Abstract
Purpose: Expressed in hepatic stellate cell (HSC), tTG is involved in fibrotic diseases including human hepatic fibrosis by promoting the cross-linking of ECM and participating in the initiation and/or progression of liver fibrosis. The purpose of this study is to identify whether depletion of tTG could attenuate liver fibrosis. Methods: In this study, primary hepatic stellate cells were isolated, purified, and cultured from rat. Expression of tTG gene was downregulated by lentivirus-mediated RNAi, and the effects on the activation, proliferation and apoptosis of HSC were investigated both in vitro and in vivo. Results: Lentivirus-mediated RNAi successfully reduced the endogenous expression of tTG in cultured cells. The down-regulation of tTG markedly inhibited the proliferation of HSC and attenuated the synthesis of Collagen-1. The downregulation of tTG also markedly reduced the level of tTG and hydroxyproline induced by CCl4 in rat livers at week 8 and week 12 after injection of CCl4. Conclusions: In summary, tTG plays an important role in liver fibrosis. Lentivirus-mediated downregulation of tTG showed a potential anti-fibrosis effect in rats, providing new evidence that the involvement of tTG in HSC activation, also suggesting that RNAi-directed targeting of tTG may be used as a potent and specific therapeutic tool for the treatment of liver fibrosis, especially in inhibiting the activation of HSC.
Keywords: Tissue transglutaminase, RNAi, hepatic stellate cells, liver fibrosis, lentiviral vector
Introduction
Tissue transglutaminase (tTG) is a member of the large transglutaminase (TG) family, which are calcium-dependent enzymes that catalyze an acyl transfer reaction between a y-carboxamide group of a peptide-bound glutamine residue and an ɛ-amino group of a lysine in another protein, being involved in numerous biochemical processes [1, 2]. Tissue transglutaminase not only displays cross-linking activity, but also functions as a GTPase in signaling processes [3]. Recently, it has been shown that tTG participates in a variety of fibrotic diseases including scleroderma, experimental renal fibrosis and human hepatic fibrosis [4, 5]. Liver fibrosis is a common disease which is characterized by increased synthesis and decreased degradation of the extracellular matrix (ECM) [6]. Decreased ECM degradation results from both irreversible cross-linking of the ECM and from increased expression of tissue inhibitor of metalloproteinase by the activated hepatic stellate cells (HSC) [7], which may account for the pathogenic process of fibrosis.
In the liver, both parenchymal and non-parenchymal cells including HSC can produce tissue transglutaminase, which appears to be released into the extracellular space [8]. Recent studies indicated that tTG may play an important role in the activation and apoptosis of HSC, and promote the cross-linking of ECM by exerting as a transglutaminase. Also, it may participate and play a particular and important role in the initiation and/or progression of liver fibrosis [9, 10]. However, how tTG initiates the fibrosis is largely unknown.
In the past decade, RNA interference (RNAi) has been proven to be a strong tool for silencing genes of interest with less toxicity, comparing with the conventional gene suppression technologies such as using chemical inhibitors [11-13]. Considering the efficiency of RNAi-mediated gene silencing and feasibility of introducing RNAi into cells and tissues, RNAi is a potential therapeutic tool for treatment of human diseases [14-16]. Thus, we studied the role of tTG in liver fibrosis and explored the possibility of using virus-mediated knock down of tTG as a new strategy in the progression of liver fibrosis.
In the present study, we isolated and cultured primary HSC of rat.We explored the functions of tTG in the activation and apoptosis of HSC, and in liver fibrosis of animal models by combining loss-of-function strategy using RNA interference (RNAi) [17] along with functional analysis
Materials and methods
Isolation and culture of rat HSC in vitro
Male Sprague-Dawley rats weighing 450 to 550 gwere purchased from the Shanghai laboratory Animal Center (Chinese Academy of Science). Hepatic stellate cells were isolated and purified based on enzymatic digestion of the perfused liver by collagenase IV (Sigma, USA), followed by the Percoll density gradient centrifugation of the crude cell suspension.
The primary hepatic stellate cells of rats were cultured in RPMI-1640 (Gibco, MD) with 10% inactivated (56°C, 30 min) calf serum at 37°C in a humidified atmosphere of 5% CO2. And then, cell morphology, viability and growth state of HSC were identified by microscopy, electro-microscopy observations and immunohistochemistry assay with the monoclonal antibody of mouse-anti-rat ED-2 (Serotec, UK).
Construction and identification of the tTG-RNAi-lentivirus vector
Small interfering RNA (siRNA) sequences targeting rat tTG (GenBank accession number NM_019386) were designed on line. Finally, we chose four siRNA targets with no homologous sequence to other genes and starting at the site of 985, 942, 1029 and 1366, respectively; and we also defined a non-specific oligo nucletides as the negative control (Scr-RNAi, TTCTCCGAACGT GTCACGT). The DNA oligos of four siRNA were constructed into the lentivirural vector (psicoR, Addgene). The recombinant lentivirus of siRNA targeting tTG (tTG-RNAi-Lentivirus, GAGTGGTGA CCAACTACAA) and the negative control lentivirus were prepared. The HSC cells were plated in 6-well plates until cell fusion reaching 30∼40%. Then the appropriate amounts of lentiviruses were added to the cells according to the different MOI values. After 12 h of infection at 37°C, the medium was replaced by fresh RPMI-1640 medium. And with a further incubation for 72 h, we observed the expression status of GFP in lentivirus-infected HSC cells. To explore the efficiency of tTG-RNAi-Lentivirus on the expression of tTG gene, we assessed the protein level of tTG after infected with lentiviruses for 5-7 days in these HSC cells by Western blotting assay.
Western blotting
After lysed with pre-cooled lysis buffer, 40 µg protein extracted from cells was loaded on 10% SDS-PAGE for each well and transferred to PVDF membrane for 2 h (Bio-Rad). Then the membrane was blocked in 5% non-fat milk for 1 h at room temperature and then probed with protein -specific antibodies overnight at 4°C. After washed three times with tris buffered saline with 0.5% Tween-20, the membrane was incubated with appropriate secondary antibody for 2 h at room temperature. Following a further washing with tris buffered saline with 0.5% Tween-20, the membrane was filmed using enhanced chemiluminescence (ECL + plus™, Amersham, UK) solution. The first antibody of mouse anti-Flag (Sigma, USA), mouse anti-GAPDH (Santa Cruz, CA) and the secondary antibody (anti-mouse IgG, Santa Cruz, CA) were all used at a 1: 4000 dilution.
Cell cycle assay
Cells were seeded into a 6-well plate and harvested after infected for 10 days. After washed with pre-cooled PBS twice, cells were fixed in 70% alcohol. Percentage of cells in each stage of cell cycle was determined by staining with propidium iodide (PI, Santa Cruz, CA). The cell cycle distribution was analyzed by FAC-Scan Flow Cytometer (BD, USA) in accordance with the manufacturer's guidelines.
Cell proliferation assay
Cell proliferation ability was assessed by methylthiazol tetrazolium (MTT). The activated HSC cells were inoculated into 96-well plates with 1×104 cells per well, and after incubation for 1 day, 2 days, 3 days, 4 days, and 5 days, 20 µl of sterile MTT (5 mg/ml, Sigma-Aldrich Corp.) was added to each well. Following a further incubation at 37° C for 4 h. The reaction was stopped by adding 150 µl of dimethyl sulfoxide (DMSO). After thoroughly mixed for 10 min at room temperature, the formazan production was determined by measurement of the spectrometric absorbance at 570 nm on an enzyme immuno-assay analyzer (1420 multi-label counter).
Effects of tTG on the liver fibrosis process
Two-hundred and four Male Sprague-Dawley rats weighing 300 to 350 g, provided by the Shanghai Laboratory Animal Center (Chinese Academy of Science), were bred in an aseptic condition with a constant humidity of 60∼70% and the room temperature of 18∼20°C. All the rats were distributed into four groups: normal, fibrosis, Scr-RNAi and tTG-RNAi groups. Chronic liver injury was induced by injection of CCl4 in each group except the normal group. The tTG-RNAi-Lentivirus (5×107 TU/ml) was administrated by portal venous injection starting 7 days before and 4 weeks after the injection of CCl4. The rats were enthanized at week 4, 8, and 12 of the CCl4 induction program, and the liver specimens were obtained from each rat. We did the histological analysis by microscopy observation. Grades of liver fibrosis were evaluated by the semi-quantitative scoring system (SSS), and hydroxyproline in these livers and the hepatic functional biochemistry were also detected.
Statistical methods
All measurement data in the text and figures are expressed as the Mean ± SD. The association analysis among groups was performed with one-way analysis of variance (ANOVA) by the statistic software SPSS12.0 (Lead Technologies, Inc, USA). And the statistical significant level was determined at a P value less than 0.05.
Results
Isolation of rat HSC in vitro
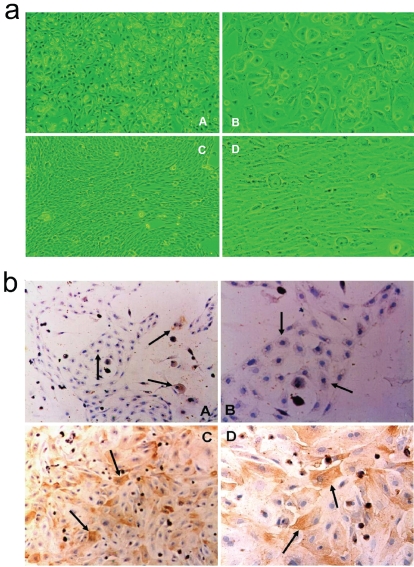
After the collagenase IV digestion and the Per-coll density gradient centrifugation, we isolated and successfully purified the rat HSC. The yield, purity and viability of HSC were 2.5∼3.2×107 per liver, (94.0 ± 1.7) % and (98.0 ± 1.8) %, respectively. After incubated in standard condition for 3 days, HSC was observed still at a quiescent stage under a light phase microscope (Figure 1a, A and B), whereas HSC was activated when cultured for another 11 days (Figure 1a, C and D). The immunostaining against desmin (a marker protein of quiescent HSCs) and α-SMA (marker proteins of activated HSCs) showed that desmin is expressed in both quiescent and activated HSCs, but α-SMA is only expressed in the activated HSC (Figure 1b).
Figure 1.
Identification for cell morphology of HSC under inverted microscopy (a) and gene expression by immunohistochemistry assay (b). (A) and (B), quiescent HSC (cultured for 3 days); (C) and (D), activated HSC (continuous cultured forl4 days). (A) and (C), ×100; (B) and (D), ×200.
Generation and validation of tTG-RNAi-lentivirus
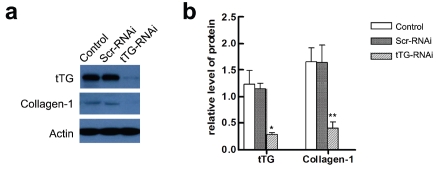
We designed four siRNA sequences targeting tTG and chose the sequence most effectively down-regulating the expression of tTG for further generation of RNAi lentivirus. The virus titer was 4×108 TU/ml for all the experiments. The cultured HSC was infected with the tTG-RNAi lentivirus and after 7 days, total protein was extracted and subjected to western blotting to validate the knockdown efficiency. The results showed that tTG-RNAi could reduce endogenous tTG protein, compared with negative control group (Scr-RNAi) infected with Scr-RNAi lentivirus (Figure 2, a and b). The expression of collagen-1 was also detected in HSC after infected with tTG-RNAi-Lentivirus by Western blotting. Results showed that the expression of Collagen-1 was significantly decreased after transfection (P < 0.01) (Figure 2, a and b).
Figure 2.
Immunodetection and quantification of tTG by Western blotting. Control, Scr-RNAi and tTG-RNAi, were groups of HSC exposed to Polybrene, GFP-RNAi-Lentivirus, and tTG-RNAi-Lentivirus, respectively, (a) A representative result of western blotting, (b) Graphical representation of relative optical density for the left image. *, P<0.01 and **, P<0.05.
The functional effect of tTG downregulation on the HSC
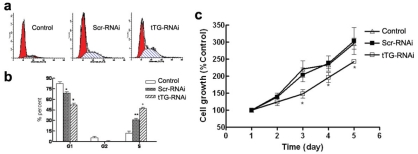
The flow cytometry assay indicated that there was no obvious apoptosis of activated HSC after tTG-RNAi-Lentivirus infection. The percentage of stage G1 cells decreased markedly while that of stage S cells increased markedly when compared to the normal control (control) and negative control group (Scr-RNAi) (Figure 3a and b). These results implied that the tTG-RNAi-Lentivirus trends to induce the apoptosis of HSC, as the higher percentage of stage S cells may be an anti-apoptosis phenomenon.
Figure 3.
Analyses for cell proliferation and apoptosis of activated HSC after infected with tTG-RNAi-Lentivirus. (a and b) Cell cycle assay by flow cytometry, *, P<0.01 and **, P<0.01 vs CON; #, P<0.01 and +, P<0.01 vs CON and GFP. (c) Cell growth curve by MTT. +, P<0.01 vs CON; #, P<0.01 vs CON and GFP;*, P<0.01 vs CON and GFP.
Furthermore, we detected the proliferative ability of HSC cells in each group by MTT assay (Figure 3c). Results showed that the proliferative ability of HSC cells infected with tTG-RNAi-Lentivirus was decreased significantly compared with the control and Scr-RNAi group (P < 0.05), indicating that the tTG-RNAi-Lentivirus inhibited the proliferation of HSC.
Down regulation of tTG in vivo prevented liver fibrosis progression
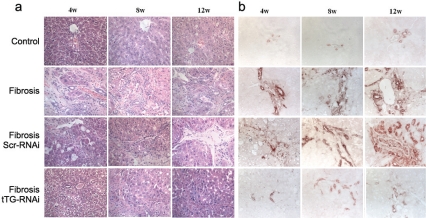
Previous studies have shown that in liver fibrosis process, tTG expression level is increased [18]. In order to investigate if down-regulation of tTG could prevent the progression of liver fibrosis in vivo, we injected animals with the tTG-RNAi lentivirus 7 days before the liver damage induced by CCl4 and applied additional injection 4 weeks later. Along with the aggravation of liver fibrosis, the expression of hydroxyproline was increased (Figure 4a). After administration of tTG-RNAi lentivirus, all of the indexes obtained were decreased than those in control groups.
Figure 4.
The pathological observation (a) and immunohistochemistry assay for tTG expression (b) in each group of rat liver tissues after tTG-RNAi-Lentivirus infection (400×).
After the injection of CCl4 for 4, 8 and 12 weeks, changes of tTG expression in rat livers was obvious, and the staining intensity against tTG protein was dramatically decreased in tTG-RNAi group compared with Scr-RNAi group (Figure 4b). The histological analysis of liver fibrosis showed a functional prevention of tTG-RNAi lentivirus to liver damage induced by CCl4 (Figure 4a).
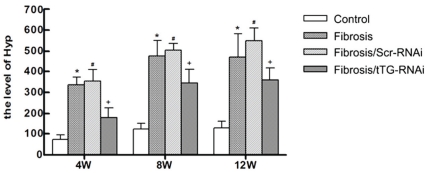
Then, the level of hydroxyproline was investigated in rat livers in each group. The level of hydroxyproline was gradually increased following the liver fibrosis (Figure 5). Results showed that hydroxyproline was down-regulated in tTG-RNAi groups compared with the Scr-RNAi group at 4, 8 and 12 weeks after the injection of CCl4 (Figure 5, P < 0.05).
Figure 5.
The level of hydroxyproline after tTG-RNAi-Lentivirus transfer in fibrosis rat liver. C, F, G and T, represents the group of normal control, liver fibrosis, fibrosis rats treated with GFP-RNAi-Lentivirus and the tTG-RNAi-Lentivirus, respectively. *, P<0.01 and #, P<0.01, contrasted to C group; +, P<0.05, contrasted to F and G group.
In a word, in the early stage of liver fibrosis, tTG-RNAi-Lentivirus may deteriorate the liver function by downregulating the protective role of the tTG. Along with the aggravation of liver fibrosis, tTG-RNAi-Lentivirus may decrease the level of Collagen-1 and hydroxyproline by inhibiting the expression of tTG, and then improve the liver function and attenuate the fibrosis.
Discussion
In this study, hepatic stellate cells from rat were infected with the tTG-RNAi lentivirus, and it could significantly suppress the expression of tTG both at mRNA and protein levels. The endogenous expression of Collagen-1 in HSC was also obviously down-regulated after infection. A previous study identified that tTG could be able to protect cells from apoptosis [19], but there was no detectable cell apoptosis after tTG knockdown in the present study. However, the cell proliferation ability of HSC was significantly decreased. The data indicated that knockdown of tTG via RNAi strategy affects HSC proliferation and the synthesis of Collagen-1 in HSC. It is proposed that tTG may play a potential role in anti-fibrogenesis.
Furthermore, we investigated the down regulation of tTG in liver fibrosis in vivo. Results showed that lentivirus-mediated down regulation of endogeous tTG is efficient, and the hydroxyproline and the SSS scores for pathological assay in rat livers were obviously decreased in rat livers infected with tTG-RNAi lentivirus at week 4, week 8 or week 12 after CCl4 injection. Moreover, the liver function was significantly improved after injection of tTG-RNAi lentivirus, revealing an anti-fibrosis function of the tTG. However, the function of tTG may be different in different stages during live fibrosis. In the early stage, knock down of tTG may deteriorate the function of rat livers by attenuating the protection of tTG. Following the aggravation of liver fibrosis, tTG depletion may decrease the level of hydroxyproline, and then improve the liver function and attenuate the fibrosis.
Tissue transglutaminase (tTG), one member of transglutaminases that could catalyze a calcium-dependent acryl transfer reaction and involved in a variety of biochemical processes, is ubiquitously expressed in many tissues such as endothelial, smooth muscle cells and fibrotic tissue [20]. Also, tTG is a multifunctional enzyme that has been implicated to several physiological processes and pathological conditions such as membrane integrity maintenance, cell adhesion, cell death or differentiation, signal transduction and tissue remodeling [21], as well as in hepatic injury and fibrosis [2, 10].
It has been reported that the mRNA and protein level of total tTG was significantly up-regulated in activated HSC and ECM during trans-differentiation of quiescent HSC to collagen-producing myofibroblasts [18], which indicated that tTG plays an important role in HSC activation. It is also demonstrated that tTG exerts crucial effect on the progression of liver fibrogenesis through its ECM cross-linking activity, which may contribute to determine the speed and extent of matrix degradation in liver fibrosis. A recent study suggested that tTG is a protective factor against liver fibrosis since hepatic fibrosis is more severe after injection of CCl4 in tTG knock-out mice compared with wild-type mice. Thus, a further exploration of the mechanism underlying how tTG plays its role in liver fibrosis is required for better understanding the progress of liver fibrosis and for the clinical treatment especially using gene therapy.
Taken together, we demonstrated that down-regulation of tTG markedly inhibited the proliferation of HSC and attenuated the synthesis of Collagen-1, a factor that participating in ECM cross-linking process. In vivo injection of lentiviral vectors carrying RNAi targeting tTG mRNA into livers in an animal model of liver fibrosis slowed down the progression of liver fibrosis. Thus, tTG may be the key for determining the reversion of liver fibrosis, and knockdown of the tTG expression by RNAi may be a new approach for the treatment of live fibrosis in the future.
References
- 1.Violante V, Luongo A, Pepe I, Annunziata S, Gentile V. Transglutaminase-dependent formation of protein aggregates as possible biochemical mechanism for polyglutamine diseases. Brain Res Bull. 2001;56:169–172. doi: 10.1016/s0361-9230(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Zern MA. Tissue transglutaminase, a key enzyme involved in liver diseases. Hepatol Res. 2004;29:1–8. doi: 10.1016/j.hepres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 4.Grenard P, Bresson-Hadni S, El Alaoui S, Chevallier M, Vuitton DA, Ricard-Blum S. Transglutaminase-mediated cross-linking is involved in the stabilization of extracellular matrix in human liver fibrosis. J Hepatol. 2001;35:367–375. doi: 10.1016/s0168-8278(01)00135-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TS, Griffin M, Thomas GL, Skill J, Cox A, Yang B, Nicholas B, Birckbichler PJ, Muchaneta-Kubara C, Meguid El Nahas A. The role of transglutaminase in the rat subtotal nephrectomy model of renal fibrosis. J Clin Invest. 1997;99:2950–2960. doi: 10.1172/JCI119490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarem M, Znaidak R, Macias M, Rey R. [Hepatic stellate cells: it's role in normal and pathological conditions] Gastroenterol Hepatol. 2006;29:93–101. doi: 10.1157/13083906. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 8.Chen JS, Mehta K. Tissue transglutaminase: an enzyme with a split personality. Int J Biochem Cell Biol. 1999;31:817–836. doi: 10.1016/s1357-2725(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 9.Nardacci R, Lo lacono O, Ciccosanti F, Falasca L, Addesso M, Amendola A, Antonucci G, Craxi A, Fimia GM, ladevaia V, Melino G, Ruco L, Tocci G, Ippolito G, Piacentini M. Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol. 2003;162:1293–1303. doi: 10.1016/S0002-9440(10)63925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza A, Liu SL, Frizell E, Zhu J, Maddukuri S, Martinez J, Davies P, Schwarting R, Norton P, Zern MA. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1997;272:G281–G288. doi: 10.1152/ajpgi.1997.272.2.G281. [DOI] [PubMed] [Google Scholar]
- 11.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 12.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 13.Arens N, Gandhari M, Bleyl U, Hildenbrand R. In vitro suppression of urokinase plasminogen activator in breast cancer cells–a comparison of two antisense strategies. Int J Oncol. 2005;26:113–119. [PubMed] [Google Scholar]
- 14.Woessmann W, Damm-Welk C, Fuchs U, Borkhardt A. RNA interference: new mechanisms for targeted treatment? Rev Clin Exp Hematol. 2003;7:270–291. [PubMed] [Google Scholar]
- 15.Jiang M, Rubbi CP, Milner J. Gel-based application of siRNA to human epithelial cancer cells induces RNAi-dependent apoptosis. Oligonucleotides. 2004;14:239–248. doi: 10.1089/oli.2004.14.239. [DOI] [PubMed] [Google Scholar]
- 16.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 17.Westhof E, Filipowicz W. From RNAi to epigenomes: how RNA rules the world. Chembiochem. 2005;6:441–443. doi: 10.1002/cbic.200400418. [DOI] [PubMed] [Google Scholar]
- 18.Schnabel C, Sawitza I, Tag CG, Lahme B, Gressner AM, Breitkopf K. Expression of cytosolic and membrane associated tissue transglutaminase in rat hepatic stellate cells and its upregulation during transdifferentiation to myofibroblasts in culture. Hepatol Res. 2004;28:140–145. doi: 10.1016/j.hepres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Fabbi M, Marimpietri D, Martini S, Brancolini C, Amoresano A, Scaloni A, Bargellesi A, Cosulich E. Tissue transglutaminase is a caspase substrate during apoptosis. Cleavage causes loss of transamidating function and is a biochemical marker of caspase 3 activation. Cell Death Differ. 1999;6:992–1001. doi: 10.1038/sj.cdd.4400573. [DOI] [PubMed] [Google Scholar]
- 20.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]