Abstract
The identification of new proliferation markers could have clinical implications in ovarian carcinoma by stratifying patients for treatment and follow-up. The aim of this study was to evaluate the diagnostic and prognostic value of the proliferation markers Ki-67/MIB-1, phosphorylated histone H3 (PHH3), and survivin in epithelial ovarian tumors. Ninety women with a pelvic mass who underwent surgery at the Department of Gynecological Oncology were included: 68 ovarian carcinomas, 11 borderline tumors, and 11 ovarian cystadenomas. We performed mitotic count and immunohistochemical analyses of Ki-67/MIB-1, PHH3, and survivin, related to clinicopathological parameters. Uni- and multivariate analyses of five-year overall survival were performed. We found statistically significant correlations between mitotic count, Ki-67/MIB-1, PHH3, and survivin. The expression of all proliferation markers was significantly higher in the carcinomas than in the borderline and benign tumors (p<0.05). There was, however an overlap of indices between the different malignancy groups. Women with advanced stage cancers (FIGO stage III and IV) had significantly higher tumor expression of all markers compared to patients with early stage cancers (FIGO stage I and II). Women with advanced disease and complete chemotherapy response had higher Ki67/MIB-1 expression than women without complete chemotherapy response. All markers had an impact on survival in the univariate analyses. In the multivariate analysis, however, only age and stage of disease reached statistical significance as prognostic factors. In conclusion, the proliferation markers Ki-67/MIB-1, PHH3, and survivin are positively correlated with each other and with tumor grade, and may contribute in the identification of aggressive ovarian carcinomas.
Keywords: Ovarian carcinoma, ovary, survival, Ki-67/MIB-1, PHH3, survivin, proliferation, diagnosis
Introduction
Ovarian cancer is the leading cause of death from gynecological malignancies in the Western world, and the highest incidence rates are found in North America and in Northern and Western Europe [1, 2]. The prognosis is poor, with an overall survival rate of about 40% in 5 years [3]. Over 70% of the women diagnosed with ovarian carcinoma have advanced disease at the time of diagnosis [4]. Important prognostic factors include stage of disease, age at diagnosis, histological type and grade, ploidity, and the amount of residual disease after primary surgery [5, 6]. Furthermore, high proliferative activity in the ovarian tumor has been shown to imply a poor prognosis [7, 8].Until now, the heterogeneous group of ovarian carcinomas has been treated with the same chemotherapy regimens [9]. In the future, sub-classification of ovarian carcinomas will be important in order to provide a more tailored therapy for this malignancy. Thus, the cellular proliferation status of a tumor may be a diagnostic, as well as a prognostic tool [8].
Mitotic count is a traditional and practical method to determine proliferative activity, but is hampered by several disturbing factors [10]. Immunohistochemical detection of proliferating cells is an alternative way to determine the proliferative potential of a tumor, and the expression of Ki-67 antigen has become a widely used marker. This antigen is expressed during all active phases of the cell cycle (G1, S, G2, and mitosis), and the monoclonal Ki-67 antibody (MIB-1) reacts with the nuclear Ki-67 antigen expressed in cycling cells [11]. High expression of Ki-67/MIB-1 has been found to indicate a poor prognosis in several cancers, including ovarian cancer [7, 12-17]. On the other hand, due to a wide range in Ki-67 expression between ovarian tumors of the same tumor grade, there is a need for novel proliferation markers.
Histone H3 is one of the five main histone proteins, which together form the major protein constituents of the chromatin in eukaryotic cells. The expression of phosphorylated histone H3 (PHH3) reaches a maximum during mitosis, and PHH3 can therefore be used as a specific mitotic marker [18-22]. High expression of PHH3 implies a poor prognosis in various human malignancies [19, 22]. There are few studies on the diagnostic and prognostic value of PHH3 in ovarian carcinoma. Scott et al. found higher PHH3 expression in serous carcinomas than in serous borderline tumors and cystadenomas [23]. Chen et al. reported higher expression of PHH3 in ovarian carcinomas compared to healthy controls, but did not demonstrate any prognostic value of PHH3 [24].
Survivin is an inhibitor of apoptosis, participating in the regulation of apoptosis and cell division, and is expressed in the G2/M phase of the cell cycle [25]. Higher expression of survivin is described in malignant and borderline ovarian tumors than in benign ovarian tumors [26]. Elevated serum survivin levels have also been described in ovarian carcinomas as compared to benign ovarian tumors [27]. Studies of the prognostic significance of survivin have however, shown conflicting results [25, 26, 28, 29].
The aim of the present study was to compare the proliferation markers Ki-67/MIB-1, PHH3, and survivin in ovarian tumors and to investigate their diagnostic and prognostic value.
Materials and methods
In the period from May 1st 2001 to April 30th 2005, 90 women with a pelvic mass operated on at the Gynecological oncology unit at St.Olavs Hospital, Trondheim University Hospital, Norway, were enrolled. An informed consent was obtained from all participants. Patient charts were reviewed to collect data regarding age at diagnosis, histology, stage of disease according to the guidelines of the International Federation of Gynecology and Obstetrics (FIGO) [30], lymph node status, and follow-up. The amount of residual disease after primary surgery was registered as <1 cm or ≥1 cm. Completeness of staging was evaluated according to the FIGO guidelines, where optimal staging requires infracolic omentectomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, selected lymphadenectomy of the pelvis and para-aortic lymph nodes, and appendectomy for mucinous tumors [30]. Completed chemotherapy was defined as ≥5 courses of a combination of carboplatine and paclitaxel or carboplatine alone. Response to chemotherapy was registered according to the RECIST criteria (Response Evaluation Criteria in Solid Tumours) [31]. All histological slides were reviewed by one pathologist (S.H.T), and classified according to the World Health Organization guidelines by histological type and tumor grade [1]. The study was approved by the Regional Committee for Medical and Health Research Ethics.
Immunohistochemistry
Formalin-fixed and paraffin-embedded sections were reviewed with selection of representative sections for immunohistochemistry. Briefly, 4-mm-thick sections were mounted on Superfrost microscopic slides, de-paraffinized, and dehydrated. Antigen retrieval was performed by pressure cooking. The slides were incubated with the following antibodies: MIB-1antibody (monoclonal Ki-67 antibody, dilution 1:100, 40 min. at room temperature, Dako, Glostrup, DK), PHH3 antibody (rabbit polyclonal Phosphohistone H3 antibody (ser10), dilution 1:2000, 60 min. at room temperature, Millipore, Billerica, MA, USA), and survivin antibody (rabbit monoclonal survivin antibody, dilution 1:250, 40 min. at room temperature, Abcam, Cambridge, UK). An automatized immunohistostainer was applied for the analyses (Dako Techmate 500). Diaminobenzidine was used as chromogene and hematoxylin as counterstain. Tissue samples of tonsils and medulloblastoma were used as positive controls. In the negative controls the primary antibody was omitted.
Evaluation of staining
Mitotic figures were counted on hematoxylin and eosin-stained slides. The number of mitosis was counted in 10 high-power fields (× 400) in the areas with highest mitotic activity.
Determination of proliferative activity by imunohistochemistry was performed quantitatively by counting immunoreactive tumor cells in the most intense staining areas. At least 1000 tumor cells at ×400 magnification were counted. The Ki-67/MIB-1 labeling index (LI) and survivin immunopositivity were defined as the percentage of immunoreactive tumor cells out of the total number of tumor cells. Evaluation of the survivin staining was based on nuclear positivity only. PHH3 immunopositivity was calculated as the number of positive mitotic figures in 10 high-power fields (× 400) in the areas with highest mitotic activity. Only distinct immunoreacitve tumor cell nuclei were counted.
In order to control for inter-observer variation, randomly selected cases were evaluated by two observers (GA, SHT).
Statistical analyses
Statistical analyses were performed in the SPSS statistical software program, version 17.0 (SPSS Inc, Chicago, IL). Figures were made in Graph Pad Prism 5. Correlations between the proliferation markers were analyzed with the Spearman correlation test. Comparison of proliferation marker positivity between carcinomas, borderline and benign ovarian tumors was performed with the Kruskal-Wallis test. Subgroup analyses were performed with the Mann-Whitney U test. In the analysis of histology, only analysis of serous versus non-serous tumors was performed. Analyses related to residual tumor volume were only calculated for stage IIIC-IV cancers (N=39).
The Cox proportional hazard regression model was used to evaluate the effect of explanatory variables on overall survival. Five-year overall survival was calculated from the date of primary surgery, to the date of death or status after five years. Univariate analysis of the prognostic significance of chemotherapy response was only calculated for advanced stage cases with complete chemotherapy, where women with complete response were compared to women with-out complete response. P<0.05 were considered statistically significant.
Results
Sixty-eight women had ovarian carcinoma, 11 women had ovarian borderline tumor, and 11 women had benign cystadenoma. Mean age at diagnosis was 64 years (±11 years) in the carcinoma group, 55 years (±16 years) in the borderline group, and 60 years (±16 years) in the benign group. The borderline group comprised 6 serous, 4 mucinous, and 1 mixed serous/endometroid tumor. The benign group comprised 6 serous and 5 mucinous cystadenomas. The group of carcinomas comprised 34 serous, 4 mucinous, 13 endometroid, 8 clear cell, 7 mixed, and 2 undifferentiated tumors.
Overview over FIGO stage, tumor grade, histological type of the carcinomas, and the results of the immunohistochemical analyses related to clinicopathological parameters are presented in Table 1. The carcinomas comprised 26 early stage cases (23 stage I and 3 stage II) and 42 advanced stage cases (35 stage III and 7 stage IV cases). Of the advanced cases, thirty-nine women had stage IIIc-IV disease. In the malignant cases, standard surgical procedures included total hysterectomy, bilateral adnexectomy, omentectomy, and lymph node sampling. At the end of primary surgery, 57 women had residual tumor volume < 1cm and 11 women had residual tumor volume ≥ 1cm. In total, 46 women (68%) completed first line chemotherapy, in advanced stage 36 (86%). Six women with advanced disease did not complete adjuvant chemotherapy as they died shortly after diagnosis. Of the women with advanced stage disease who completed chemotherapy, 26 (72%) had complete response, six (17%) had partial response, and three (8%) had stable disease after first line chemotherapy. One woman died before response to treatment could be evaluated.
Table 1.
Differences in median mitotic count, Ki-67/MIB-1, PHH3, and survivin immunostainingin ovarian carcinomas related to clinicopathological parameters (N=68)
| Characteristics | N | Mitotic count | Ki-67/MIB | PHH3 | Survivin |
|---|---|---|---|---|---|
| (+) cells/10 HPF | LI | (+) cells/10 HPF | (+) cells % | ||
| Carcinoma | 68 | 21 (2-108) | 36.7 (3.6-70.3) | 48.5 (0-198) | 13.7 (0-34.6) |
| FIGO stage | |||||
| I&II | 26 | 9.5 (2-108)* | 27.2 (7.7-58.1)* | 35 (0-198)* | 7.0 (1.8-27.4)* |
| III&IV | 42 | 28.0 (5-81) | 42.1 (3.6-70.3) | 63.5 (0-125) | 15.3 (0-34.6) |
| Grade†,‡ | |||||
| G1 | 8 | 10 (3-27)* | 30.7 (7.0-58.1)* | 24 (0-43)* | 8.2 (2.3-18.7)* |
| G2&G3 | 52 | 27 (4-108) | 41.6 (3.6-70.3) | 63 (0-198) | 14.5 (0-34.6) |
| Histology | |||||
| Serous | 34 | 15.5 (2-108)* | 41.6 (3.6-70.3) | 65 (0-198)* | 15.5 (0-30.9)* |
| Mucinous | 4 | 13.5 (9-18) | 41.7 (16.2-58.1) | 30.5 (4-38) | 8.6 (3.6-18.7) |
| Endometroid | 13 | 22 (4-56) | 33.0 (10.6-67.5) | 49 (4-96) | 10.5 (3.6-34.6) |
| Clear cell | 8 | 8.5 (2-16) | 24.1 (15.5-45.1) | 9.5 (0-44) | 6.1 (1.8-15.3) |
| Mixed | 7 | 28 (5-108) | 29.6 (7.8-47.8) | 41 (0-110) | 7.5 (1.8-19.9) |
| Undiff. | 2 | 76 (71-81) | 53.3 (50.8-55.8) | 85.5 (56-115) | 21.7 (15.3-28.2) |
LI:Labeling Index; HPF:High Power Fields
Clear cell tumors are not graded
Computed for stage IIIC-IV
Statistically significant differences (p<0.05)
Mitotic count
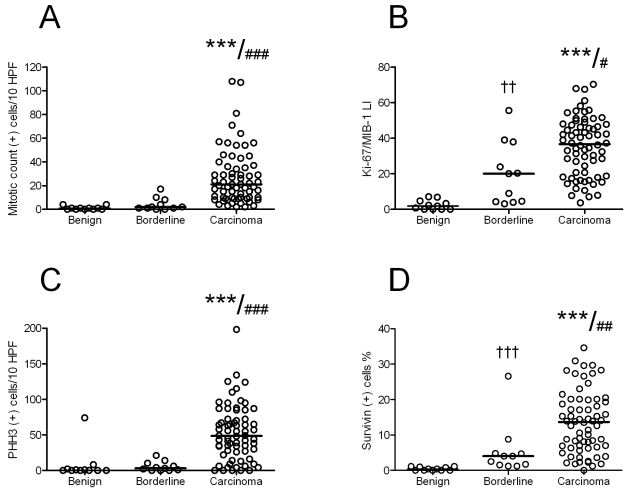
There was a statistically significant difference in the median number of mitosis between carcinomas, borderline tumors, and benign tumors (median (+) cells/10 HPF; 21, 2, and 1, respectively, p<0.001) (Figure 1A).
Figure 1.
Mitotic count (A) and expression of Ki-67/MIB-1 (B), PHH3 (C), and survivin (D) in ovarian carcinomas, borderline, and benign ovarian tumors. ***: Carcinoma vs. benign, p<0.001; ###, ##, #: Carcinoma vs. borderline, p<0.001, <0.01, and <0.05, respectively ; ††, †: Borderline vs. benign, <0.01, and <0.05, respectively. LI: Labeling Index, Line marks median.
We found a significant positive correlation between mitotic count and tumor grade (r=0.622, p<0.001), and between mitotic count and FIGO stage (r=0.590, p<0.001). Serous carcinomas had higher mitotic count than non-serous carcinomas (median (+) cells/10 HPF; 28 and 15.5, respectively, p=0.043) (Table 1).
In advanced disease, women with complete response to first-line chemotherapy had a significantly higher mitotic count as compared to women with only partial response or stable disease after first line chemotherapy (p=0.050).
Immunohistochemistry
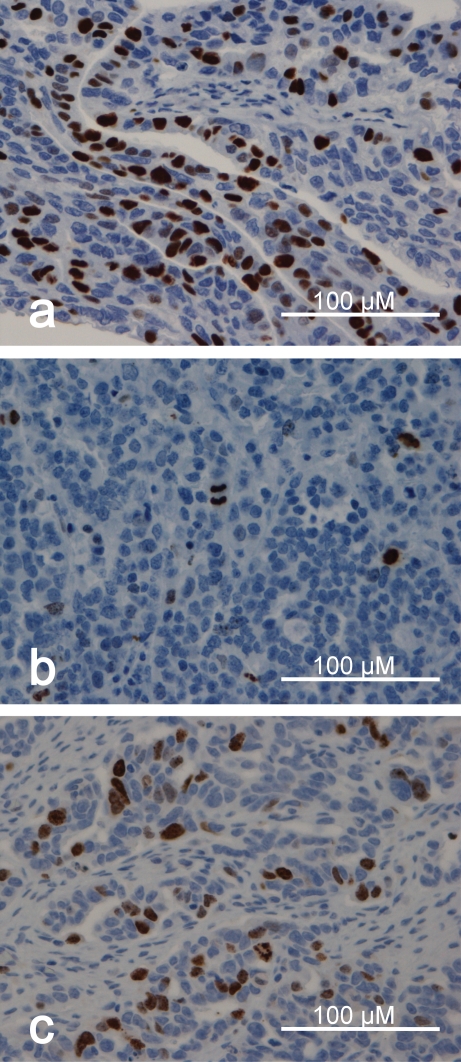
Images of the immunohistochemical stainings are presented in Figure 2. Differences in mitotic count, Ki-67/MIB-1, PHH3, and survivin immunostaining in ovarian carcinomas related to clinicopathological parameters are presented in Table 1.
Figure 2.
Images of the MIB-1/Ki-67, PHH3, and survivin immunohistochemical stainings in ovarian serous carcinoma: Expression of MIB-1/Ki-67, PHH3, and survivin, (a), (b) and (c), respectively. The images are from representative specimen.
Ki-67/MIB-1
The Ki-67/MIB-1 immunostaining was confined to the nucleus. The positive tumor cell nuclei were heterogeneously distributed. There was a statistically significant difference in the expression of Ki-67/MIB-1 between carcinomas, borderline tumors, and benign tumors (median LI; 36.7, 20, and 1.8, respectively, p<0.001), (Figure 1B).
Ki-67/MIB-1 expression was positively correlated with mitotic count (r=0.771, p<0.001), PHH3 (r=0.632, p<0.001), survivin (r=0.654, p<0.001), tumor grade (r=0.366, p<0.001), and stage of disease (r=0.427, p<0.001). There was no difference in the expression of Ki-67/MIB-1 between serous and non-serous carcinomas (median (+) cells/10 HPF; 42 and 32, respectively, p=0.141)
In advanced disease, women with complete response to first-line chemotherapy had significantly higher Ki-67/MIB-1 expression as compared to women with only partial response or stable disease after first line chemotherapy (p=0.003).
PHH3
The PHH3 immunostaining made it easy to localize mitotic figures. We found a higher amount of mitotic figures with the PHH3 staining method than by the mitotic count (median 35 vs. 15 MF/10 HPF, respectively). PHH3-positive non-mitotic nuclei in both low and high graded tumors were also observed. There was a statistically significant difference in the expression of PHH3 between carcinomas, borderline tumors, and benign tumors (median (+) cells/10 HPF; 48.5, 3, and 0, respectively, p<0.001), (Figure 1C). Serous carcinomas had higher PHH3 expression than non-serous carcinomas (median (+) cells/10 HPF; 65 and 35, respectively, p=0.003) (Table 1).
There were positive correlations between PHH3 and mitotic count (r=0.794, p<0.001), and between PHH3 and survivin expression (r=0.610, p<0.001). PHH3 expression was also positively
correlated to tumor grade (r=0.515, p<0.001), and FIGO stage (r=0.475, p<0.001).
Survivin
Survivin immunoreactivity was demonstrated in both nucleus and cytoplasm. There was a statistically significant difference in the expression of survivin between carcinomas, borderline tumors, and benign tumors (median (+) cells %; 13.7, 4.0, and 0.4, respectively, p<0.001) (Figure 1D). Serous carcinomas had higher survivin expression than non-serous carcinomas (median (+) cells/10 HPF; 15.5 and 8.2, respectively, p=0.006) (Table 1). Survivin expression was significantly correlated to mitotic count (r=0.688, p<0.001), tumor grade (r=0.433, p<0.001), and stage of disease (r=0.507, p<0.001).
Survival analyses
In univariate analyses of five-year overall survival advanced stage disease, residual tumor volume ≥1 cm, serous histology, age, and high expression of Ki-67/MIB-1, PHH3, and survivin implied a poor prognosis (Table 2). Mitotic count, tumor grade, and response to first line chemotherapy were not statistically significant parameters in the univariate analysis of survival.
Table 2.
Univariate analyses of potential prognostic factors for survival in women with ovarian carcinomas (N=68)
| Variable | Overall survival | ||
|---|---|---|---|
| HR | 95%CIforHR | p-value | |
| Advanced stage | 9.008 | 3.168-25.611 | <0.001 |
| Residual tumor ≥lcm | 3.494 | 1.628-7.490 | 0.004 |
| Serous histology | 3.329 | 1.634-6.783 | 0.001 |
| High grade tumor | 2.118 | 0.648-6.292 | 0.214 |
| Not CR chemo† | 1.309 | 0.558-3.071 | 0.536 |
| Age | 1.032 | 1.001-1.063 | 0.040 |
| Survivin | 1.066 | 1.028-1.106 | <0.001 |
| Ki-67/MIB-1 | 1.024 | 1.004-1.045 | 0.018 |
| Mitotic count | 1.009 | 0.998-1.02 | 0.113 |
| PHH3 | 1.007 | 1.0-1.014 | 0.047 |
HR: Hazard Ratio; CI: Confidence Interval; CR: Complete Responders
computed for cases with completed chemotherapy
Parameters with p-values <0.2 in the univariate analyses were included in the multivariate analysis of survival. In the multivariate analysis of survival only advanced stage disease and age turned out to be statistically significant parameters (Table 3).
Table 3.
Multivariate analysis of potential prognostic factors for survival in women with ovarian carcinomas (N=68)
| Variable | Overall survival | ||
|---|---|---|---|
| Multivariate analysis | |||
| HR | 95%CIforHR | p-value | |
| Advanced stage | 6.883 | 2.190-21.634 | 0.001 |
| Residual tumor ≥1cm | 1.879 | 0.753-4.687 | 0.176 |
| Serous histology | 1.080 | 0.459-2.543 | 0.860 |
| Age | 1.051 | 1.013-1.090 | 0.007 |
| Survivin | 1.021 | 0.973-1.072 | 0.395 |
| Ki-67/MIB-1 | 1.009 | 0.982-1.036 | 0.517 |
| Mitotic count | 0.997 | 0.976-1.019 | 0.812 |
| PHH3 | 1.002 | 0.990-1.014 | 0.782 |
HR: Hazard Ratio; CI:Confidence Interval
Discussion
The introduction of new biomarkers could facilitate a simpler and more consistent method to determine the proliferation potential of a tumor. This could have clinical implications, both in indicating prognosis and tailoring chemotherapy. In the present study we found that the proliferation markers Ki-67/MIB-1, PHH3, and survivin were positively correlated with histological malignancy grade.
Ki-67/MIB-1 expression is an established method for evaluation of proliferation in ovarian tumors. In accordance with previous studies, we found higher expression of Ki-67/MIB-1 in carcinomas than in borderline and benign ovarian tumors [32-34]. Furthermore, the expression of Ki-67/MIB-1 was positively correlated to stage of disease and tumor grade in the group of carcinomas. This has also been reported by others [17]. There was, however, a considerable overlap of indices between the different malignancy groups, which represent a serious drawback of Ki67/MIB-1 immunostaining. This has been experienced in other human neoplasms as well [35]. Thus, in an individual case, a low index does not necessarily imply a biologically benign tumor, and vice versa. Other troublesome aspects of this immunostaining are the lack of standardization of the procedure and the pronounced inter-laboratory variation of indices. These factors make it difficult to establish cutoff values for identification of more aggressive ovarian tumors.
PHH3 immunostaining has gained much interest as a more distinct proliferation marker, and we found that this marker easily displayed mitotic figures in the tumor tissue. The expression of PHH3 was higher in carcinomas than in ovarian borderline tumors and cystadenomas, which is in accordance with a previous study in serous tumors [23]. Furthermore, PHH3 was positively correlated with mitotic count, Ki-67/MIB-1, and survivin-expression, tumor grade, and stage of disease. We were also able to locate a larger number of mitotic figures in the PHH3 stained slides than by traditional mitotic count on hematoxylin and eosin-stained slides. These results indicate that PHH3 is a reliable marker of proliferative activity in ovarian tumors. We observed, however, PHH3 positive non-mitotic nuclei in both low and high graded tumors. This phenomenon has also been described by others [23], and may constitute a weakness of this marker. Further, PHH3 immunostaining can not simply replace mitotic count, as PHH3 values related to clinical parameters have to be established. Finally, PHH3 also revealed overlap of indices between the different grades of ovarian carcinomas, and larger studies are required to establish any threshold value.
Regarding the survivin immunostaining, we demonstrated a higher percentage of survivin-expressing cells in the ovarian carcinomas and borderline tumors as compared to the benign cases [36]. In contrast to previous studies, we found that the expression of survivin was positively correlated to stage of disease [25, 36, 37]. We experienced disturbing background staining with the survivin antibody, despite optimalization of the staining method. Further, we observed both nuclear and cytoplasmic immunoreactivity, and the clinical significance of this phenomenon is not fully clarified [25, 28, 36, 37]. Only the nuclear staining was recorded, as this pattern is obviously related to proliferation, whereas the role of the cytoplasmatic immuno-reactivity is not fully clarified [38, 39]. Furthermore, the survivin indices paralleled those of Ki-67/MIB-1 and PHH3, with considerable overlap between the malignancy groups. Additional studies are necessary to optimalize this immunostaining and define its definite role in the histopathological diagnosis of ovarian carcinomas.
In univariate analyses of five year overall survival, age, stage of disease, residual tumor volume, histological type, and proliferation marker status seemed to have an impact on prognosis. However, in a multivariate analysis, the impact of residual tumor volume, histological type, and proliferation marker status was not statistically significant. Age, stage of disease, and residual tumor volume are well documented prognostic factors in ovarian cancer [2]. Surprisingly, residual tumor volume was not found to have an impact on prognosis in the multivariate analysis of survival in the present study. This may be due to a relatively small number of patients. Altogether, stage of disease was the strongest prognostic factor in the present study.
Previous studies have reported conflicting results regarding the association between proliferation status and prognosis in ovarian cancer. Many studies have reported a worse outcome for patients with highly proliferative tumors [34, 40, 41]. It has been argued that some of these studies were limited by a small number of patients, and little information about chemotherapy. On the other hand, Kommoss et al. reported a longer survival for women with highly proliferative tumors, explained by a better response to chemotherapy [42]. In the present study, we found that highly proliferative tumors seemed to respond better to first line chemotherapy. Unlike Kommoss et al. we did not find prolonged survival in women with high proliferative tumors, probably because stage of disease is such a powerful prognostic factor. It seems likely that women with highly proliferative tumors have an increased chemosensitivity, and the clinical value of this observation should be further investigated.
In conclusion, we found that Ki-67/MIB-1, PHH3, and survivin can serve as reliable markers of proliferation in ovarian tumors. However, because of the overlap of proliferation indices between the different malignancy groups, further studies are needed to clarify their role in the histopatholigical diagnosis of ovarian neoplasms. It is possible that a proliferative profile composed of several markers can be useful. Further, the positive associations between these proliferation markers and survival and chemotherapy response are intriguing, pointing to a prognostic role of these markers for women with ovarian tumors.
References
- 1.Tavassoli FA, Devilee P. In: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Kleihues P, Sobin LH, editors. Lyon: International Agency for Research on Cancer; 2003. [Google Scholar]
- 2.Colombo N, Van Gorp T, Parma G, Amant F, Gatta G, Sessa C, Vergote I. Ovarian cancer. Crit Rev Oncol Hematol. 2006;60:159–179. doi: 10.1016/j.critrevonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK, Brady MF. Intraperitoneal therapy for ovarian cancer: a treatment ready for prime time. J Clin Oncol. 2006;24:4531–4533. doi: 10.1200/JCO.2006.06.7140. [DOI] [PubMed] [Google Scholar]
- 6.Clark TG, Stewart ME, Altman DG, Gabra H, Smyth JF. A prognostic model for ovarian cancer. Br J Cancer. 2001;85:944–952. doi: 10.1054/bjoc.2001.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garzetti GG, Ciavattini A, Goteri G, De Nictolis M, Stramazzotti D, Lucarini G, Biagini G. Ki67 antigen immunostaining (MIB 1 monoclonal antibody) in serous ovarian tumors: index of proliferative activity with prognostic significance. Gynecol Oncol. 1995;56:169–174. doi: 10.1006/gyno.1995.1026. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta PS, McGown AT, Bajaj V, Blackhall F, Swindell R, Bromley M, Shanks JH, Ward T, Buckley CH, Reynolds K, Slade RJ, Jayson GC. p53 and related proteins in epithelial ovarian cancer. Eur J Cancer. 2000;36:2317–2328. doi: 10.1016/s0959-8049(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 9.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 10.Linden MD, Torres FX, Kubus J, Zarbo RJ. Clinical application of morphologic and immunocytochemical assessments of cell proliferation. Am J Clin Pathol. 1992;97:S4–13. [PubMed] [Google Scholar]
- 11.Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 12.Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME, Venkatesan V, Lawton CA, Roach M, 3rd, Shipley WU, Hanks GE, Sandler HM. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133–2140. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 13.Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C, Neven P, Orosz Z, Olszewski WP, Knox F, Thurlimann B, Price KN, Castiglione-Gertsch M, Gelber RD, Gusterson BA, Goldhirsch A. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margulis V, Lotan Y, Karakiewicz PI, Fradet Y, Ashfaq R, Capitanio U, Montorsi F, Bastian PJ, Nielsen ME, Muller SC, Rigaud J, Heukamp LC, Netto G, Lerner SP, Sagalowsky AI, Shariat SF. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009;101:114–119. doi: 10.1093/jnci/djn451. [DOI] [PubMed] [Google Scholar]
- 15.Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, Kohne CH, Hillebrand T, Daniel PT, Fong Y, Lorenz M. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27:80–87. doi: 10.1053/ejso.2000.1029. [DOI] [PubMed] [Google Scholar]
- 16.Kritpracha K, Hanprasertpong J, Chandeying V, Dechsukhum C, Geater A. Survival analysis in advanced epithelial ovarian carcinoma in relation to proliferative index of MIB-1 immunostaining. J Obstet Gynaecol Res. 2005;31:268–276. doi: 10.1111/j.1447-0756.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 17.Mishra SK, Crasta JA. An immunohistochemical comparison of P53 and Bcl-2 as apopto-tic and MIB1 as proliferative markers in low-grade and high-grade ovarian serous carcinomas. Int J Gynecol Cancer. 2010;20:537–541. doi: 10.1111/IGC.0b013e3181d6de3f. [DOI] [PubMed] [Google Scholar]
- 18.Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN. The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol. 2004;28:1532–1536. doi: 10.1097/01.pas.0000141389.06925.d5. [DOI] [PubMed] [Google Scholar]
- 19.Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP. Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol. 2007;20:1307–1315. doi: 10.1038/modpathol.3800972. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima S, Terasaki M, Sakata K, Miyagi N, Kato S, Sugita Y, Shigemori M. Sensitivity and usefulness of anti-phosphohistone-H3 antibody immunostaining for counting mitotic figures in meningioma cases. Brain Tumor Pathol. 2009;26:51–57. doi: 10.1007/s10014-009-0249-9. [DOI] [PubMed] [Google Scholar]
- 21.Tapia C, Kutzner H, Mentzel T, Savic S, Baumhoer D, Glatz K. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol. 2006;30:83–89. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Murai Y, Tsuneyama K, Nomoto K, Okada E, Fujita H, Takano Y. Overexpression of phosphorylated histone H3 is an indicator of poor prognosis in gastric adenocarcinoma patients. Appl Immunohistochem Mol Morphol. 2006;14:296–302. doi: 10.1097/00129039-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Scott IS, Heath TM, Morris LS, Rushbrook SM, Bird K, Vowler SL, Arends MJ, Coleman N. A novel immunohistochemical method for estimating cell cycle phase distribution in ovarian serous neoplasms: implications for the histopathological assessment of paraffin-embedded specimens. Br J Cancer. 2004;90:1583–1590. doi: 10.1038/sj.bjc.6601660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YJ, Chen CM, Twu NF, Yen MS, Lai CR, Wu HH, Wang PH, Yuan CC. Overexpression of Aurora B is associated with poor prognosis in epithelial ovarian cancer patients. Virchows Arch. 2009;455:431–440. doi: 10.1007/s00428-009-0838-3. [DOI] [PubMed] [Google Scholar]
- 25.Ferrandina G, Legge F, Martinelli E, Ranelletti FO, Zannoni GF, Lauriola L, Gessi M, Gallotta V, Scambia G. Survivin expression in ovarian cancer and its correlation with clinico-pathological, surgical and apoptosis-related parameters. Br J Cancer. 2005;92:271–277. doi: 10.1038/sj.bjc.6602332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002;21:315–320. [PubMed] [Google Scholar]
- 27.No JH, Jeon YT, Kim YB, Song YS. Quantitative detection of serum survivin and its relationship with prognostic factors in ovarian cancer. Gynecol Obstet Invest. 2011;71:136–140. doi: 10.1159/000316049. [DOI] [PubMed] [Google Scholar]
- 28.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Expression of survivin is associated with malignant potential in epithelial ovarian carcinoma. Int J Mol Med. 2002;10:211–216. [PubMed] [Google Scholar]
- 29.Qian X, Xi X, Li L. Nuclear survivin is associated with malignant potential in epithelial ovarian carcinoma. Appl Immunohistochem Mol Morphol. 2011;19:126–132. doi: 10.1097/PAI.0b013e3181e30dcd. [DOI] [PubMed] [Google Scholar]
- 30.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 31.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.Huettner PC, Weinberg DS, Lage JM. Assessment of proliferative activity in ovarian neoplasms by flow and static cytometry. Correlation with prognostic features. Am J Pathol. 1992;141:699–706. [PMC free article] [PubMed] [Google Scholar]
- 33.Wong WS, Tattersall MH. Immunohistochemical determination of tumour growth fraction in human ovarian carcinoma. Br J Obstet Gynaecol. 1989;96:720–724. doi: 10.1111/j.1471-0528.1989.tb03289.x. [DOI] [PubMed] [Google Scholar]
- 34.Isola J, Kallioniemi OP, Korte JM, Wahlstrom T, Aine R, Helle M, Helin H. Steroid receptors and Ki-67 reactivity in ovarian cancer and in normal ovary: correlation with DNA flow cytometry, biochemical receptor assay, and patient survival. J Pathol. 1990;162:295–301. doi: 10.1002/path.1711620404. [DOI] [PubMed] [Google Scholar]
- 35.Abry E, Thomassen IO, Salvesen OO, Torp SH. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract. 2010;206:810–815. doi: 10.1016/j.prp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Sui L, Dong YY, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. International Journal of Oncology. 2002;21:315–320. [PubMed] [Google Scholar]
- 37.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cellular and Molecular Life Sciences. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005;92:212–216. doi: 10.1038/sj.bjc.6602340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F, Yang J, Ramnath N, Javle MM, Tan D. Nuclear or cytoplasmic expression of survivwhat is the significance? Int J Cancer. 2005;114:509–512. doi: 10.1002/ijc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layfield LJ, Saria EA, Berchuck A, Dodge RK, Thompson JK, Conlon DH, Kerns BJ. Prognostic value of MIB-1 in advanced ovarian carcinoma as determined using automated immunohistochemistry and quantitative image analysis. J Surg Oncol. 1997;66:230–236. doi: 10.1002/(sici)1096-9098(199712)66:4<230::aid-jso2>3.0.co;2-c. discussion 236-237. [DOI] [PubMed] [Google Scholar]
- 41.Mita S, Nakai A, Maeda S, Takeshita T. Prognostic significance of Ki-67 antigen immunostaining (MIB-1 monoclonal antibody) in ovarian cancer. J Nippon Med Sch. 2004;71:384–391. doi: 10.1272/jnms.71.384. [DOI] [PubMed] [Google Scholar]
- 42.Kommoss S, du Bois A, Schmidt D, Parwaresch R, Pfisterer J, Kommoss F. Chemotherapy may be more effective in highly proliferative ovarian carcinomas–a translational research subprotocol of a prospective randomized phase III study (AGO-OVAR 3 protocol) Gynecol Oncol. 2006;103:67–71. doi: 10.1016/j.ygyno.2006.01.037. [DOI] [PubMed] [Google Scholar]