I form part of the interface between the monomers of CLC membrane domains.
In addition to helices D and R, CBS2 interacts with a short intracellular domain connecting helices H and I (Fig. 2). The H and I helices form part of the dimer interface between CLC monomers.5 The interaction of CBS2 with this dimer interface suggests a structural mechanism by which conformational changes in the cytoplasmic C-terminus could mediate or modulate slow or common gating.
Now that we have a complete CLC structure, what does it tell us about how phosphorylation and nucleotide binding regulate CLC proteins? Our studies on CLH-3b provide a foundation for addressing this important question. Deletion of the CLH-3b regulatory domain gives rise to channel activity similar to that observed after phosphorylation by GCK-3.45 The simplest model to explain this result is that the regulatory domain interacts with another part of the channel, and that this interaction is disrupted by phosphorylation or regulatory domain deletion. Recent studies on CLC-5 using small angle X-ray scattering suggest that ATP binding to the cytoplasmic C-terminus increases protein compactness and suggest that CBS1 and CBS2 undergo a clamp-like closure around an ATP molecule.46 Given the location of the CLH-3b regulatory domain between CBS1 and CBS2, it is easy to envision how changes in phosphorylation may cause a similar change in CBS architecture.
As noted earlier, the linker between CBS1 and CBS2 shows little or no sequence conservation between various CLC proteins and appears to be largely unstructured.17–19,41,42 However, NMR studies on the CLC-0 CBS linker suggest that located within this disordered domain are small islands of secondary structure.42 Analysis of the sequence of the CLH-3b CBS linker suggests that it is also mostly disordered. Located upstream from the regulatory domain though is a densely charged region predicted to form α-helices.
Unstructured domains are common in proteins and are thought to play critical functional roles.47,48 For example, phosphorylation sites occur predominately in disordered domains. Small structured regions within disordered motifs may function as nucleation points that mediate regulated folding events. The so-called “fly-casting” hypothesis posits that unstructured domains located within well-structured protein regions function to “fish” in or “sample” a large volume of the cytoplasm for binding partners such as kinases, phosphatases and ligands. With the CmCLC crystal structure and extensive functional studies in hand, we can now imagine and test how the CLH-3b CBS linker may serve as a sensor for intracellular regulatory signals, how these signal alter CBS conformation, and how CBS conformational changes are transmitted to intramembrane domains that control channel/transporter activity. As the first CLC structures4,5 demonstrated, crystals provide a clear window into protein function and an important foundation for new hypothesis-driven mutagenesis and structural studies.
Acknowledgements
I thank Drs. Jerod Denton and Michael Pusch for critically reading the manuscript and helpful comments, and Dr. Christine Bear for many helpful and stimulating discussions. This work was supported by NIH R01 grant DK51610.
References
- 1.Chen TY, Hwang TC. CLC-0 and CFTR: chloride channels evolved from transporters. Physiol Rev. 2008;88:351–387. doi: 10.1152/physrev.00058.2006. [DOI] [PubMed] [Google Scholar]
- 2.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 3.Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 4.Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- 5.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 6.Estevez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron. 2003;38:47–59. doi: 10.1016/s0896-6273(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 7.Accardi A, Pusch M. Conformational changes in the pore of CLC-0. J Gen Physiol. 2003;122:277–294. doi: 10.1085/jgp.200308834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traverso S, Elia L, Pusch M. Gating competence of constitutively open CLC-0 mutants revealed by the interaction with a small organic inhibitor. J Gen Physiol. 2003;122:295–306. doi: 10.1085/jgp.200308784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engh AM, Maduke M. Cysteine accessibility in ClC-0 supports conservation of the ClC intracellular vestibule. J Gen Physiol. 2005;125:601–617. doi: 10.1085/jgp.200509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estevez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol. 2004;557:363–378. doi: 10.1113/jphysiol.2003.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong P, Rehfeldt A, Jentsch TJ. Determinants of slow gating in ClC-0, the voltage-gated chloride channel of Torpedo marmorata. Am J Physiol. 1998;274:966–973. doi: 10.1152/ajpcell.1998.274.4.C966. [DOI] [PubMed] [Google Scholar]
- 12.Chen TY. Extracellular zinc ion inhibits ClC-0 chloride channels by facilitating slow gating. J Gen Physiol. 1998;112:715–726. doi: 10.1085/jgp.112.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. J Gen Physiol. 1997;109:105–116. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennetts B, Roberts ML, Bretag AH, Rychkov GY. Temperature dependence of human muscle ClC-1 chloride channel. J Physiol. 2001;535:83–93. doi: 10.1111/j.1469-7793.2001.t01-1-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bykova EA, Zhang XD, Chen TY, Zheng J. Large movement in the C terminus of CLC-0 chloride channel during slow gating. Nat Struct Mol Biol. 2006;13:1115–1119. doi: 10.1038/nsmb1176. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Olivares J, Alekov A, Boroumand MR, et al. Gating of human ClC-2 chloride channels and regulation by carboxy-terminal domains. J Physiol. 2008;586:5325–5336. doi: 10.1113/jphysiol.2008.158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markovic S, Dutzler R. The structure of the cytoplasmic domain of the chloride channel ClC-Ka reveals a conserved interaction interface. Structure. 2007;15:715–725. doi: 10.1016/j.str.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Meyer S, Dutzler R. Crystal structure of the cytoplasmic domain of the chloride channel ClC-0. Structure. 2006;14:299–307. doi: 10.1016/j.str.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Meyer S, Savaresi S, Forster IC, Dutzler R. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat Struct Mol Biol. 2007;14:60–67. doi: 10.1038/nsmb1188. [DOI] [PubMed] [Google Scholar]
- 20.Ignoul S, Eggermont J. CBS domains: structure, function and pathology in human proteins. Am J Physiol. 2005;289:1369–1378. doi: 10.1152/ajpcell.00282.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 22.Martinez GQ, Maduke M. A cytoplasmic domain mutation in ClC-Kb affects long-distance communication across the membrane. PLoS ONE. 2008;3:2746. doi: 10.1371/journal.pone.0002746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuddapah VA, Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem. 2010;285:11188–11196. doi: 10.1074/jbc.M109.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao KM, Huang RY, Tang PH, Lin MJ. Functional study of CLC-1 mutants expressed in Xenopus oocytes reveals that a C-terminal region Thr891-Ser892-Thr893 is responsible for the effects of protein kinase C activator. Cell Physiol Biochem. 2010;25:687–694. doi: 10.1159/000315088. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Ogura T, Zheng YJ, et al. Phosphorylation and functional regulation of ClC-2 chloride channels expressed in Xenopus oocytes by M cyclin-dependent protein kinase. J Physiol. 2002;540:883–893. doi: 10.1113/jphysiol.2001.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaus F, Laufer J, Czarkowski K, et al. PIKfyve-dependent regulation of the Cl− channel ClC-2. Biochem Biophys Res Commun. 2009;381:407–411. doi: 10.1016/j.bbrc.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 27.Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol. 1999;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasaki M, Ye L, Duan D, Horowitz B, Hume JR. Intracellular cyclic AMP inhibits native and recombinant volume-regulated chloride channels from mammalian heart. J Physiol. 2000;523:705–717. doi: 10.1111/j.1469-7793.2000.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang P, Liu J, Di A, et al. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 2001;276:20093–20100. doi: 10.1074/jbc.M009376200. [DOI] [PubMed] [Google Scholar]
- 30.Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol. 2004;556:353–368. doi: 10.1113/jphysiol.2003.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XD, Tseng PY, Chen TY. ATP inhibition of CLC-1 is controlled by oxidation and reduction. J Gen Physiol. 2008;132:421–428. doi: 10.1085/jgp.200810023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niemeyer MI, Yusef YR, Cornejo I, et al. Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol Genomics. 2004;19:74–83. doi: 10.1152/physiolgenomics.00070.2004. [DOI] [PubMed] [Google Scholar]
- 33.Zifarelli G, Pusch M. Intracellular regulation of human ClC-5 by adenine nucleotides. EMBO Rep. 2009;10:1111–1116. doi: 10.1038/embor.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutledge E, Bianchi L, Christensen M, et al. CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C. elegans oocytes. Curr Biol. 2001;11:161–170. doi: 10.1016/s0960-9822(01)00051-3. [DOI] [PubMed] [Google Scholar]
- 35.Rutledge E, Denton J, Strange K. Cell cycle- and swelling-induced activation of a C. elegans ClC channel is mediated by CeGLC-7α/β phosphatases. J Cell Biol. 2002;158:435–444. doi: 10.1083/jcb.200204142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falin R, Morrison R, Ham A, Strange K. Identification of regulatory phosphorylation sites in a Ste20 kinase regulated cell cycle- and volume-senstive ClC anion channel. J Gen Physiol. 2009;133:29–42. doi: 10.1085/jgp.200810080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol. 2005;125:113–125. doi: 10.1085/jgp.200409215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falin RA, Miyazaki H, Strange K. C. elegans STK39/SPAK ortholog mediated inhibition of ClC anion channel activity is regulated by WNK independent ERK kinase signaling. Am J Physiol. 2010 doi: 10.1152/ajpcell.00343.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 40.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 41.Feng L, Campbell EB, Hsiung Y, MacKinnon R. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 2010;330:635–641. doi: 10.1126/science.1195230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alioth S, Meyer S, Dutzler R, Pervushin K. The cytoplasmic domain of the chloride channel ClC-0: structural and dynamic characterization of flexible regions. J Mol Biol. 2007;369:1163–1169. doi: 10.1016/j.jmb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Denton J, Nehrke K, Rutledge E, Morrison R, Strange K. Alternative splicing of N- and C-termini of a C. elegans ClC channel alters gating and sensitivity to external Cl− and H+ J Physiol. 2004;555:97–114. doi: 10.1113/jphysiol.2003.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dave S, Sheehan JH, Meiler J, Strange K. Unique gating properties of C. elegans ClC anion channel splice variants are determined by altered CBS domain conformation and the R-helix linker. Channels. 2010;4:289–301. doi: 10.4161/chan.4.4.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Denton J, Nehrke K, Strange K. Carboxy terminus splice variation alters ClC channel gating and extracellular cysteine reactivity. Biophys J. 2006;90:3570–3581. doi: 10.1529/biophysj.105.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellhauser L, Luna-Chavez C, D'Antonio C, Tainer J, Bear CE. ATP binding induces conformational changes in the carboxy terminal region of ClC-5. J Biol Chem. 2011;286:6733–6741. doi: 10.1074/jbc.M110.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 48.Gsponer J, Babu MM. The rules of disorder or why disorder rules. Prog Biophys Mol Biol. 2009;99:94–103. doi: 10.1016/j.pbiomolbio.2009.03.001. [DOI] [PubMed] [Google Scholar]