Abstract
Drosophila embryonic hemocytes have emerged as a potent system to analyse the roles of key regulators of the actin and microtubule cytoskeletons live and in an in vivo context (Table 1 and references therein). The relative ease with which live imaging can be used to visualize the invasive migrations of these highly motile macrophages and their responses to wound and chemoattractant signals make them a particularly appropriate and genetically tractable cell type to study in relation to pathological conditions such as cancer metastasis and inflammation.1–3 In order to understand how signaling pathways are integrated for a coordinated response, a question with direct relevance to autoimmune dysfunction, we have sought to more fully characterize the inputs these cells receive in vivo over the course of their developmental dispersal. These studies have recently revealed that hemocyte migration is intimately associated with the development of the ventral nerve cord (VNC ), a structure used by hemocytes to disperse over the embryo that itself requires this association for its correct morphogenesis. Crucially the VNC must separate from the epidermis to create a channel for hemocyte migration, revealing how constriction of extracellular space can be used to control cell migration in vivo.4
Key words: Drosophila, development, migration, hemocyte, ventral nerve cord, Slit
slit and robo are Required for the Developmental Dispersal of Hemocytes
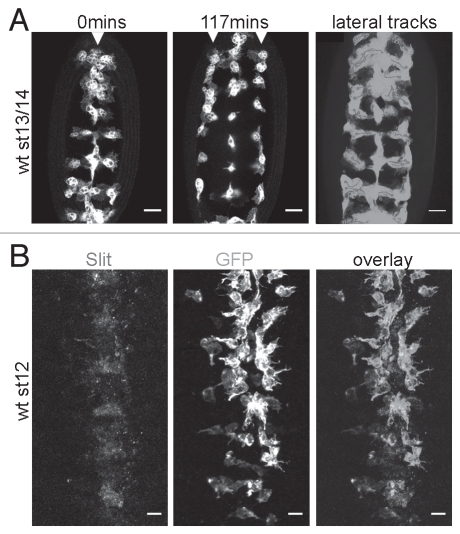
It has long been recognized that hemocytes require trophic stimulation by Pdgf/Vegf-related ligands (Pvfs) to activate the Pdgf/Vegf-related receptor Pvr for their survival and that these cues are also used as guidance signals to direct hemocytes along their highly stereotyped migratory routes through the developing embryo.5–7 However, the identity of other instructive cues remains unknown, for example that of the cues that pattern the characteristic ‘lateral migration’ of hemocytes from the midline to the edges of the VNC (Fig. 1A). Given the repulsive role of Slit in vertebrate leukocyte migration8 and its expression along the ventral midline (Fig. 1B), we examined whether it might act similarly on Drosophila hemocytes and drive their migration laterally.
Figure 1.
Lateral migration of hemocytes away from the ventral midline. (A) Live movies of the ventral surface of stage 13/14 embryos revealed that GFP-labelled wild type hemocytes migrate from the ventral midline (arrowhead, first part) to the edges of the ventral nerve cord (arrowheads, second part) in a highly directional manner; third part shows tracks of hemocytes as they migrate laterally (purple; dots mark where tracking ceased) superimposed over a projection showing every timepoint from a timelapse movie (green). (B) Co-immunostaining wild type embryos with GFP-labelled hemocytes for Slit (C555.6D anti-Slit from DHSB—first part; red in overlay) and GFP (anti-GFP from Abcam—second part; green in overlay) showed that Slit, expressed along the ventral midline, could potentially drive lateral migration of hemocytes. Scale bars represent 20 µm; see Evans et al.2 for methodology and fly lines used.
Surprisingly we found that both Slit and its receptors Robo and Robo2 were actually required for migration along the VNC,4 suggesting an attractive role with respect to hemocyte migration (compare the position of hemocytes in Fig. 2A and B).4 However, misexpression of Slit within hemocytes failed to cause retention of hemocytes in the embryonic head, which was observed when Pvf2 was similarly misexpressed. Likewise hemocyte-specific expression of Comm, a negative regulator of the Robo receptors, did not affect progression along the ventral midline, therefore it seemed that neither slit nor robo were required autonomously by hemocytes.
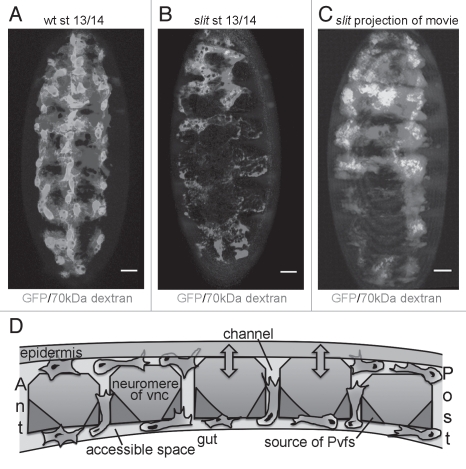
Figure 2.
Slit controls separation of the VNC from the epidermis, indirectly regulating hemocyte migration. (A–C) 70 kDa rhodamine-labelled dextran (red) was injected into live wild type and slitGA178 null mutant embryos with GFP-labelled hemocytes (green) to reveal extracellular spaces accessible to these migrating blood cells. (A) The VNC and epidermis separate in wild type embryos creating a channel for hemocytes to migrate along. (B) In slit mutants these structures fail to separate creating physical barriers to hemocyte movement along the ventral midline; note how dextran fails to pool ventrally. (C) Projection showing all time points from a movie of a slit mutant embryo; hemocytes remain tightly constricted to fluid-accessible regions and are unable to penetrate the physical barriers caused by the failure of the VNC and epidermis to separate. (D) Longitudinal schematic through the VNC depicting a model for hemocyte migration along this structure. Hemocytes (green) reach the VNC at the anterior (ant) from the head and at the posterior (post) having entered the extended germ band. These migrations must be coordinated with the production of the Pvfs (red) and the separation (arrows) of the VNC (blue) from the overlying epidermis (grey) to create a channel for hemocytes to enter, contact the Pvfs and subsequently progress along this migratory substrate. Hemocytes can reside in and move between the ventral and dorsal surfaces of the VNC using dorsoventral channels between neuromeres, which may facilitate the diffusion of the Pvfs or other regulatory molecules (pink). Scale bars represent 20 µm; see Evans et al.2 for methodology and fly lines used.
Interdependence of Hemocyte Migration and Ventral Nerve Cord Development
The dispersal of hemocytes is crucial for the correct morphogenesis of the embryo,9–12 in particular the patterning of the VNC, a process in which Slit is a key molecule.13 In turn the VNC is used by hemocytes as a migratory substrate to disperse over the embryo. We wondered if the perturbed VNC observed in slit mutants might be responsible for the defects in hemocyte dispersal, since Pvf expression was retained in these mutants.4 Normally, the VNC separates from the epidermis as hemocytes migrate out from the head, however by injecting fluorescently labelled dextran we found that the channel normally created by this morphogenetic movement was highly constricted in slit mutants, forming a physical barrier to hemocytes (Fig. 2A and B).4 Hemocytes remained tightly constrained within areas that are accessible to the injected dye (Fig. 2C).4 Unlike the entry of hemocytes into the germ band through an epithelial barrier,2 hemocytes themselves did not appear to facilitate this separation, as it still occurred when they were artificially retained in the head via expression of dominant negative Rac.4
Thus Slit-Robo signaling enables the separation of the VNC and epidermis at the precise time that hemocytes exit the head and the attractive Pvf ligands are expressed along their future routeways. If these carefully integrated events go awry and hemocytes fail to cover the VNC, then the morphogenesis of the VNC is perturbed, owing to their role in removal of apoptotic corpses.12,14 Hemocyte-derived matrix may also be crucial for the generation of traction and subsequent condensation of the VNC and formation of the blood-brain barrier. These migrations are vital to the developing embryo and are consequently robustly hard-wired, as hemocytes will even ignore wound signals to follow their pre-determined routes during this period of development.15
It is currently unclear exactly which cells require Slit-Robo signaling to trigger separation of the epidermis and VNC. In one potential model Slit-Robo signaling might control de-adhesion of midline cells from the epidermis. Alternatively, midline cells might fail to migrate appropriately owing to the defective patterning of the axonal scaffold in slit mutants, leading to a failure in separation of epidermis and VNC, with which they are intimately associated. slit genetically interacts with a number of regulators of adhesion such as ena16,17 and neurexin IV18 and crosstalk with the integrins themselves has previously been reported.19 Therefore it is possible that signaling downstream of Robo receptors regulates complex shifts in cell-cell interactions within the VNC.
The interaction of hemocytes with the VNC reiterates an important point for screening and subsequent analysis of candidate genes—that defects in the surrounding tissues can have powerful indirect effects on the behavior of distinct cells. For instance several deficiencies found to disrupt hemocyte dispersal20 cover genes important for VNC development and homozygotes for these deficiencies exhibit clear VNC defects (I. Evans, J. Howes and W. Wood, unpublished data). Nevertheless such screens remain useful, as highlighted by the key role of RhoL in hemocyte penetrance of the germ band epithelium,2 a gene covered by the deficiency Df(2L)by496 previously identified as perturbing hemocyte migration.20
Control of Hemocyte Developmental Dispersal
We are now beginning to be able to draw a more complete picture of how hemocyte developmental migrations are controlled: hemocytes are born in the head and can either reach their VNC substrate by moving ventrally or more invasively by forcing their way through an epithelial barrier to penetrate the extended germ band in a RhoL-dependent fashion.2 Both of these routes and the subsequent movement of hemocytes along the VNC require the activation of Pvr by its Pvf ligands. However Pvf expression alone is insufficient for appropriate migration along the ventral midline as revealed by slit mutants that maintain Pvf expression yet have perturbed hemocyte migration. The study of these mutants revealed hemocyte-independent separation of the VNC and epidermis to be fundamental in creating a void for hemocytes to enter, encounter the Pvfs and move along the ventral midline (Fig. 2D).4 This corralling of hemocytes by attracting them into a restricted extracellular space resembles the migration of vertebrate dendritic cells, which is controlled in a similar fashion, with pre-formed ‘portals’ enabling these cells to enter afferent lymph vessels.21 Similarly some tumor cells migrate invasively by following matrix-remodelling fibroblasts that create a less spatially-constrained environment.22 Therefore it seems that for many cell types restricting available space is an efficient way to ensure that their migration remains ‘on track’.
Once on their VNC substrate hemocytes move along tracks of Pvf ligands expressed by the midline cells (Fig. 2D), which may be tethered to components of the extracellular matrix secreted by preceding hemocytes. Hemocytes might be further inclined to follow the paths of leading cells by binding to the matrix itself as Laminin and integrins have been shown to play an important role in hemocyte migration (Table 1 and references therein). Hemocyte-derived matrix also clearly plays an important role in morphogenesis, for example it was recently shown that Collagen IV deposition potentiates BMP signaling, required for correct formation of the renal tubules,9 whilst hemocyte-specific expression of SPARC was sufficient to rescue many basal lamina-associated defects of SPARC mutants.11
Table 1.
Genes implicated in the migration of embryonic hemocytes
| Gene | Function in hemocytes | Reference |
| pvr | Pdgf/Vegf-related receptor; mediates attraction to areas of Pvf ligand expression and proliferation and survival of hemocytes | Cho, et al.6 Bruckner, et al.5 |
|
pvf2 pvf3 |
Attractive Pdgf/Vegf-related ligands; expressed along the ventral midline (pvf2 and pvf3), dorsal vessel (pvf2) and by the renal tubules (pvf2 and pvf3) to direct hemocyte migration and survival | Cho, et al.6 Bruckner, et al.5 Wood, et al.7 Bunt, et al.9 |
| rho1 | Small GTPase; required for hemocyte migration to wounds but not developmental migration; hemocytes lacking Rho1 fail to retract their tails and are tethered together | Paladi and Tepass20 Stramer, et al.3 |
|
rac1 rac2 mtl |
The three Rac GTPase genes of Drosophila; required for hemocyte dispersal along posterior VNC and migration to wounds; hemocytes lacking Rac function fail to produce their characteristic large lamellipodia | Paladi and Tepass20 Stramer, et al.3 |
| cdc42 | Small GTPase; required for directional migration to wounds and hemocyte polarity; required for migration along entire VNC, most likely due to indirect effects on surrounding tissues | Stramer, et al.3 Paladi and Tepass20 |
| PI3K | Required for migration to epithelial wounds but not developmental migrations* | Wood, et al.7 |
| dizzy | PDZ-GEF; drives the formation of Rap1 and integrin-dependent protrusions and is required for penetration of the germ band | Huelsmann, et al.26 Siekhaus, et al.2 |
| singed | Fascin; required for developmental dispersal of hemocytes along the posterior of the VNC; hemocytes in sn mutants lack polarity with their lamellipodia extending around the entire cell | Zanet, et al.27 |
| lanB1 | No laminin trimers can be made in lanB1 mutants; laminin, a component of the extracellular matrix, is required for developmental dispersal of hemocytes along the entire VNC | Urbano, et al.28 |
| duox | Dual Oxidase; produces the chemotactic cue H2O2 at wounds, which is required to attract hemocytes | Moreira, et al.15 |
| orbit | Clasp homolog; microtubule binding protein required to stabilize a microtubule ‘arm’ within hemocytes that is required for cell-cell repulsion and polarity | Stramer, et al.25 |
| slit | Repulsive matrix component; required for the correct formation of the VNC and its separation from the epidermis, creating a channel for hemocytes to migrate ventrally | Evans, et al.4 |
|
robo robo2 |
Receptors for Slit; required for the correct formation of the VNC and its separation from the epidermis, creating a channel for hemocytes to migrate ventrally | Evans, et al.4 |
| sim | Transcription factor required for midline identity; sim mutants lack the expression of Pvf ligands along the VNC, which remains intercalated with the epidermis. Hemocyte migration routes through and along the VNC are therefore blocked and attractants are absent | Cho, et al.6 Evans, et al.4 |
| rhoL | Small GTPase; required for developmental dispersal; rhoL mutant hemocytes cannot penetrate an epithelial barrier and fail to enter the extended germ band | Siekhaus, et al.2 |
| inflated | α-integrin subunit; required for penetration of the germ band | Siekhaus, et al.2 |
| ena | Sole Ena/VASP family member in Drosophila; enhances migration speeds during developmental dispersal and to epithelial wounds and drives the formation of filopodia | Tucker, et al.24 |
The PI3K inhibitor LY294002 and expression of a dominant negative version of the Pi3K92E/Dp110 catalytic subunit were used to show requirement of PI3K function
The migration of hemocytes is driven by the actin cytoskeleton as witnessed by the importance of the Rho GTPase family in these cells (Table 1), with the formation and/or stabilization of actin-rich lamellipodia and filopodia (the machinery of hemocyte migration) presumably occurring downstream of Pvr signaling. Exactly how this is achieved and the full complement of downstream effectors is yet to be characterized but it is interesting to note that, unlike border cells,23 another cell type that uses Pvr to migrate, myoblast city, a DOCK180/ced-5 homolog and component of a Rac GEF complex with ELMO/ced-12, does not appear essential for hemocyte responses to Pvfs.20 Interestingly, despite the similarity between Pvr and the Pdgf receptor tyrosine kinases, PI3K activity is not required for hemocyte developmental migrations but is necessary for migration to epithelial wounds.7 One important regulator of actin dynamics in both of these settings is Ena, which binds the barbed ends of actin filaments and seems to accelerate hemocyte migration,24 although it is yet to be determined whether Ena is actively targeted downstream of any guidance molecules that regulate hemocyte migration. Microtubules have also recently been shown to play an important role in migration, with the microtubule-binding protein Orbit/Clasp facilitating their bundling into an ‘arm-like’ structure that is important for maintaining polarity.25
Later in development hemocytes leave the ventral midline and migrate laterally following downregulation of the Pvfs.7 The highly directional nature of this migration suggests a lateral cue but the reduction in constriction following greater separation of VNC and epidermis4 and the cell-cell repulsion exhibited by hemocytes at this stage of development25 may also help to drive this characteristic migration. It is possible that this separation actually leads to the formation of ‘lateral channels’ that help shape lateral migration in much the same way as hemocytes are channeled along the midline earlier in development; alternatively separation may unmask contactdependent cues at the lateral edges of the VNC or simply remove a physical barrier. Further work will reveal how directional cues, cell-cell repulsion events and physical barriers are coordinated to orchestrate this beautifully choreographed migration of blood cells in vivo.
Acknowledgements
This work was funded by a Wellcome Trust Career Development Fellowship to Will Wood, at the University of Bath. We thank Adrian Rogers and the Bioimaging suite at the University of Bath for technical assistance and Philippa Tucker and Kiri Tan for feedback on the manuscript.
Abbreviations
- VNC
ventral nerve cord
Extra View to: Ev H, Wood W. Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos. Development. 2010;137:1625–1633. doi: 10.1242/dev.046797.
References
- 1.Evans IR, Zanet J, Wood W. Stramer BM, Live imaging of Drosophila melanogaster embryonic hemocyte migrations. J Vis Exp. 2010 doi: 10.3791/1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siekhaus D, Haesemeyer M, Moffitt O, Lehmann R. RhoL controls invasion and Rap1 localization during immune cell transmigration in Drosophila. Nat Cell Biol. 2010;12:605–610. doi: 10.1038/ncb2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, et al. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans IR, Hu N, Skaer H, Wood W. Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos. Development. 2010;137:1625–1633. doi: 10.1242/dev.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, et al. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- 7.Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defaye A, Evans I, Crozatier M, Wood W, Lemaitre B, Leulier F. Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J Innate Immun. 2009;1:322–334. doi: 10.1159/000210264. [DOI] [PubMed] [Google Scholar]
- 11.Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- 12.Olofsson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 14.Sears HC, Kennedy CJ, Garrity PA. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130:3557–3565. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- 15.Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 17.Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J Cell Sci. 2009;122:4363–4374. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S, Blauth K, Peters K, Rogers SL, Fanning AS, Bhat MA. Drosophila neurexin IV interacts with Roundabout and is required for repulsive midline axon guidance. J Neurosci. 2010;30:5653–5667. doi: 10.1523/JNEUROSCI.6187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens A, Jacobs JR. Integrins regulate responsiveness to slit repellent signals. J Neurosci. 2002;22:4448–4455. doi: 10.1523/JNEUROSCI.22-11-04448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paladi M, Tepass U, Function o. Function of Rho GTPase in embryonic blood cell migration in Drosophila. J Cell Sci. 2004;117:6313–6326. doi: 10.1242/jcs.01552. [DOI] [PubMed] [Google Scholar]
- 21.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 23.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 24.Tucker P, Evans I, Wood W. DMM. 2010. Ena Regulates the Invasive Migration of Drosophila Macrophages. In Press. [Google Scholar]
- 25.Stramer B, Moreira S, Millard T, Evans I, Huang CY, Sabet O, et al. Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J Cell Biol. 2010;189:681–689. doi: 10.1083/jcb.200912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huelsmann S, Hepper C, Marchese D, Knoll C, Reuter R. The PDZ-GEF dizzy regulates cell shape of migrating macrophages via Rap1 and integrins in the Drosophila embryo. Development. 2006;133:2915–2924. doi: 10.1242/dev.02449. [DOI] [PubMed] [Google Scholar]
- 27.Zanet J, Stramer B, Millard T, Martin P, Payre F, Plaza S. Fascin is required for blood cell migration during Drosophila embryogenesis. Development. 2009;136:2557–2565. doi: 10.1242/dev.036517. [DOI] [PubMed] [Google Scholar]
- 28.Urbano JM, Torgler CN, Molnar C, Tepass U, Lopez-Varea A, Brown NH, et al. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–4176. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]