Abstract
The overexpression of the transcription factor, E2F1, induces hypertrophy and apoptosis with cell cycle re-entry in cardiomyocytes in vitro and in vivo, suggesting that targeting E2F1 may have therapeutic potential. Accordingly, we tested the hypothesis that blocking the E2F1-mediated signal transduction pathway prevents cardiac hypertrophy by treating E2F1 knockout mice (E2F1-/-) with either isoproterenol (ISO) or Angiotensin II (ANG). Echocardi-ography was used to measure left ventricular mass index and myocardial performance index, a measure of combined systolic and diastolic left ventricular function. In control mice (E2F1+/+) both ISO and ANG treatments induced cardiac hypertrophy, and impaired ventricular function in ANG treated mice. In contrast to previously published work, E2F1-/- mice also demonstrated a similar pattern of cardiac hypertrophy and function after either treatment. Atrial natriuretic peptide, a molecular marker of hypertrophy and necropsy-determined body weight-normalized left ventricle mass were similarly increased in ISO and ANG treated E2F1+/+ and E2F-/- mice, supporting the echocardiographic data. These data indicate that E2F1 is not necessary for the development of cardiac hypertrophy although studies using an overexpression approach suggest a causal role of E2F1. The reason for this discrepancy is unclear, although it is possible that other E2F-family members (e.g., E2F2) may play a compensatory role. In conclusion, our data demonstrate that cardiac hypertrophy can be induced in an E2F1-independent fashion and suggest that in contrast to previous reports, targeting E2F1 may not be a good therapeutic approach.
Keywords: E2F1, cardiac hypertrophy, isoproterenol, angiotensin II, cardiomyopathy, cell cycle
Introduction
The family of E2F transcription factors is comprised of seven members (E2F1 to E2F7) that are involved in transcriptional activation and repression, cell cycle regulation and DNA synthesis (reviewed in [1]). E2F1 in particular has been shown to play a key role in inducing S-phase of cell cycle in quiescent cells [2]; thus, E2F1 complexed with the pocket protein Rb prevents the cell from entering S phase by inhibiting the transfer and binding of E2F1 to its transcriptional regulation sites of DNA [3]. E2F1 is known to be expressed in the myocardium during development and becomes downregulated when cells become terminally differentiated [4], but in myocardial samples from patients with heart failure, protein expression of E2F1 and Rb were increased and correlated to cardiomyocyte hypertrophy [5].
Inhibition of E2F1 has been shown to prevent the development of hypertrophy in cardiomyocytes. Specifically, a blocking peptide to E2F prevented cell hypertrophy, an increase in total cellular protein and induction of hypertrophic markers in neonatal cardiomyocytes stimulated with either serum or phenylephrine [4]. Moreover, when E2F1 was overexpressed by adenoviral delivery into either adult ventricular or neonatal myocytes in vitro or by direct injection into mouse myocardium in vivo, markers of cell cycle, DNA synthesis and apoptosis were induced [6, 7].
Taken together, these studies suggest a critical role for E2F1 in the development of hypertrophy in adult myocardium. Therefore, we utilized a mouse model to test the hypothesis that inhibition of E2F1 in vivo can prevent the development of cardiomyopathy induced by pharmacologic agents causing ventricular hypertrophy.
Materials and methods
Animals
Male E2F1 Knockout (E2F1-/-) and control littermate mice [8] from Jackson Labs (Bar Harbor, ME), aged 3-5 months old, were treated with either isoproterenol (ISO, 60mg/kg/day for 7 days) or angiotensin II (ANG, 3 mg/kg/day for 14 days) subcutaneously administered via mini-osmotic pumps (Durect Corp, Cupertino, CA).
Echocardiography analysis
Mice were weighed and anesthetized with isoflurane (induced at 3% and maintained at ∼1% by nosecone). Echocardiography was performed and analyzed as previously described [9] before and after induction of hypertrophy. Studies were performed by a sonographer who was blinded to genotype.
Gravimetry
When mice were sacrificed, body weights were taken in grams and hearts were harvested and weighed (in mg). Heart weight to body weight (HW/BW) ratio was determined.
Histology
Formalin fixed, paraffin embedded myocardial tissue was sectioned at 5mm and mounted on slides. Tissue was rehydrated using standard protocols and stained with hematoxylin (Fisher Scientific, Pittsburgh, PA) and eosin-Y (Richard-Allen Scientific, Kalamazoo, MI), dehydrated and mounted using Permount (Fisher). Images were captured on an upright Zeiss Axiovert with an attached Axiovision camera using Axiovision software (Thornwood, NY).
Real Time PCR
Ventricles of E2F1+/+ and E2F1-/- mice treated with either ANG, ISO or untreated controls were isolated and homogenized with Tri-Reagent Solution (Ambion, Austin, TX) then purified using RNAeasy columns (Qiagen, Valencia, CA). RNA was quantified on a NanoDrop Spectrophotometer (Wilmington, DE) and 1 µg of RNA was used to make cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following manufacturer's protocol. Subsequently, 5ng of cDNA was used with Taqman gene expression assays and Taqman Fast Master Mix for atrial natriuretic peptide and GAPDH on a Step One Plus Real Time machine (Applied Biosystems).
Statistics
Statistical comparisons were performed by parametric analysis using a two-way analysis of variance (ANOVA) for analysis of genotype versus drug and their interactions, and one-way ANOVA and/or Student's t-test where appropriate. Statistical significance was determined when p<0.05.
Results
Effects of isoproterenol treatment in E2F1 knockout mice
Isoproterenol (ISO) treatment increased LVMI and RWT (indicating concentric hypertrophy), and fractional shortening, Vcf and heart rate in both E2F1 -/- and E2F1 +/+ (Table 1). The index of combined systolic and diastolic function, MPI, was unchanged by ISO in both groups. Analysis of effect of drug on phenotype by two-way ANOVA indicated E2F1 -/- and +/+ mice had similar levels of hypertrophy.
Table 1.
Effect of isoproterenol treatment in E2F1 knockout mice. Echocardiography analysis of E2F1 +/+ wild type mice and E2F1 -/- knockout mice before (pre) and after (post) treatment with isoproterenol. 2-way ANOVA analysis comparing E2F1 +/+ to E2F1 -/- (genotype), pre and post drug treatment (drug) and the interaction between genotype and drug (interaction). Data are expressed as the mean +/- SD. Significance was determined when p<0.05 and is indicated not significant (NS) if value was higher than 0.05.
| E2F1 +/+ | (n=6) | E2F1 -/- | (n=6) | 2-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Genotype | Drug | Interaction | |
| Weight (g) | 31.1±2.9 | 32.7±2.4 | 25.6±3 | 27.3±2.4 | p<0.05 | NS | NS |
| HR (bpm) | 410±60 | 538±46 | 425±33 | 506±54 | NS | p<0.05 | NS |
| LA (cm) | 0.21±0.02 | 0.21±0.02 | 0.21±0.02 | 0.21±0.02 | NS | NS | NS |
| MPI | 0.36±0.03 | 0.34±0.06 | 0.31±0.02 | 0.38±0.08 | NS | NS | NS |
| CO (cc) | 16±4 | 26±2 | 15±2 | 26±8 | NS | p<0.05 | NS |
| EDD (cm) | 0.36±0.02 | 0.38±0.03 | 0.34±0.02 | 0.32±0.03 | p<0.05 | NS | p<0.05 |
| ESD (cm) | 0.21±0.03 | 0.17±0.02 | 0.17±0.02 | 0.14±0.02 | p<0.05 | p<0.05 | NS |
| FS | 0.42±0.07 | 0.56±0.06 | 0.5±0.04 | 0.56±0.03 | p<0.05 | p<0.05 | NS |
| E/A | 1.18±0.11 | 1.11±0.36 | 1.22±0.2 | 1.11±0.28 | NS | NS | NS |
| LVMI (mg/g) | 4.6±0.5 | 6.8±1.8 | 5.2±1.3 | 6.8±1.3 | NS | p<0.05 | NS |
| RWT | 0.59±0.07 | 0.69±0.08 | 0.62±0.17 | 0.88±0.17 | NS | p<0.05 | NS |
| Vcf | 6.5±2.3 | 12.9±2.2 | 8.8±2 | 10.9±1.6 | NS | p<0.05 | p<0.05 |
Abbreviations: heart rate (HR), left atrial (LA), myocardial performance index (MPI), cardiac output (CO), end diastolic dimension (EDD), end systolic dimension (ESD), fractional shortening (FS), the ratio between early and late ventricular filling velocity (E/A), left ventricular mass index (LVMI), relative wall thickness (RWT), velocity of circumferential fiber shortening (Vcf).
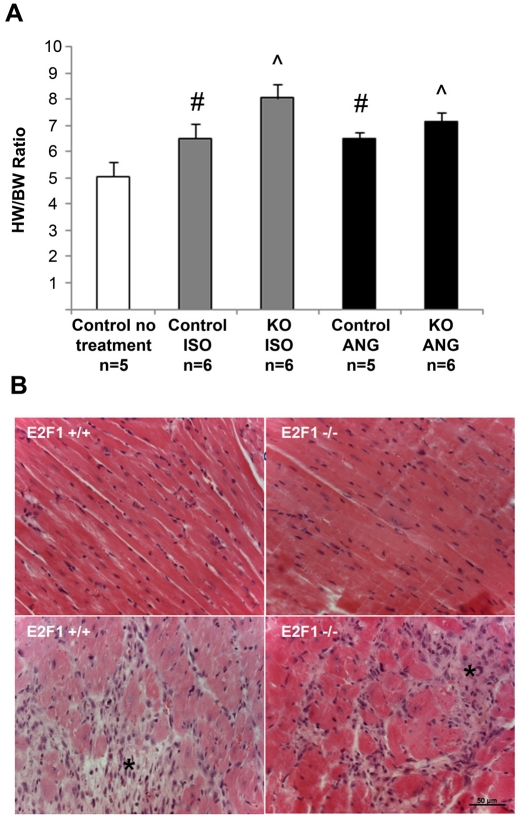
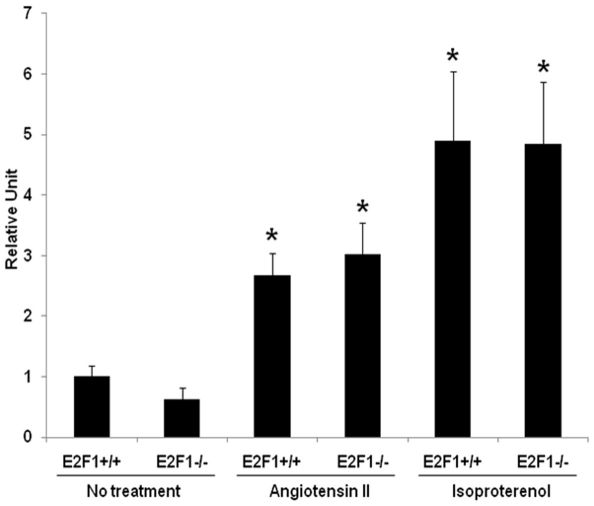
Hearts (which include left and right ventricles) were significantly larger in the E2F1 -/- as compared to WT treated with ISO as measured by HW/BW ratio (Figure 1A), indicating no attenuation of hypertrophy in the E2F1 -/- when treated with ISO. Histological analysis of ISO treated E2F1 +/+ and -/- myocardial sections showed myocyte hypertrophy and an increase in fibrosis consistent with previous ISO studies [10] (Figure 1B). Gene expression of the hypertrophy marker, atrial natriuretic peptide (ANP), was increased with ISO similarly in both control and E2F1 knockout mice (Figure 2).
Figure 1.
Both control and E2F1 knockout mice exhibit signs of hypertrophy and fibrosis. A. Heart weight (HW, in mg) was divided by body weight (BW, in g) to determine the HW/BW ratio. #p<0.05 versus control no treatment, ˇp<0.0001 versus control no treatment. B. Myocardial sections from E2F1 +/+ and E2F1 -/- mice were stained with hematoxylin and eosin after treatment with isoproterenol. Hypertrophic changes are not significantly different between the two strains (bottom panel). Fibrosis (*) is evident in both genotypes after isoproterenol treatment but no difference is detected between the two groups. Scale bar = 50µm.
Figure 2.
Atrial natriuretic peptide is increased in both control and E2F1 knockout mice after treatment with either isoproterenol or angiotensin II. Expression of atrial natriuretic peptide (ANP), a molecular marker of hypertrophy, was significantly increased upon treatment with isoproterenol of angiotensin II in both E2F1 +/+ and E2F1 -/- compared to non treated animals. Real time expression of ANP was normalized to expression of GAPDH, a housekeeping gene, and presented as fold change. No difference was seen between E2F1 +/+ and E2F1 -/-. *p<0.05 versus no treatment
Effects of angiotensin II treatment in E2F1 knockout mice
Angiotensin II (ANG) treatment increased LVMI and RWT in both E2F1 -/- and E2F1 +/+ (Table 2). Fractional shortening, Vcf, and HR were unchanged, but MPI was increased (indicating worse function) by ANG in both groups. Analysis of effect of drug on phenotype by two-way ANOVA indicated E2F1 -/- and +/+ mice had similar levels of hypertrophy.
Table 2.
Effect of angiotensin II treatment in E2F1 knockout mice. Echocardiography analysis of E2F1 +/+ wild type mice and E2F1 -/- knockout mice before and after treatment with Angiotensin II. 2-way ANOVA analysis comparing E2F1 +/+ to E2F1 -/- (genotype), pre and post drug treatment (drug) and the interaction between genotype and drug (interaction). Significance was determined when p<0.05 and is indicated not significant (NS) if value was higher than 0.05
| E2F1 +/+ | (n=6) | E2F1-/- | (n=6) | 2-way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Genotype | Drug | Interaction | |
| Weight | 28.1±1.4 | 26.8±1.8 | 26.8±3.1 | 24±3.3 | NS | NS | NS |
| HR | 360±83 | 442±70 | 469±28 | 488±90 | p<0.05 | NS | NS |
| LA | 0.17±0.03 | 0.18±0.03 | 0.19±0.03 | 0.20±0.03 | NS | NS | NS |
| MPI | 0.32±0.06 | 0.41±0.04 | 0.32±0.02 | 0.41±0.03 | NS | p<0.05 | NS |
| CO | 13±2 | 17±6 | 15±1 | 16±4 | NS | NS | NS |
| EDD | 0.38±0.04 | 0.33±0.05 | 0.34±0.02 | 0.32±0.05 | NS | p<0.05 | NS |
| ESD | 0.22±0.04 | 0.19±0.04 | 0.18±0.02 | 0.18±0.07 | NS | NS | NS |
| FS | 0.42±0.06 | 0.45±0.11 | 0.48±0.05 | 0.49±0.08 | NS | NS | NS |
| E/A | 1.3±0.11 | 1.21±0.36 | 1.41±0.36 | 1.43±0.7 | NS | NS | NS |
| LVMI | 5±0.5 | 5.9±0.7 | 5.1±1 | 6.6±1.1 | NS | p<0.05 | NS |
| RWT | 0.53±0.13 | 0.71±1 | 0.61±0.07 | 0.78±0.2 | NS | p<0.05 | NS |
| Vcf | 5.9±1.8 | 8±3.4 | 9±1.7 | 8.6±3.2 | NS | NS | NS |
Hearts were significantly larger in the E2F1 -/- as compared to E2F1 +/+ treated with ANG as measured by HW/BW ratio (Figure 1A). Indicating no attenuation of hypertrophy due to E2F1 -/ - in the ANG treated mice. Histological analysis of ANG treated E2F1 +/+ and -/- myocardial sections showed myocyte hypertrophy and an increase in fibrosis (Figure 1B). Gene expression of ANP was increased with ANG similarly in both E2F1 +/+ and E2F1 -/- mice (Figure 2).
Discussion
The major finding of our study is that ablation of E2F1 failed to prevent the development of LV hypertrophy induced by either ANG or ISO when continuously given for 14 or 7 days, respectively. In addition, changes in ejection phase (FS, Vcf) and isovolumic phase indices (MPI) were similar in WT and knockout animals, suggesting that E2F1 does not play a significant role in maintenance of cardiac function.
Previous studies have reported that blocking the function of E2F using an inhibitory peptide prevented myocyte hypertrophy and induction of hypertrophic markers [4, 11]. Moreover, when E2F1 was overexpressed in serum starved, quiescent cells, DNA synthesis was activated [2]. Taken together, these data suggest that the general inhibition of E2F functional pathways may treat diseases caused by hypertrophic growth. However, our data suggests targeting specific E2F proteins may not be effective in preventing ventricular hypertrophy.
The reasons for these disparate findings are not clear. It is possible that compensation by other E2F family members (e.g., E2F3) may be responsible. For example, when a specific E2F member is knocked out from birth, the other E2F family members are able to compensate for its function [12]. It is also possible that the specific hypertrophic pathway or the mechanism of E2F knockdown explains the disparity. Thus, while inhibition of E2F signaling through gene-targeting knockout of E2F1 did not show a decrease in beta-adrenergic agonist and angiotensin II-mediated hypertrophy, exogenous treatment with a blocking peptide that disrupted E2F binding prevented hypertrophy in cells stimulated with either serum or the alpha adrenergic receptor agonist, phenylephrine. Future experiments with knockout mice targeting multiple E2F proteins and using multiple hypertrophy inducing stimuli may provide a more complete inhibition of induced hypertrophy through pathways involved in regulation of cell cycle re-entry and DNA synthesis.
Acknowledgments
This research was supported by the National Institutes of Health [AG028679 (HGL); HL057506(BDH)].
References
- 1.Polager S, Ginsberg D. E2F - at the cross-roads of life and death. Trends Cell Biol. 2008;18:528–535. doi: 10.1016/j.tcb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 3.Shirodkar S, Ewen M, DeCaprio JA, Morgan J, Livingston DM, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- 4.Vara D, Bicknell KA, Coxon CH, Brooks G. Inhibition of E2F abrogates the development of cardiac myocyte hypertrophy. J Biol Chem. 2003;278:21388–21394. doi: 10.1074/jbc.M212612200. [DOI] [PubMed] [Google Scholar]
- 5.Wohlschlaeger J, Schmitz KJ, Takeda A, Takeda N, Vahlhaus C, Stypmann J, Schmid C, Baba HA. Reversible regulation of the retinoblastoma protein/E2F-1 pathway during “reverse cardiac remodelling” after ventricular unloading. J Heart Lung Transplant. 2010;29:117–124. doi: 10.1016/j.healun.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100:2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira EM, Krieger JE. Chronic beta-adrenoceptor stimulation and cardiac hypertrophy with no induction of circulating renin. Eur J Pharmacol. 2005;520:135–141. doi: 10.1016/j.ejphar.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 9.Lee HG, Chen Q, Wolfram JA, Richardson SL, Liner A, Siedlak SL, Zhu X, Ziats NP, Fujioka H, Felsher DW, Castellani RJ, Valencik ML, McDonald JA, Hoit BD, Lesnefsky EJ, Smith MA. Cell cycle re-entry and mitochondrial defects in myc-mediated hypertrophic cardiomyopathy and heart failure. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007172. e7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD. Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol. 2005;289:H30–36. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
- 11.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 12.Tsai SY, Opavsky R, Sharma N, Wu L, Naidu S, Nolan E, Feria-Arias E, Timmers C, Opavska J, de Bruin A, Chong JL, Trikha P, Fernandez SA, Stromberg P, Rosol TJ, Leone G. Mouse development with a single E2F activator. Nature. 2008;454:1137–1141. doi: 10.1038/nature07066. [DOI] [PMC free article] [PubMed] [Google Scholar]