Abstract
Recently, we investigated the molecular mechanisms of the smoking cessation drug varenicline, a nicotinic acetylcholine receptor (nAChR) partial agonist, in its ability to decrease voluntary ethanol intake in mice. Previous to our study, other labs had shown that this drug can decrease ethanol consumption and seeking in rat models of ethanol intake. Although varenicline was designed to be a high affinity partial agonist of nAChRs containing the α4 and β2 subunits (designated as α4β2*), at higher concentrations it can also act upon α3β2*, α6*, α3β4* and α7 nAChRs. Therefore, to further elucidate the nAChR subtype responsible for varenicline-induced reduction of ethanol consumption, we utilized a pharmacological approach in combination with two complimentary nAChR genetic mouse models, a knock-out line that does not express the α4 subunit (α4 KO) and another line that expresses α4* nAChRs hypersensitive to agonist (the Leu9′Ala line). We found that activation of α4* nAChRs was necessary and sufficient for varenicline-induced reduction of alcohol consumption. Consistent with this result, here we show that a more efficacious nAChR agonist, nicotine, also decreased voluntary ethanol intake, and that α4* nAChRs are critical for this reduction.
Key words: alcoholism, ethanol, nicotine, varenicline, nicotinic acetylcholine receptors, mice
Introduction
Similar to all drugs of abuse, ethanol administration causes an increase of dopamine (DA) release in the nucleus accumbens (NAcc). This increase in DA release is thought to underlie the rewarding or reinforcing properties of the drug.1 Although ethanol has been found to modulate several receptors and ion channels, emerging evidence indicates that nicotinic acetylcholine receptors (nAChRs) may play a role in ethanol-induced accumbal DA release and ethanol reinforcement.2 Pre-application of mecamylamine, a nAChR antagonist, either by direct infusion into the ventral tegmental area (VTA) or an intraperitoneal (i.p.) injection, decreases ethanol-induced DA release in the NAcc3,4 as well as decreases ethanol self-administration in rats5 and ethanol voluntary intake in mice.6 Thus, nAChRs are now a focus of intense investigations as molecular targets for not only nicotine addiction, but alcoholism as well.
For example, the nAChR partial agonist varenicline is an FDA approved smoking cessation aid that has also shown promise to reduce ethanol consumption in several rodent models and one clinical model.7–10 The molecular mechanism by which varenicline acts to decrease nicotine intake is well understood, while on the other hand, until recently, its role in decreasing ethanol intake was not entirely clear due to the vast array of nAChR subtypes it may act upon.
Neuronal nAChRs are pentameric, ligand-gated cation channels that are activated by the endogenous neurotransmitter acetylcholine (ACh) as well as exogenous agonists such as nicotine. To date, twelve mammalian neuronal nAChR subunits have been identified (α2–α10, β2–β4), which can combine to form either hetero- or homomeric receptors, producing multiple functional subtypes of receptors, each with distinct pharmacological and biophysical properties.11
Given the large number of nAChR subtypes with which varenicline may interact,12 we sought to identify which nAChR subtype underlies varenicline's effect on ethanol intake. Because varenicline was designed to be a partial agonist of nAChRs containing α4 and β2 subunits (designated as α4β2*),13,14 we examined the effect of the drug on ethanol intake in α4 KO and α4 KI mice hypersensitive to agonist (Leu9′Ala line). We found that activation of α4* nAChRs was necessary and sufficient for varenicline-induced reduction of voluntary ethanol intake in a limited access ethanol-drinking assay.7 Here, we extend our previous findings and present further evidence for the activation of α4* nAChRs in modulating the rewarding effects of ethanol.
Results
Alpha4* nAChRs are necessary for nicotine-induced reduction of ethanol consumption.
To examine the role of α4* nAChRs in baseline ethanol intake and after a pre-injection of nicotine, we used α4 KO and WT mice in the Drinking in the Dark (DID) assay15 (Fig. 1). Two-way ANOVA revealed that there was a significant main effect of treatment (F(2,72) = 7.77, p < 0.001) but not genotype and a significant treatment × genotype interaction (F(2,72) = 4.57, p < 0.05). Similar to our previous observations, nicotine dose dependently decreased voluntary ethanol intake in WT mice at doses of 0.25 mg/kg and 0.5 mg/kg nicotine compared to a saline injection (p < 0.05, p < 0.001, respectively).6 In the α4 KO mice, neither dose of nicotine had any significant effect on ethanol intake, indicating that α4* nAChRs are necessary for the nicotine-induced effect on ethanol drinking. In addition to nicotine having no effect in decreasing ethanol intake in the α4 KO mice, there was also a significant reduction in baseline ethanol consumption after a saline injection when compared to WT mice (Fig. 1 and p < 0.01) suggesting that α4* nAChRs may also be involved in generalized ethanol consumption.
Figure 1.
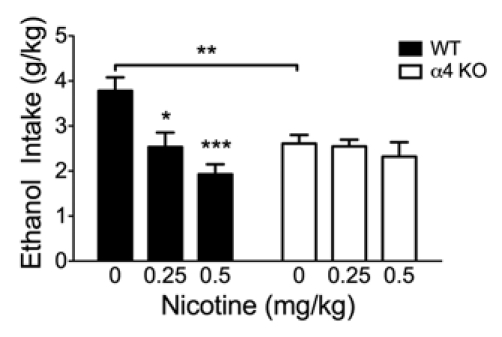
Alcohol intake after nicotine treatment in WT and α4 KO mice. A pre-injection of nicotine (0.25 mg/kg and 0.5 mg/kg) dose dependently decreased 20% ethanol intake in WT mice compared to a saline injection, but had no effect on α4 KO mice (n = 9–18/genotype). Additionally, α4 KO mice consumed significantly less 20% ethanol compared to WT mice after a saline injection. *p < 0.05, **p < 0.01, ***p < 0.001.
Activation of α4* nAChRs is sufficient for nicotine-induced reduction of ethanol consumption.
To determine if activation of α4* nAChRs was sufficient for nicotine-induced reduction of ethanol consumption, we used the hypersensitive Leu9′Ala mice in the 20% ethanol DID assay (Fig. 2). In this experiment however, the concentration of nicotine was lowered such that the dose used would have no effect on WT nAChRs, but would selectively activate mutant Leu9′Ala α4* nAChRs.16 Two-way ANOVA showed a significant main effect of genotype (F(1,28) = 4.56, p < 0.05) but not treatment and there was no significant interaction between these two factors. Low doses of nicotine (0.05 mg/kg) significantly decreased ethanol intake in Leu9′Ala mice, but had no effect on WT mice (Fig. 2 and p < 0.05). These data indicate that selective activation of α4* nAChRs is sufficient for nicotine-induced reduction of ethanol consumption.
Figure 2.
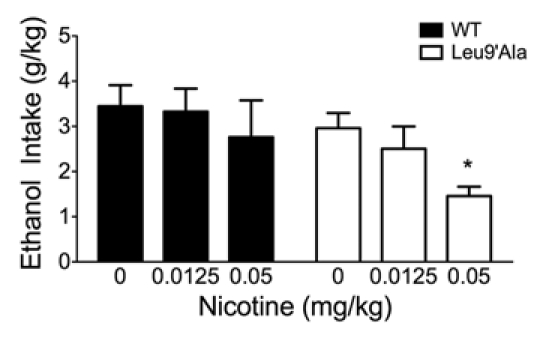
Alcohol intake in WT and Leu9′Ala mice after nicotine pre-treatment. Low doses of nicotine (0.0125 mg/kg and 0.05 mg/kg) did not significantly decrease 20% ethanol intake in WT mice compared to a saline injection. Low dose nicotine (0.05 mg/kg) did significantly decrease 20% ethanol intake in the hypersensitive Leu9′Ala mice compared to a saline injection (n = 5–12/genotype). *p < 0.05.
Discussion
It is widely accepted that α4β2* nAChRs are critical for mediating the rewarding properties of nicotine and nicotine addiction.16,17 It is also known that nicotine and alcohol are often co-abused and that 70–75% of alcoholics are also dependent on nicotine,18,19 suggesting a functional interaction between these drugs. Varenicline, acting as partial agonist of α4β2* nAChRs, is a successful smoking cessation aid and has also been shown to attenuate acute ethanol-induced DA release as well as reduce ethanol craving in heavy drinking smokers.10,20
Previously, several labs including our own have shown that varenicline can decrease ethanol intake in several rodent models.7–9 Because of the pharmacological promiscuity of varenicline,12 we sought to identify the nAChR subtype responsible for varenicline-induced reduction of alcohol consumption. While most of the previous studies primarily used pharmacology alone, our study combined pharmacology with genetically modified nAChR knock-out and knock-in mice. We showed that both varenicline and ethanol can activate DAergic neurons of the posterior VTA and that these activated neurons express higher levels of the α4, α6 and β3 nAChR subunit gene transcripts. Additionally, we showed that nAChRs containing the α4 subunit, and possibly the α4α6β2β3 subtype, are critical for varenicline's effect on drinking.7
It is likely that the mechanism of action underlying varenicline's ability to decrease ethanol intake is similar to how it is thought to decrease nicotine use. That is, acting as a partial agonist, varenicline selectively binds and partially activates the nAChRs that modulate ethanol intake (i.e., α4* nAChRs) thus occupying or desensitizing the relevant receptors, precluding any further activation by ethanol. Additionally, the selective activation of α4* nAChRs themselves may act to increase DA release in the NAcc such that ethanol has no further enhancing effect.
In line with this idea, here we show that the nAChR full agonist nicotine also decreases ethanol consumption in an α4* nAChR-dependent mechanism. Nicotine reduced ethanol consumption in WT mice but had no effect in α4 KO mice implying that expression of α4* nAChRs is necessary for nicotine-induced reduction of ethanol consumption. In contrast, low doses of nicotine that have no effect in WT mice significantly reduced ethanol intake in the Leu9′Ala hypersensitive mice indicating that the selective activation of α4* nAChRs is sufficient for the effect on alcohol consumption.
One common argument against a role for α4β2* nAChRs in alcohol consumption is that the selective competitive antagonist, dihydro-β-erythroidine (DHβE), fails to reduce ethanol intake in both rats and mice. However, caution should be used in interpreting these data for at least two reasons. First, the ability of DHβE to block α4β2* nAChRs depends on the stoichiometry of the target receptor population,21 which is unknown in vivo. And second, the subunit composition of nAChRs involved in alcohol reward, while likely containing α4 and β2 subunits, has not been fully elucidated. For example, high affinity α4β2* herteromeric nicotinic receptors that contain additional α or β subunits may be insensitive to DHβE.22
Taken together, our results show that the selective activation of α4* nAChRs is necessary and sufficient to reduce acute ethanol consumption. This result also further supports the hypothesis that selective agonists or partial agonists of α4* nAChRs may be valuable therapeutics for the treatment of alcoholism.
Materials and Methods
Animals.
Adult (8–10 week-old) male α4 KO mice and their WT litter mates, as well as heterozygous Leu9′Ala knock-in mice and their WT litter mates, all bred on site, were used. The genetic engineering of both α4 KO and Leu9′Ala mouse lines have been described previously in references 16 and 23. Both lines have been back-crossed to a C57BL/6J background > 9 generations. Mice were individually housed on a reversed 12 h light/dark cycle (lights on 10 PM, off 10 AM) with ad libitum access to food and water (except during experiments as described below). All experiments were conducted in accordance with the guidelines for care and use of laboratory animals provided by the National Research Council,24 as well as with an approved animal protocol from the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Drugs and drinking solutions.
Ethanol drinking solutions were prepared from 190 proof absolute anhydrous ethanol (Pharmco-Aaper) diluted to 20% ethanol (v/v) using tap water. Nicotine hydrogen bitartrate (Sigma-Aldrich), was dissolved in 0.9% saline and was administered via intraperitoneal (i.p.,) injections at the indicated doses. Nicotine concentrations are reported as nicotine base.
Drinking in the dark (DID).
Ethanol consumption was measured using a DID procedure as previously described in reference 7. Animals were singly housed in experimental chambers for 1 week prior to the beginning of the DID sessions. The mice received a 15 ml graduated cylinder water bottle fitted with a one-hole rubber stopper with a stainless steel double-ball-bearing sipper tube that was sealed with Parafilm to prevent leakage. For the first three nights, two hours after the lights were off, mice were i.p., injected with saline immediately before their water bottle was replaced with the 20% ethanol bottle, and allowed to drink for two hours. This procedure was used to acclimatize the mice to the experimental conditions and allow them to reach a baseline of ethanol intake prior to drug administration. On the fourth night, the mice received their first dose of drug immediately before placement of the ethanol bottle. The amount of ethanol consumed was recorded immediately after each two-hour session and converted to g/kg per each animal's ethanol consumption and body weight. The mice were given two days of rest (no injections or ethanol) and then began the saline injection/ethanol consumption assay for 2–3 days until a stable ethanol intake was reached. Once the baseline returned, a second, higher dose of drug was administered prior to the ethanol bottle being placed in the cage. In this design, all mice in one group drink a single concentration of ethanol throughout the experiment, but receive two doses of drug, 4–5 days apart, with the lower concentration of drug first.
Data analysis.
The effect of nicotine on ethanol intake was compared to ethanol intake after a saline injection from the previous day using Two-way ANOVA with genotype and treatment as variables followed by Bonferroni post hoc tests. Data were analyzed using Graphpad software (Graphpad Software, Inc.). Results were considered significant at p < 0.05. All data are expressed as means ± standard errors of means (SEM).
Acknowledgements
This study was supported by National Institute on Alcohol Abuse and Alcoholism award numbers R01AA017656 (Andrew R. Tapper) and F31AA018915 (Linzy M. Hendrickson) and from National Institute On Neurological Disorders and Stroke award number R01NS030243 (Paul D. Gardner). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Neurological Disorders and Stroke or the National Institutes of Health.
Abbreviations
- α4 KO
α4 knock-out
- ACh
acetylcholine
- ANOVA
analysis of variance
- DA
dopamine
- DHβE
dihydro-β-erythroidine
- DID
drinking in the dark
- i.p.
intraperitoneal
- MLA
methyllycaconitine
- NAcc
nucleus accumbens
- nAChR
nicotinic acetylcholine receptor
- Leu9′Ala
leucine 9′ alanine knock-in
- VTA
ventral tegmental area
References
- 1.Soderpalm B, Lof E, Ericson M. Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry. 2009;42:87–94. doi: 10.1055/s-0029-1220690. [DOI] [PubMed] [Google Scholar]
- 2.Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 3.Blomqvist O, Ericson M, Engel JA, Soderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- 4.Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- 5.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of α4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-009-1759-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 13.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 14.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 17.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 18.Room R. Smoking and drinking as complementary behaviours. Biomed Pharmacother. 2004;58:111–115. doi: 10.1016/j.biopha.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Bobo JK, Husten C. Sociocultural influences on smoking and drinking. Alcohol Res Health. 2000;24:225–232. [PMC free article] [PubMed] [Google Scholar]
- 20.Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- 21.Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- 22.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 23.Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, et al. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council, author. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]


