Abstract
The funny current, If, in sinoatrial myocytes is thought to contribute to the sympathetic fight-or-flight increase in heart rate. If is produced by hyperpolarization-activated cyclic nucleotide sensitive-4 (HCN4) channels, and it is widely believed that sympathetic regulation of If occurs via direct binding of cAMP to HCN4, independent of phosphorylation. However, we have recently shown that Protein Kinase A (PKA) activity is required for sympathetic regulation of If, and that PKA can directly phosphorylate HCN4.1 In the present study, we examined the effects of a myristoylated PKA inhibitory peptide (myr-PKI) on If in mouse sinoatrial myocytes. We found that myr-PKI and another myristoylated peptide potently and specifically potentiated If via a mechanism that did not involve PKA inhibition and that was independent of the peptide sequence, Protein Kinase C or phosphatidylinositol-4,5-bisphosphate. The off-target activation of If by myristoylated peptides limits their usefulness for studies of pacemaker mechanisms in sinoatrial myocytes.
Key words: sinoatrial node, hyperpolarization-activated cyclic nucleotide sensitive channel, HCN4, cAMP-dependent protein kinase, cardiac pacemaking, heart rate regulation
Introduction
The sympathetic fight-or-flight increase in heart rate involves an increase in intracellular cAMP in pacemaker myocytes in the sinoatrial node; however, the molecular mechanisms for this signaling are not well understood. Hyperpolarization-activated, cyclic nucleotide sensitive-4 (HCN4) channels are among the downstream targets of cAMP in sinoatrial myocytes. HCN4 channels produce the “funny current” (If), a mixed Na+/K+ current with a reversal potential of ∼−30 mV. Open HCN4 channels thus conduct a net inward current during diastole, and are thereby thought to contribute to the spontaneous “diastolic depolarization” that drives pacemaking. Sympathetic stimulation of β adrenergic receptors on sinoatrial myocytes shifts the voltage-dependence of activation of If to more depolarized potentials, and the ensuing increase in If during diastole can contribute to heart rate acceleration.
It is widely believed that β adrenergic activation of If occurs via direct binding of cAMP to HCN4 channel proteins, independent of phosphorylation. However, we recently demonstrated that the cAMP-dependent protein kinase (Protein Kinase A, PKA) is essential for sympathetic regulation of If in mouse sinoatrial myocytes.1 Inhibition of PKA by intracellular perfusion with the PKA pseudosubstrate inhibitory peptide, PKI6–22 amide (PKI), almost abolished the ability of the β adrenergic agonist isoproterenol (ISO) to shift the voltage-dependence of If. Moreover, PKA caused a depolarizing shift in the voltage-dependence of activation of heterologously-expressed HCN4 channels. In vitro phosphorylation assays and mass spectrometry revealed that PKA can directly phosphorylate HCN4 at numerous sites in the intracellular amino and carboxyl terminals. A cluster of four serine/threonine residues within a single consensus PKA site in the distal carboxyl terminus was found to mediate the PKA-dependent shift in voltage-dependence of If.
We wanted to follow-up on two significant questions that were raised by our study: (1) does PKA phosphorylation of HCN4 contribute to sympathetic regulation of heart rate? and (2) how do direct binding of cAMP and PKA phosphorylation interact in regulation of If in sinoatrial myocytes? In initial experiments directed at these questions, we discovered that a myristoylated version of PKI (myr-PKI) potentiated If, an effect opposite to that of the non-myristoylated PKI peptide. The present study is an aside that examines the basis for this unexpected effect. We report that myr-PKI and another myristoylated peptide specifically and potently activate If via a mechanism that does not involve inhibition of PKA, and that is independent of the peptide sequence, protein kinase C (PKC) activity or phosphatidylinositol-4,5-bisphosphate (PIP2) synthesis. The off-target activation of If by myr-PKI precludes use of this reagent in studies of pacemaker mechanisms in sinoatrial myocytes.
Results
Myr-PKI increases If via a PKA-independent mechanism.
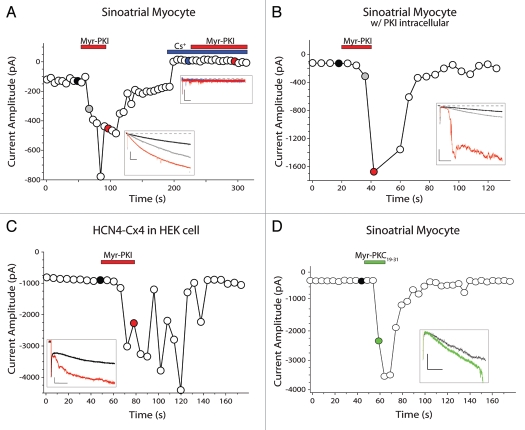
As a follow-up to our studies of PKA regulation of If in sinoatrial myocytes, we attempted to do internally-controlled experiments in single cells by recording the current before and after wash-on of myristoylated PKI14–22 amide (myr-PKI, 2 µM), which is marketed as a cell permeable version of PKI. Surprisingly, myr-PKI rapidly and potently increased the amplitude of If in whole cell recordings from isolated sinoatrial myocytes from mice (n = 13; Fig. 1A). In most cells, the increase in If was followed quickly by a loss of voltage clamp control (e.g., Fig. 1B and C). Both the increase in If and loss of voltage control were generally reversible, however the loss of voltage clamp control prevented measurement of the voltage-dependence for If in the presence of myr-PKI, even at a lower concentration (200 nM; data not shown). These effects of myr-PKI appeared to result specifically from activation of If—rather than from non-specific effects on the membrane or activation of other ionic conductances—because myr-PKI had no effect on currents elicited by hyperpolarizing voltage steps when If was blocked with either Cs+ (5 mM, n = 5, Fig. 1A) or ZD7288 (100 µM, n = 4, data not shown).
Figure 1.
Myristoylated peptides increase If via a PKA-independent mechanism. If current amplitude as a function of time for sinoatrial myocytes exposed to 2 µM myr-PKI or 2 µM myr-PKC19–31. Whole-cell currents were elicited by 2 s voltage steps to −120 mV from a holding potential of −50 mV every 6 s. Filled symbols indicate the traces shown in the insets. Dashed lines indicate zero current. (A) Myr-PKI effects on hyperpolarization-activated currents in the absence or presence of 5 mM Cs+. Scale bars in insets, 100 pA/100 ms. (B) If amplitude for a myocyte perfused with myr-PKI in the presence of 10 µM non-myristoylated PKI in the patch pipette. Scale bars in inset, 400 pA/100 ms. (C) Current amplitude for HCN4-Cx4 channels expressed in HEK293 cells during perfusion of myr-PKI. Scale bars in inset, 500 pA/200 ms. (D) If amplitude for a myocyte perfused with 2 µM Myr-PKC19–31. Scale bars in inset, 100 pA/100 ms.
The potentiation of If by myr-PKI was surprising because it was opposite to effects we observed in our previous study1 for the non-myristoylated PKI6–22 peptide, which was shown to (1) inhibit β adrenergic regulation of If in sinoatrial myocytes, (2) prevent PKA activation of heterologously-expressed HCN4 channels, (3) inhibit PKA phosphorylation of HCN4 in vitro and (4) have no effect on voltage clamp control. Thus, we next asked whether the myr-PKI potentiation of If actually involved inhibition of PKA. We addressed this question first by including the non-myristoylated PKI peptide (10 µM) in the patch pipette during whole-cell recordings, as we had done in our previous study. As shown in Figure 1B, myr-PKI still increased If even in the presence of a 5-fold excess of the non-myristoylated PKI peptide (n = 4), suggesting that PKI and myr-PKI affect If via different mechanisms.
In our earlier study, we demonstrated that PKA regulation of If was mediated by a site in the distal carboxyl terminus of HCN4; PKA had no effect on mutant HCN4-Cx4 channels, in which four serine/threonine residues within a single PKA site were neutralized to alanine (T1153A, S1154A, S1155A and S1157A).1 As shown in Figure 1C, myr-PKI potentiated If produced by HCN4-Cx4 channels expressed in HEK cells (n = 4), demonstrating not only that myr-PKI acts via a mechanism distinct from that of PKA in its regulation of HCN4, but also that this mechanism is present in HEK and CHO (data not shown) cells as well as in mouse sinoatrial myocytes.
To determine whether the potentiation of If by myr-PKI depended on the PKI peptide sequence itself, we next examined the effects on If of another myristoylated peptide, myristoylated protein kinase C 19–31 inhibitory peptide (myr-PKC19–31). We found that, like myr-PKI, myr-PKC19–31 dramatically increased If (n = 3; Fig. 1D). Thus, the mechanism by which myr-PKI activates HCN4 channels is independent of the exact peptide sequence, and is clearly independent of PKA inhibition. Given this, and the observation that non-myristoylated PKI or PKC19–31 peptides do not activate If (ref. 1, Fig. 2B), we next asked whether the myristoyl group itself was sufficient to mediate this effect. Accordingly, we applied to isolated sinoatrial myocytes myristic acid (2 µM, n = 5 or 100 µM, n = 3), myristamide (2 µM, n = 6), or methyl myristate (5 µM, n = 4 or 10 µM, n = 3). None of these compounds had any effect on If (data not shown), suggesting that both the myristoyl and peptide moieties of myristoylated peptides are necessary for their activation of HCN4 channels.
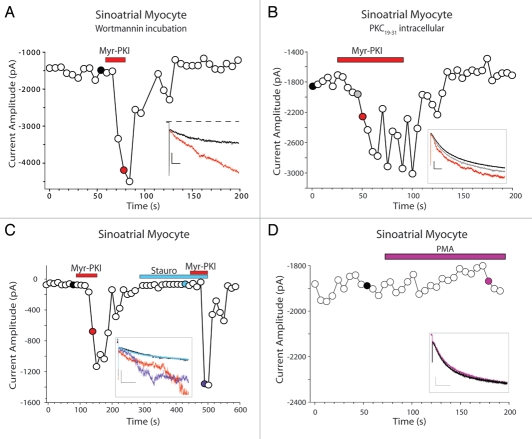
Figure 2.
Potentiation of If by myristoylated peptides is independent of PIP2 or PKC. If current amplitude as a function of time for sinoatrial myocytes exposed to 2 µM myr-PKI in the presence of (A) 15 µM wortmannin (scale bars in inset 1 nA/100 ms); (B) 10 µM PKC19–31 (scale bars in inset 400 pA/100 ms) or (C) 1 µM staurosporine (scale bars in inset 100 pA/200 ms). (D) If amplitude for a cell exposed to 500 nM PMA (scale bars in inset 400 pA/200 ms). Whole-cell currents were elicited by 2 s voltage steps to −120 mV from a holding potential of −50 mV every 6 s. Filled symbols indicate the traces shown in the insets.
Myr-PKI potentiation of If is independent of PIP2 and PKC.
The membrane lipid PIP2 causes a large depolarizing shift in the voltage dependence of activation of HCN channels.2,3 We investigated whether myr-PKI increases If via an acute increase in PIP2 levels by exposing sinoatrial myocytes to wortmannin, which blocks PIP2 synthesis by inhibiting phosphatidylinositol 4-kinase. We found that pre-incubation of sinoatrial myocytes in wortmannin (15 µM, 30–60 min) had no effect on the ability of myr-PKI to potentiate If (n = 5; Fig. 2A). Acute wash-on of wortmannin (15 µM, 1–3 minutes) also did not attenuate the ability of myr-PKI to increase If, although it slightly decreased the amplitude of If, as expected for a modest reduction in PIP2 levels (n = 4; data not shown). These data suggested that myristoylated peptides increase If via a mechanism that is independent of PIP2 synthesis.
It has previously been shown that a variety of myristoylated peptides—including myr-PKI—can non-specifically inhibit PKC.4 We therefore hypothesized that the mechanism for the potentiation of If by myr-PKI and myr-PKC19–31 could be via inhibition of PKC. To test this hypothesis, we inhibited PKC using either the non-myristoylated PKC19–31 inhibitory peptide in the patch pipette (10 µM; n = 10; Fig. 2B) or staurosporine, a broad-spectrum kinase inhibitor, which was applied acutely by perfusion for ∼3 min (1 µM; n = 3; Fig. 2C) or by pre-incubation for 30–60 min (1 µM; n = 3; data not shown). Neither PKC19–31 nor staurosporine mimicked the potentiation of If caused by myristoylated peptides—indeed wash-on of staurosporine reduced the amplitude of If in some cells, perhaps via inhibition of PKA—and neither inhibitor altered the ability of myr-PKI to increase If (Fig. 2B and C). These data indicate that myristoylated peptides increase If via a PKC-independent mechanism. In addition, the data further support the conclusion that myr-PKI potentiation of If is independent of PKA inhibition, since staurosporine also blocks PKA (and a number of other kinases) with nanomolar affinity.5
In the course of these experiments, we also asked whether PKC activity can regulate If in sinoatrial myocytes, independent of the effects of myristoylated peptides. We found that phorbol 12-myristate 12-acetate (PMA, an activator of conventional and novel PKC isoforms) had no effect on If when it was perfused onto the cells for ∼3 min (500 nM; Fig. 2D; n = 8). In addition, pre-incubation of the cells with PMA (500 nM, 30 min), or inclusion in the patch pipette of either the catalytic subunit of PKC (PKCc; 30 nM) or the PKC19–31 inhibitory peptide (10 µM) had no significant effect on the voltage-dependence of activation for If (V1/2 = −126.3 ± 3.1 mV, n = 6 in control intracellular; −123.9 ± 2.6 mV, n = 7 with PMA pre-incubation; −124.5 ± 5.0 mV, n = 10 with PKCc intracellular; −120.1 ± 2.2 mV, n = 9 with PKC19–31 intracellular; p > 0.05 for all pairwise comparisons). Taken together, these data suggest that PKC is not a major modulator of If in sinoatrial myocytes.
Discussion
In this study we demonstrated that myristoylated peptides activate If in mouse sinoatrial myocytes via a mechanism that is independent of the peptide sequence, PKA, PKC or PIP2. We also demonstrated that PKC has little effect on If in sinoatrial myocytes, at least under the conditions used in this study.
Myristoylated peptides, including myr-PKI, myristoylated CaMKII inhibitory peptide and myristoylated control peptides have been previously shown to completely but reversibly halt spontaneous firing of isolated sinoatrial myocytes from rabbits or mice.6,7 We have also observed rapid and reversible cessation of spontaneous activity in mouse sinoatrial myocytes exposed to 2 µM myr-PKI or 2 µM myr-PKC19–31 (data not shown). Our present study raises the possibility that potentiation of If is the mechanism by which myristoylated peptides stop spontaneous firing. Although modest activation of If would be expected to increase the spontaneous firing rate, excessive activation would simply clamp the cells near the If reversal potential (∼−30 mV), thereby preventing compensation by other pacemaker mechanisms. This idea underscores the fundamental point that pacemaker activity in sinoatrial myocytes depends on a finely-tuned balance among many different ionic conductances working in concert, and that dysregulation of any part of the system can cause it to fail.
There are many possible mechanisms by which myristoylated peptides could activate If. For example, they could mimic, activate or interfere with an endogenous signaling pathway, or they could interact directly with HCN4 channels. Two physiological signaling molecules that are known to regulate If are cAMP and PIP2. However, we consider it unlikely that myristoylated peptides act via either of these pathways. The effects of myristoylated peptides on If are quite different from any reported manipulations of cAMP in sinoatrial myocytes, including acute wash-on of the membrane permeant cAMP analogue 8-Br-cAMP (data not shown). And, our present data argue against myristoylated peptide activation of PIP2 synthesis (Fig. 2A). There are of course a multitude of other signaling pathways in sinoatrial cells that could regulate If. We have here ruled out PKC signaling as a mechanism for myr-PKI activation of If. Our experiments also suggested that If may be relatively unaffected by PKC activity in mouse sinoatrial cells. Investigation of other regulatory pathways that control HCN channel activity in the heart and brain is the focus of much ongoing work.
Overall, the major finding of this study is that myristoylated peptides have off-target effects that render them unsuitable for use in studies of pacemaker mechanisms in sinoatrial myocytes. The possibility that the dramatic activation of If by myristoylated peptides echoes a physiological regulatory mechanism awaits further study. However, the lack of requirement for a specific peptide sequence and the similar activation of HCN4 channels in sinoatrial myocytes and HEK cells indicate that any such mechanism does not involve myocyte-specific signaling pathways.
Materials and Methods
Materials.
Wortmannin, staurosporine, PMA, PKCc and myristic acid were obtained from Sigma-Aldrich; PKI6–22 amide, myr-PKI14–22 amide and PKC19–31 from EMD Chemicals; myr-PKC19–31 from AnaSpec; n-tetradecanamide and methyl tetradecanoate from Alfa Aesar; and ZD7288 from Tocris Bioscience.
Sinoatrial myocyte isolation and electrophysiology.
Sinoatrial myocytes were isolated from adult (>7 weeks) male C57BL/6J mice as described previously in reference 1. Briefly, the sinoatrial node region was dissected at 35°C in a heparinized (10 U/ml) solution (in mM: 140 NaCl, 5.4 KCl, 1.2 KH2PO4, 5 HEPES, 5.55 Glucose, 1 MgCl2, 1.8 CaCl2, pH adjusted to 7.4 with NaOH) and myocytes were isolated by digestion for 25–30 min in collagenase type II, protease type XIV and elastase.
Hyperpolarization-activated If currents were recorded from sinoatrial myocytes in the whole cell patch clamp configuration with pipettes that had resistances of ∼1.5 to 3 MΩ when filled with an intracellular solution consisting of (in mM) 135 K-aspartate, 6.6 Na-phosphocreatine, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 EGTA, 0.1 Na-GTP, 4 Mg-ATP, pH adjusted to 7.2 with KOH. Cells were constantly perfused (∼1–2 ml/min) with Tyrode's solution (140 NaCl, 5.4 KCl, 5 HEPES, 5.55 Glucose, 1 MgCl2, 1.8 CaCl2, pH adjusted to 7.4 with NaOH) containing 1 mM BaCl2. All experiments were conducted at room temperature.
If was measured as the time-dependent component of current elicited by hyperpolarizing voltage steps. Conductance (G) for If was calculated as:
G = I/(Vm − Vr)
where I is the time-dependent component of inward current, Vm is the applied membrane voltage (corrected for a +14 mV junction potential error), and Vr is the reversal potential for If (−30 mV; ref. 8; Liao Z, Proenza C, unpublished). Conductance-voltage plots were fit with a Boltzmann equation to determine midpoint activation voltages (V½).
HEK cell culture, transfection and electrophysiology.
HEK293 cells were transiently transfected with cDNA encoding HCN4 or HCN4-CX4 plus CD8 using Fugene6 (Roche) according to the manufacturer's directions. Transfected cells were identified by anti-CD8 antibody coated beads (Invitrogen), and were used for electrophysiology 24–48 hours post-transfection. Cells were voltage-clamped in the whole cell configuration using pipettes with resistances of ∼1.5 to 3 MΩ when filled with an intracellular solution that contained (in mM) 130 K-aspartate, 10 NaCl, 1 EGTA, 5 HEPES, 0.5 MgCl2, 2 MgATP, pH adjusted to 7.2 with KOH. Cells were constantly perfused (∼1–2 ml/min) with extracellular solution consisting of (in mM) 115 NaCl, 30 KCl, 1 MgCl2, 1.8 CaCl2, 5.5 glucose, 5 HEPES, pH adjusted to 7.4 with NaOH.
Statistics.
All results are reported as mean ± SEM. Comparisons were performed using unpaired two-tailed t-tests.
Acknowledgements
We thank Roger Bannister and Kurt Beam for critical reading of the manuscript. This work was supported in part by a grant to Catherine Proenza from the National Institutes of Health (HL088427).
References
- 1.Liao Z, Lockhead D, Larson ED, Proenza C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J Gen Physiol. 2010;136:247–258. doi: 10.1085/jgp.201010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pian P, Bucchi A, Robinson RB, Siegelbaum SA. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J Gen Physiol. 2006;128:593–604. doi: 10.1085/jgp.200609648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, et al. Pace-making by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–1036. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Harris TE, Persaud SJ, Jones PM. Pseudosubstrate peptide inhibitors of beta-cell protein kinases: altered selectivity after myristoylation. Mol Cell Endocrinol. 1999;155:61–68. doi: 10.1016/s0303-7207(99)00114-8. [DOI] [PubMed] [Google Scholar]
- 5.Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, et al. Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem. 1995;234:317–322. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- 6.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci USA. 2009;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangoni ME, Nargeot J. Properties of the hyperpolarization-activated current (If) in isolated mouse sino-atrial cells. Cardiovasc Res. 2001;52:51–64. doi: 10.1016/s0008-6363(01)00370-4. [DOI] [PubMed] [Google Scholar]