Abstract
Myogenesis in Drosophila embryos requires fusion between Founder cells (FCs) and Fusion Competent myoblasts (FCMs) to form multinucleate myotubes. Myoblast fusion is well characterized in embryos, and many factors required for this process have been identified; however, a number of questions pertaining to the mechanisms of fusion remain and are challenging to answer in the embryo. We have developed a modified primary cell culture protocol to address these questions in vitro. Using this system, we determined the optimal time for examining fusion in culture and confirmed that known fusion proteins are expressed and localized as in embryos. Importantly, we disrupted the actin and microtubule networks with the drugs latrunculin B and nocodazole, respectively, confirming that actin is required for myoblast fusion and showing for the first time that microtubules are also required for this process in Drosophila. Finally, we show that myotubes in culture adopt and maintain specific muscle identities.
Key words: Drosophila, primary culture, myoblast fusion, muscle morphogenesis, identity genes
Introduction
In the Drosophila melanogaster larva, each muscle fiber is a single multinucleate cell. This syncytium forms from myoblast fusion during embryonic development and creates the myofiber. Fusion occurs between two types of myoblasts: a single Founder cell (FC) that undergoes multiple rounds of fusion with surrounding Fusion Competent myoblasts (FCMs) to form the final muscle. FCs contain the information required to generate muscles of specific identities; this information is encoded in the combinatorial expression of particular transcription factors, known as identity genes, in the FC and nascent myotube.1–3 Genetic analyses have highlighted several key myoblast behaviors as critical for the fusion process, including FC/FCM specification, FCM migration, recognition and adhesion between these two cell types, formation of an actin focus at the site of fusion and membrane breakdown leading to cytoplasmic continuity.4–6 While the large number of in vivo studies have uncovered many players and processes important for myoblast fusion and muscle morphogenesis, there are questions that have proven difficult to answer within the context of the intact fly embryo. For this reason, we have developed a Drosophila primary myoblast culture system to examine early steps in myotube development, including myoblast fusion and maintenance of muscle identity. Primary cell culture has several advantages over in vivo methods, as it provides a more efficient platform for experiments including: live imaging, directed migration assays and high-throughput screenings using UAS-RNAi fly lines or small molecules. Primary cell culture may also represent a closer-to-physiological state than do immortalized insect cell lines, the origins and behaviors of which can be uncertain.
In the last several decades, a number of studies reported methods to examine myogenesis ex vivo.7,8 More recently, Bai and colleagues developed a method for the establishment of primary cultures that could be treated and screened with dsRNA.9–11 These protocols allowed investigators to examine later features of muscle differentiation in culture, such as final nuclear number and sarcomere organization;9 however, several critical aspects of early muscle differentiation have not been examined, including myotube identity and the myoblast fusion process. We have analyzed these critical features in primary cell culture, using a modified protocol so that we dissociate embryos as early as possible before the onset of fusion.
We now report the characterization of the early developmental steps of muscle in our primary culture system. For this study, we quantified the progression of myoblast fusion in primary myoblast cultures (hereafter primary cultures/primary cells) and tested for the presence and localization of known fusion proteins. Because dsRNA treatment is not a viable approach to perturb fusion in these cultures,10,11 we developed a protocol for drug treatment of primary cultures that permits disruption of myoblast fusion. Finally, we examined primary cells for expression and maintenance of specific muscle identity transcription factors. We found that primary cultures recapitulate numerous aspects of myotube differentiation as seen in the embryo, making them useful for further studies of myoblast fusion ex vivo. Our drug treatment studies also confirmed the role of actin in myoblast fusion and newly implicated microtubules in this multistep process in Drosophila. This new treatment protocol additionally offers an effective platform for drug screening in primary cultures. Taken together, our work provides a foundation for more complex studies of myoblast fusion in vitro.
Results
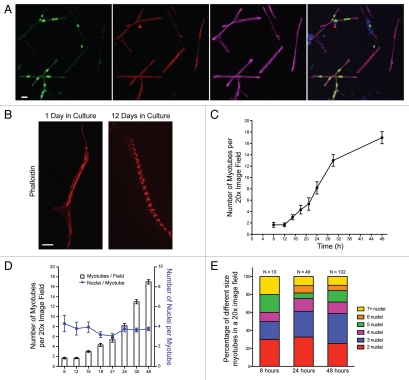
We modified earlier Drosophila primary cell culture protocols (see Materials and Methods) to examine muscle fiber formation and differentiation, with a focus on myoblast fusion and muscle identity. Briefly, we dissociate late stage 8/stage 9 embryos (3.25–4.25 hours), remove debris, plate and grow for 8 hours. We begin analysis 8 hours after plating, since it takes this amount of time for the cells to settle and adhere to the culture dish. To confirm that our protocol could be used for studies of muscle differentiation, we first determined that myotubes formed in culture as evidenced by the presence of multinucleate cells grown for 24 hours expressing myosin heavy-chain (MHC) (Fig. 1A). We next visualized the actin cytoskeleton with phalloidin and found that actin becomes striated by day 12, indicating that these myotubes undergo terminal differentiation to form myofibers with sarcomeres (Fig. 1B). To establish the ideal time for assessing myoblast fusion in culture, we performed a time course analysis of fusion by counting the number of multinucleate myotubes in a given 20x image field. We found that myoblast fusion commences in the initial 8 hours after plating and increases rapidly between 12–30 hours post-plating, reaching a plateau around 48 hours after plating (Fig. 1C). The myotubes in culture adopt a variety of morphologies including long, finger-like cells as well as branched and polygon-shaped myotubes (Figs. 1 and 3 and data not shown). The number of nuclei in the myotubes ranges from two to greater than seven, with a mean of 3.7 nuclei per myotube (Fig. 1D). We quantified the number of nuclei per myotube as a function of time (Fig. 1E) and found that, although the total number of myotubes increases over time, the distribution of myotubes containing 2, 3, 4, 5, 6 or 7+ nuclei remains consistent. These results are consistent with previous cell culture experiments11 and delineated\ a discrete period of time, between 12–48 hours after plating, as optimal for studying myoblast fusion.
Figure 1.
Primary embryonic Drosophila cells fuse and form muscles in culture. (A) Representative image from a field of myotubes in a culture of primary cells grown for 24 h from twipromoter-actin-GFP, apME-NLS::GFP embryos. The culture was immunostained for GFP (green) and Myosin Heavy Chain ([MHC], red) and labeled with phalloidin (magenta) and DAPI (blue). Scale bar, 10 µm. (B) Images of single myotubes stained with phalloidin (red) that have been in culture for either 1 or 12 days. Scale bar, 10 µm. (C) Quantification of myotube formation over a 48 h period measured by the average number of myotubes present in a 20x image field. Six image fields were counted for each time point. (D) Quantification of the average number of nuclei per myotube (blue line) over a 48 h period measured by the average number of DAPI-stained nuclei/myotube present in a 20x image field. Six image fields were counted for each time point. The average number of myotubes per field at each time point is represented by the bars. (E) Relative distribution of the number of nuclei per myotube at 8, 24 and 48 h in culture. The distribution of myotubes in a culture with given numbers of nuclei remained relatively consistent over time as new myotubes are formed.
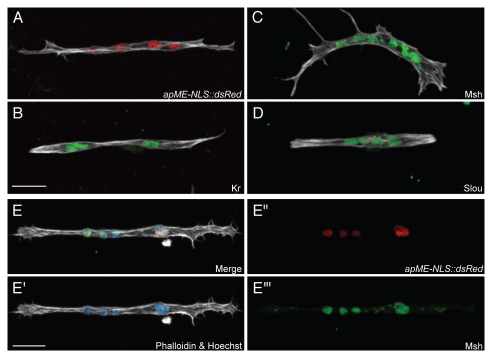
Figure 3.
Muscle identity is preserved in myotubes in primary cell culture. (A–D) Confocal maximum intensity projections of primary myotubes formed from cells isolated from apME-NLS::dsRed embryos at 24 h after plating. Myotube identity was determined by monitoring nuclei for expression of the apME-NLS::dsRed transgene as a marker for Ap expression (red), or by antibody staining for Msh, Kr or Slou (green). Cultures were labled with phalloidin (white). Scale bars, 10 µm. (E-E′″) Confocal maximum intensity projections of a primary myotube at 24 h after plating formed from cells isolated from apME-NLS::dsRed embryos. The nuclei co-label with an antibody against Msh (green), and also express the apME-NLS::dsRed transgene (red). The myotube is co-labeled with phalloidin (white) and Hoechst (blue). Scale bars, 10 µm.
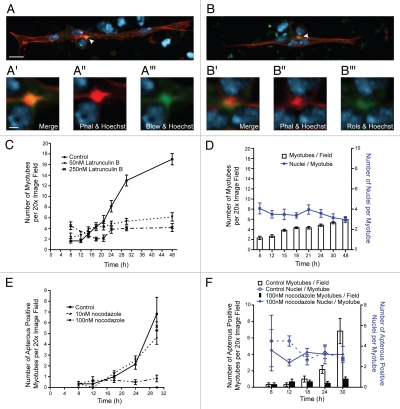
Despite previous work demonstrating that multinucleate myotubes form in primary cell cultures;7–12 how the process of myoblast fusion proceeds in culture and compares to what is observed in embryos has not been studied. We therefore sought to determine whether embryonic myoblast fusion proteins were similarly expressed and localized in our primary culture system. We focused on two well-characterized proteins that are required for fusion in the embryo: the intracellular adaptor protein Rolling pebbles/Antisocial (Rols/Ants) and the fusion protein Blown-fuse (Blow).13–20 In the embryo, Rols is found at the fusion site in FCs and myotubes, while Blow is found initially in FCMs and subsequently in myotubes after fusion.4 Blow and Rols/Ants are expressed in primary cells and localize to the sites of attachment between myoblasts and nascent myotubes as has been shown in embryos (Fig. 2A and B). Additionally, staining with phalloidin revealed an F-actin structure at the myoblast attachment site (Fig. 2A and B and white arrowheads). This accumulation of F-actin has been shown through time lapse microscopy in embryos to mark the site of fusion19 and is designated the actin focus (Fig. 2A″ and B″).15,19,21 We measured the size of the actin foci that formed in culture. These foci ranged in size from 1.2 µm2 to 3.4 µm2 with a mean area of 2.3 µm2 (SD ± 0.7 µm). This size range is within the previously observed range for normal foci of 0.7 µm2 to 4.5 µm2 and the mean size we observed is not significantly different from the reported mean of 1.9 µm2 (SD ± 0.7 µm) observed in embryos (p = 0.18). The localization of these fusion proteins and the actin focus at the site of fusion is similar to what our lab and others have observed in Drosophila embryos.13–21 Therefore, based on the localization of fusion proteins and the size of the actin focus, fusion between myoblasts and multinucleate myotubes in primary culture appears to proceed via mechanisms similar to fusion in embryos.
Figure 2.
Proteins important for myoblast fusion are properly expressed and localized in primary myoblasts in culture. (A and B) Confocal maximum intensity projections of representative myotubes generated from apME-NLS::dsRed embryos after 24 h in culture immunostained for myoblast fusion proteins (Blown Fuse [Blow], Rolling Pebbles [Rols], green) and co-labeled with phalloidin (red) to label actin and Hoecsht (blue) to label nuclei. Each arrowhead marks the co-localization of the fusion protein with the actin focus at the site of adhesion between the myotube and myoblast. Scale bars, 5 µm. (A′-A′″ and B′-B′″) Insets show a single confocal section of the actin focus and site of fusion. Scale bars, 1 µm. (C) Myotube formation over a 48-h period measured by the average number of myotubes present in six 20x image fields. At 8 h after plating, media was changed and parallel cultures were control treated (ethanol), or treated with the actin depolymerization drug latrunculin B at two different concentrations (50 nM or 250 nM). (D) Quantification of the average number of nuclei per myotube (blue line) in cultures treated with 50 nM latrunculin B over a 48-h period measured by the average number of DAPI-stained nuclei/myotube present in a 20x image field. Six image fields were counted for each time point. The average number of myotubes per field at each time point is represented by bars. (E) Graph of myotube formation over a 30-h period measured by the average number of apME-NLS::GFP positive myotubes present in six 20x image fields. Eight hours after plating, media was changed and parallel cultures were control treated (DMSO), or treated with the microtubule depolymerization drug nocodazole at two different concentrations (10 nM or 100 nM). (F) Quantification of the average number of nuclei per myotube (blue line) in cultures treated with 100 nM nocodazole over a 48-h period measured by the average number of apME-NLS::GFP-positive nuclei/myotube present in a 20x image field. Six image fields were counted for each time point. The average number of myotubes per field at each time point is represented by bars.
Having established that the early events of myoblast fusion in primary culture are analogous to those observed in embryos, we set out to perturb myotube formation and myoblast fusion by treating primary cultures with drugs. Recognizing that the actin cytoskeleton and actin regulators are integral components of the fusion process, we grew primary cells in the presence of the actin polymerization inhibitor latrunculin B.15,19,21–26 We performed a time course and quantified the number of myotubes in primary cultures that were treated with two different concentrations of latrunculin B 8 hours after plating (Fig. 2C). We found that fusion was severely decreased in cultures treated with the drug and that fusion was reduced to a greater extent in the cultures treated with a higher concentration of drug.
To further characterize this reduction in fusion, we counted the number of nuclei per myotube at time points from 8 to 48 hours after plating (Fig. 2D). The mean number of nuclei/myotube was 3.7 in cultures treated with 250 nM of latrunculin B and 3.5 in cultures treated with 50 nM latrunculin B (p = 0.25), numbers which are not significantly different from the 3.7 nuclei/myotube observed in untreated controls (Fig. 1D). The total number of myotubes in the drug-treated culture increases only slightly, if at all, between 8 hours and 48 hours after plating; we do not observe any significant change to the average number of nuclei/myotube during this time period. These data indicated that drug treatment beginning 8 hours after plating prevented the formation of new myotubes, as well as additional rounds of myoblast fusion to existing myotubes. Moreover, any myotubes that formed prior to the drug treatment at 8 hours post-plating remained intact, and disruption of the actin cytoskeleton with these drug concentrations had no effect on the viability of these myotubes, their morphology, or their attachment to the coverslip (Fig. 2C and data not shown). This disruption of fusion in primary cells was consistent with the known role for actin during fusion in embryos. In addition, these data indicated that the primary cell culture system is suitable for drug treatment experiments aimed at understanding the mechanisms of myoblast fusion.
Prior work in mammalian C2C12 cells has implicated the microtubule-binding proteins EB1 and EB3 in myoblast fusion;27,28 we therefore tested whether microtubules were essential for myoblast fusion in Drosophila cells. The role of microtubules in fusion has not been examined in Drosophila because embryos completely lacking tubulin do not survive long enough to make muscle. Therefore the ability to transiently and reversibly interfere with microtubules in primary culture is an ideal system to determine whether microtubules have a function during fusion. Eight hours after plating, we treated cells with the microtubule depolymerization drug nocodazole. By performing a time course analysis of myotube formation, we observed that treatment with nocodazole resulted in a reduction of fusion and myotube formation (Fig. 2E), and that an increased concentration of drug in the media further decreased fusion. We counted the average number of GFP-positive nuclei per myotube in cultures treated with 100 nM nocodazole, and found that the mean number of nuclei per myotube was 4.0 (sd ± 0.3), which was not significantly different (p = 0.44) from untreated cells (3.7, sd ± 0.4) (Fig. 2F). Similar to treatment with latrunculin B, the average number of nuclei per myotube did not change over time. While our data, thus far, do not pinpoint which step(s) in the fusion process require microtubules, these results provide the first evidence that microtubule integrity is important for myoblast fusion in Drosophila.
An additional aspect of muscle differentiation in Drosophila embryos is the adoption of specific muscle identities, the result of the expression of particular factors known as FC identity genes. These identity genes are transcriptional regulators that are expressed in incompletely overlapping subsets of FCs and muscles, and are thought to determine final muscle properties such as size, shape and orientation.1–3 The earliest identity gene expression can be detected in stage 10 embryos (4.5–5.5 hours), and expression of some identity genes continues in the developing myotube and final muscle. To determine whether specific muscle identities are established and maintained in primary cell culture, we examined expression of five such identity genes: Krüppel (Kr), apterous (ap), slouch (slou), Muscle segment homeobox Msh) and even-skipped (eve) (Fig. 3A–D and data not shown).29–33 We detected myotubes expressing Kr, Slou, Msh or Eve proteins, as well as myotubes expressing the ap promoter-driven transgene, apME-NLS::dsRed. Since identity genes are expressed in incompletely overlapping patterns within the 30 muscles per hemisegment in the embryo, we expected to detect expression of single identity genes in myotubes as well as overlapping expression.1–3,34 As predicted, we observe myotubes that co-express Msh and apME-NLS::dsRed in myotubes (Fig. 3E), as well as co-expression of Kr and apME-NLS::dsRed (data not shown). We therefore conclude that aspects of muscle identity are established and maintained in our primary culture system.
Discussion
In this work we have characterized the early steps of muscle fiber differentiation in a Drosophila primary cell culture system. We have shown that both fusion proteins as well as Founder Cell identity genes are expressed in multinucleate myotubes grown in culture. Furthermore, we have treated primary myoblast cultures with drugs to disrupt the actin and microtubule cytoskeletons and have demonstrated the requirement of these networks for fusion in culture. While prior work with primary cell culture has looked later in myogenesis at the differentiation steps of sarcomere formation and maintenance,9,11 this work establishes primary myoblast cultures as a system in which the early developmental steps of myoblast fusion and specification can be studied.
We have characterized fusion events beginning at 8 hours post-plating. Since primary cells are dissociated from embryos long before fusion would occur, we did not expect any fusion events at the time of plating. It is clear from our results, however, that a limited amount of fusion takes place in the initial 8 hours following plating of the cells while the cells are in suspension. We are unable to examine primary cultures at earlier time points since cells do not securely adhere to the glass coverslips prior to 8 hours. Interestingly, although our protocol dissociates embryos approximately 2 hours earlier than Bai and colleagues, we observe the same numbers of fusion events over time as they do.11 This similarity is most likely due to the fact that no matter how early cells are dissociated, they require 8 hours after plating to recover from embryonic dissociation, adhere to the plate, and begin fusing with their neighbors. The majority of fusion events are concentrated between 12 and 30 hours post-plating. We therefore have selected 24 hours post-plating as an optimal time period to observe the process of myoblast fusion.
Similar to Bai et al.11 we observe that the average number of nuclei/myotube is between three and four, and this number remains consistent over time. As has been previously noted, this number of nuclei/myotube is fewer than what is observed for most muscles in the embryo. If we examine the distribution of nuclear number in culture (that is, how many myotubes at a particular time point have a certain number of nuclei/myotube), we find that the absolute number of myotubes containing greater than five nuclei increases over time. Interestingly, though, the percentage of myotubes with greater than five nuclei does not change between 24 and 48 hours, because the number of myotubes in all categories increases over the same time period. These data suggest that FCMs in culture are equally as likely to fuse with a single-nucleated FC as with a growing multinucleate myotube. We hypothesize that reduced nuclear number is a result of a limited number of FCMs being in close enough spatial proximity to fuse with FCs and nascent myotubes, a limitation resulting from the cell density required to distinguish individual myotubes in the cell field.
Primary cell culture provides unique experimental advantages compared to intact Drosophila embryos. One advantage of a cell culture system is the ability to treat cells with drugs or other small molecules. Previous studies have shown that dsRNA treatment of primary myoblasts is not a viable approach for early studies of fusion and specification, since several hours of serum starvation followed by a day-long incubation with dsRNA are required.11 An alternative method is the preparation of primary cultures from embryos derived from crosses between a mesodermal Gal4 enhancer trap stock and UAS-RNAi fly stocks, a technique we will attempt in the future. In this study, we have demonstrated the feasibility of treating primary myoblasts with the drugs latrunculin B and nocodazole. While we have shown that the actin and microtubule networks are important for myoblast fusion in primary culture, further characterization will be required to pinpoint the exact role these cytoskeletal components play in the fusion process. Further experiments will be required to determine which aspect(s) of the fusion process in culture (i.e., migration, adhesion and membrane breakdown) are blocked by drug treatment. In particular, though actin has been previously shown to be important for myoblast fusion in Drosophila embryos, this study provides the first indication that the microtubule network is vital for fusion in this system. This result raises many questions, such as whether microtubules are enriched at the site of fusion, like actin is, or whether known fusion mutants disrupt the microtubule network. We are eager to image the actin and microtubule cytoskeletal networks in mutant primary cells by taking advantage of fluorescently-marked balancer chromosomes and embryo sorting prior to the culture protocol.
Another benefit of studying primary myoblasts in culture is the ease of imaging provided by growing myoblasts on glass coverslips, since muscles in intact embryos are 30–150 microns below the embryo surface. We have been able to obtain detailed images of myotubes in culture and plan to use this system to perform time-lapse confocal imaging of the fusion process in culture, as well as high-resolution three-dimensional imaging of the site of fusion. One caveat of this two-dimensional growth environment is how well the cellular context in vitro approximates the in vivo system, and whether cellular processes and behaviors observed in culture can be extrapolated to the embryo. Cell culture preparation of necessity disrupts the cellular environment, displacing mesodermal cells from their overlying epidermis, and disturbing the extracellular matrix. These primary cultures represent a mixed population of cells containing epidermal and neuronal cells as well as myoblasts, in juxtaposition. We are therefore encouraged to find in this study that cell fusion proteins are similarly localized to the site of fusion in culture, and that an actin focus of similar size forms as it does in embryos.
Cellular context plays an important role in the adoption of particular muscle identities, since it is known that signaling between cells in the mesoderm, as well as signaling from the overlying ectodermal cells, are both necessary for proper specification.35,36 Specific identity genes are expressed in spatially delineated patterns under the control of Hox genes, as well as Wingless, Decapentaplegic, RTK-RAS-MAPK and Notch signaling pathways.1,35,37–46 It was thus important to establish that myoblasts in culture could adopt particular fates in the absence of this context, and that embryo dissociation did not result in a homogenous population of myoblasts. We have shown that myotubes in culture express at least five different identity genes, in incompletely overlapping patterns, similar to what is observed in the embryo. At the time of the cell preparation, our embryos are between 3 hours 15 minutes old and 4 hours 15 minutes old. This time window would put the embryos at roughly stage 9, prior to the formation of Lethal of scute-expressing equivalence groups.40 We were intrigued by this result, since it suggested that cells in culture can adopt distinct fates despite being prepared from embryos at a time in which FCs have not been obviously specified and identity genes are not yet expressed in the mesoderm. This finding may therefore provide additional insight to how spatial awareness and muscle identity are established in the embryo, by suggesting that muscle identity could be specified at a time prior to embryonic dissociation. Our experiments cannot distinguish whether the information needed to direct fate is imparted to the myoblast prior to dissociation, or whether this information is transmitted from the surrounding cells in culture. In either case, these results support a model whereby the spatial information necessary to direct myoblast identity fate decisions is in place within the embryo prior to the onset of FC specification.
The experiments described herein have laid the groundwork for subsequent studies of myoblast fusion and myotube differentiation using primary myoblast cultures. The success of our drug treatment experiments has been twofold: in addition to having shown for the first time that microtubules are important for myoblast fusion, we have developed a system that can be used for large-scale small molecule and chemical genetic screens to further our understanding of fusion. Similar screens can also be performed to look for factors that alter muscle identity in culture. Taken together, our data validate the cell culture system for the examination of early muscle developmental events and provide a platform for future work.
Materials and Methods
Drosophila genetics.
Drosophila stocks were grown on standard cornmeal medium at 25°C. Fly stocks used were: twipromoter- actin-GFP, apME-NLS::GFP,19 and apME-NLS::dsRed. The apME-NLS::dsRed and apME-NLS::GFP19 transgenes are expressed predominantly in the lateral transverse (LT) muscles 1–4 in the embryo.
Cell culture.
Primary cultures of Drosophila embryonic cells were made using a modified homogenization protocol.7,8,10–12 Flies in laying pots were maintained at 25°C on a 12 hour light/12 hour dark cycle in an incubator and fed with yeast paste spread on apple juice agar plates. After a 1-hour pre-lay, flies were allowed to lay for 1 hour; these agar plates were removed and aged for an additional 3 hours and 15 minutes. The embryos were then collected and dechorionated for 5 minutes in 50% bleach. Subsequently, embryos were rinsed in 80% ethanol and transferred to the tissue culture hood, where they were rinsed three times in modified Schneider's media (pH 6.9 and supplemented with 1 mU/ml insulin, 1% penicillin/streptomycin and 0.005 mg/ml of gentamicin unless otherwise specified) containing 2% fetal bovine serum (FBS).12 Embryos were dounce homogenized in modified Schneider's media with 20% FBS and 3 mM EGTA, pelleted in a microcentrifuge at 5,000 rpm for 5 minutes at room temperature and resuspended in unsupplemented modified Schneider's media with no serum, containing 0.01% trypsin. Cells were trypsinized for 5 minutes, passed through a filter-topped FACS tube, and then rinsed with modified Schneider's media containing 20% FBS. Cells were pelleted again and resuspended in 20% modified Schneider's media and plated on autoclaved glass coverslips inside the wells of 24-well cell culture plates at a concentration of 1 × 106 cells/ml and a plating density of 2.5 × 105 cells/cm2. Cell cultures were maintained at 18°C for the indicated lengths of time.
Immunohistochemistry.
Cells were fixed in 4% paraformaldehyde/PBS for all immunohistochemistry. Antibodies were preabsorbed (PA) 1:10 against fixed wild-type embryos where stated. Antibody dilutions used were: rabbit anti-Myosin Heavy Chain (1:10,000, gift of D. Kiehart),47 rabbit anti-Rols/Ants (1:3,000; PA; gift of E. Chen),48 rabbit anti-Blown Fuse (1:500; PA 1:20; gift or R. Renkawitz-Pohl),14 mouse anti-GFP (1:200; PA; Clontech), guinea pig anti-Kruppel (1:2,000; PA; gift of J. Reinitz),49 rat anti-Slouch (1:200; PA 1:10),40 and rabbit anti-Muscle segment homeobox (1:500; gift of C. Doe).50 Alexa488, Alexa555 and Alexa647 conjugated secondary antibodies were used (1:400; Invitrogen). Alexa647 conjugated phalloidin was used to visualize F-actin (1:100; Invitrogen) and DAPI (0.5 µg/ml; Sigma) or Hoechst 33258 (1 ng/ml; Invitrogen) were used to visualize nuclei as indicated. Cells were mounted in ProLong Gold antifade reagent (Invitrogen).
Quantification of myotubes.
Initial characterization of our cultured cells revealed that when multiple nuclei (visualized by DAPI staining or expressing apME-NLS::GFP) were detected within a single cell (visualized by phalloidin-labeled cortical actin), these cells also expressed myosin heavy chain, indicating that these cells were differentiated myotubes. A myotube was therefore counted as any multinucleate cell with greater than or equal to two nuclei contained within a continuous cortical actin belt visualized using phalloidin or actin-GFP. Each multinucleate cell was only counted it if was not in contact with any other cells or cell clusters. For each experimental condition and time point, a minimum of six 20x image fields were quantified, and fields were sampled from six areas on each coverslip. Similar locations were sampled on each coverslip.
Confocal imaging.
Fluorescent images were acquired on either a Zeiss LSM 510 confocal microscope with a 63x 1.2 NA C-Apochromat water objective (Fig. 1) or a Leica SP5 confocal microscope with a 100x 1.46 NA ProApochromat oil objective (Figs. 2 and 3). Pinholes were set to capture an optical slice of 1.0 airy unit (AU). Lasers (405 nm, 488 nm, 543 nm and 633 nm) were used to excite the fluorochromes. Images were processed using Improvision Volocity software 5 (Perkin Elmer) and Adobe Photoshop CS4.
Drug treatment.
Latrunculin B (Calbiochem), solubilized in ethanol or nocodazole (Sigma), solubilized in DMSO, were added 8 hours after cells had been plated by removing the 20% FBS modified Schneider's media and replacing it with media containing the specified concentration of drug.
Acknowledgements
We would like to thank J. Botas, D. Kiehart, E. Chen, R. Renkawitz-Pohl, C. Doe and J. Reinitz for generously sharing fly stocks, reagents and antibodies. Additionally, we would like to thank members of the Baylies lab for assistance and helpful discussions, and in particular, E. Folker for comments on the manuscript. This research was supported by NIH grants GM056989 and GM078318 to Mary K. Baylies, NIH Kirschstein-NRSA post-doctoral fellowship F32AR057290 to Krista C. Dobi and NIH Training grant T32 GM008539 to Thomas Metzger.
Abbreviations
- FC
founder cell
- FCM
fusion competent myoblast
- Rols/Ants
Rolling pebbles/Antisocial
- blow
blown-fuse
- Msh
Muscle segment homeobox
- Kr
Krüppel
- ap
apterous
- twi
twist
- S59/slou
slouch
- eve
even-skipped
- MHC
myosin heavy chain
- FBS
fetal bovine serum
References
- 1.Baylies MK, Bate M, Ruiz-Gomez M. Myogenesis: A view from Drosophila. Cell. 1998;93:921–927. doi: 10.1016/s0092-8674(00)81198-8. [DOI] [PubMed] [Google Scholar]
- 2.Beckett K, Baylies MK. The development of the Drosophila larval body wall muscles. Int Rev Neurobiol. 2006;75:55–70. doi: 10.1016/S0074-7742(06)75003-6. [DOI] [PubMed] [Google Scholar]
- 3.Frasch M. Controls in patterning and diversification of somatic muscles during Drosophila embryogenesis. Curr Opin Genet Dev. 1999;9:522–529. doi: 10.1016/s0959-437x(99)00014-3. [DOI] [PubMed] [Google Scholar]
- 4.Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: when it takes more to make one. Dev Biol. 2010;341:66–83. doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haralalka S, Abmayr SM. Myoblast fusion in Drosophila. Exp Cell Res. 2010;316:3007–3013. doi: 10.1016/j.yexcr.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schejter ED, Baylies MK. Born to run: creating the muscle fiber. Curr Opin Cell Bio. 22:566–574. doi: 10.1016/j.ceb.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein SI, Fyrberg EA, Donady JJ. Isolation and partial characterization of Drosophila myoblasts from primary cultures of embryonic cells. J Cell Biol. 1978;78:856–865. doi: 10.1083/jcb.78.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seecof RL, Gerson I, Donady JJ, Teplitz RL. Drosophilia myogenesis in vitro: the genesis of “small” myocytes and myotubes. Dev Biol. 1973;35:250–261. doi: 10.1016/0012-1606(73)90022-5. [DOI] [PubMed] [Google Scholar]
- 9.Bai J, Hartwig JH, Perrimon N. SALS, a WH2-domain-containing protein, promotes sarcomeric actin filament elongation from pointed ends during Drosophila muscle growth. Dev Cell. 2007;13:828–842. doi: 10.1016/j.devcel.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Bai J, Sepp KJ, Perrimon N. Culture of Drosophila primary cells dissociated from gastrula embryos and their use in RNAi screening. Nat Protoc. 2009;4:1502–1512. doi: 10.1038/nprot.2009.147. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Binari R, Ni JQ, Vijayakanthan M, Li HS, Perrimon N. RNA interference screening in Drosophila primary cells for genes involved in muscle assembly and maintenance. Development. 2008;135:1439–1449. doi: 10.1242/dev.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seecof RL, Teplitz RL, Gerson I, Ikeda K, Donady J. Differentiation of neuromuscular junctions in cultures of embryonic Drosophila cells. Proc Natl Acad Sci USA. 1972;69:566–570. doi: 10.1073/pnas.69.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen E, Olson E. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev Cell. 2001;1:705–715. doi: 10.1016/s1534-5807(01)00084-3. [DOI] [PubMed] [Google Scholar]
- 14.Doberstein S, Fetter R, Mehta A, Goodman C. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, et al. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Menon S, Chia W. Drosophila Rolling pebbles: a multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast atractant Dumbfounded. Dev Cell. 2001;1:691–703. doi: 10.1016/s1534-5807(01)00075-2. [DOI] [PubMed] [Google Scholar]
- 17.Menon SD, Osman Z, Chenchill K, Chia W. A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J Cell Biol. 2005;169:909–920. doi: 10.1083/jcb.200501126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rau A, Buttgereit D, Holz A, Fetter R, Doberstein SK, Paululat A, et al. rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development. 2001;128:5061–5073. doi: 10.1242/dev.128.24.5061. [DOI] [PubMed] [Google Scholar]
- 19.Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroter RH, Lier S, Holz A, Bogdan S, Klambt C, Beck L, et al. kette and blown fuse interact genetically during the second fusion step of myogenesis in Drosophila. Development. 2004;131:4501–4509. doi: 10.1242/dev.01309. [DOI] [PubMed] [Google Scholar]
- 21.Kesper DA, Stute C, Buttgereit D, Kreiskother N, Vishnu S, Fischbach KF, et al. Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS) Dev Dyn. 2007;236:404–415. doi: 10.1002/dvdy.21035. [DOI] [PubMed] [Google Scholar]
- 22.Berger S, Schafer G, Kesper DA, Holz A, Eriksson T, Palmer RH, et al. WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J Cell Sci. 2008;121:1303–1313. doi: 10.1242/jcs.022269. [DOI] [PubMed] [Google Scholar]
- 23.Gildor B, Massarwa R, Shilo BZ, Schejter ED. The SCAR and WASp nucleation-promoting factors act sequentially to mediate Drosophila myoblast fusion. EMBO Rep. 2009;10:1043–1050. doi: 10.1038/embor.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Geisbrecht ER, Haralalka S, Swanson SK, Florens L, Washburn MP, Abmayr SM. Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev Biol. 2008;314:137–149. doi: 10.1016/j.ydbio.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onel SF, Renkawitz-Pohl R. FuRMAS: triggering myoblast fusion in Drosophila. Dev Dyn. 2009;238:1513–1525. doi: 10.1002/dvdy.21961. [DOI] [PubMed] [Google Scholar]
- 27.Straube A, Merdes A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr Biol. 2007;17:1318–1325. doi: 10.1016/j.cub.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T, Zaal KJ, Sheridan J, Mehta A, Gundersen GG, Ralston E. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J Cell Sci. 2009;122:1401–1409. doi: 10.1242/jcs.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knirr S, Azpiazu N, Frasch M. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development. 1999;126:4525–4535. doi: 10.1242/dev.126.20.4525. [DOI] [PubMed] [Google Scholar]
- 30.Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4:2098–2111. doi: 10.1101/gad.4.12a.2098. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Gomez M, Romani S, Hartmann C, Jäckle H, Bate M. Specific muscle identities are regulated by Krüppel during Drosophila embryogenesis. Development. 1997;124:4857–4866. doi: 10.1242/dev.124.17.3407. [DOI] [PubMed] [Google Scholar]
- 32.Bourgouin C, Lundgren SE, Thomas JB. apterous is a Drosophila LIM domain gene required for the development of a subset of embryonic muscles. Neuron. 1992;9:549–561. doi: 10.1016/0896-6273(92)90192-g. [DOI] [PubMed] [Google Scholar]
- 33.Nose A, Isshiki T, Takeichi M. Regional specification of muscle progenitors in Drosophila: the role of the msh homeobox gene. Development. 1998;125:215–223. doi: 10.1242/dev.125.2.215. [DOI] [PubMed] [Google Scholar]
- 34.Tixier V, Bataille L, Jagla K. Diversification of muscle types: recent insights from Drosophila. Exp Cell Res. 2010;316:3019–3027. doi: 10.1016/j.yexcr.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Baylies M, Michelson A. Invertebrate myogenesis: looking back to the future of muscle development. Curr Opin Genet Dev. 2001;11:431–439. doi: 10.1016/s0959-437x(00)00214-8. [DOI] [PubMed] [Google Scholar]
- 36.Furlong EE. Integrating transcriptional and signalling networks during muscle development. Curr Opin Genet Dev. 2004;14:343–350. doi: 10.1016/j.gde.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Baylies MK, Martínez-Arias A, Bate M. wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development. 1995;121:3829–3837. doi: 10.1242/dev.121.11.3829. [DOI] [PubMed] [Google Scholar]
- 38.Halfon MS, Carmena A, Gisselbrecht S, Sackerson CM, Jimenez F, Baylies MK, et al. Ras pathway specificity is determined by the integration of multiple signal-activated and tissue-restricted transcription factors. Cell. 2000;103:63–74. doi: 10.1016/s0092-8674(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 39.Staehling-Hampton K, Hoffman FM, Baylies MK, Rushton E, Bate M. dpp induces mesodermal gene expression in Drosophila. Nature. 1994;372:783–786. doi: 10.1038/372783a0. [DOI] [PubMed] [Google Scholar]
- 40.Carmena A, Bate M, Jiménez F. lethal of scute, a proneural gene, participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev. 1995;9:2373–2383. doi: 10.1101/gad.9.19.2373. [DOI] [PubMed] [Google Scholar]
- 41.Carmena A, Gisselbrecht S, Harrison J, Jimenez F, Michelson A. Combinatorial signaling codes for the progressive determination of cell fates in the Drosophila embryonic mesoderm. Genes Dev. 1998;15:3910–3922. doi: 10.1101/gad.12.24.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azpiazu N, Lawrence PA, Vincent JP, Frasch M. Segmentation and specification of the Drosophila mesoderm. Genes Dev. 1996;10:3183–3194. doi: 10.1101/gad.10.24.3183. [DOI] [PubMed] [Google Scholar]
- 43.Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- 44.Riechmann V, Irion U, Wilson R, Grosskortenhaus R, Leptin M. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124:2915–2922. doi: 10.1242/dev.124.15.2915. [DOI] [PubMed] [Google Scholar]
- 45.Capovilla M, Kambris Z, Botas J. Direct regulation of the muscle-identity gene apterous by a Hox protein in the somatic mesoderm. Development. 2001;128:1221–1230. doi: 10.1242/dev.128.8.1221. [DOI] [PubMed] [Google Scholar]
- 46.Baker R, Schubiger G. Ectoderm induces muscle-specific gene expression in Drosophila embryos. Development. 1995;121:1387–1398. doi: 10.1242/dev.121.5.1387. [DOI] [PubMed] [Google Scholar]
- 47.Kiehart DP, Feghali R. Cytoplasmic myosin from Drosophila melanogaster. J Cell Biol. 1986;103:1517–1525. doi: 10.1083/jcb.103.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN. Control of myoblast fusion by a guanine nucleotide exchange factor, loner and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- 49.Kosman D, Small S, Reinitz J. Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Genes Evol. 1998;208:290–294. doi: 10.1007/s004270050184. [DOI] [PubMed] [Google Scholar]
- 50.Jagla T, Bellard F, Lutz Y, Dretzen G, Bellard M, Jagla K. ladybird determines cell fate decisions during diversification of Drosophila somatic muscles. Development. 1998;125:3699–3708. doi: 10.1242/dev.125.18.3699. [DOI] [PubMed] [Google Scholar]