Abstract
Subcorneal pustular dermatosis (SPD) represents a chronic, relapsing sterile pustular eruption, involving the trunk and flexoral proximal extremities. A 54-year-old female presented with recurrent, flaccid pustules measuring several millimeters in diameter, on normal and mildly erythematous skin of the groin and submammary areas. Biopsies for hematoxylin and eosin (H&E) examination, direct immunofluorescence (DIF) and immunohistochemistry (IHC) analysis were performed. The H&E staining demonstrated typical features of SPD, including some damage within dermal pilosebaceous units subjacent to the subcorneal blistering process. DIF revealed strong deposits of immunoreactants IgG, IgM, fibrinogen and complement/C3, present in a shaggy pattern within the subcorneal disease areas; in focal, areas of the basement membrane junction and in focal pericytoplasmic areas of epidermal keratinocytes. IHC revealed strong positivity to HLA-DPDQDR, mast cell tryptase, CD68, and ZAP-70 in the subcorneal inflammatory infiltrate, and surrounding dermal blood vessels. Myeloperoxidase was also positive. Positive staining with the anti-ribosomal protein S6-pS240 at the edges of hair follicles and sebaceous glands subjacent to the subcorneal blisters was also noted. Conclusions: We conclude that this disorder may have several components in its etiopathology, including a possible restricted immune response and a possible genetic component; these possibilities warrant further investigation.
Keywords: Subcorneal pustular dermatosis, anti-ribosomal protein S6-pS240, mast cell tryptase, HLA-DPDQDR, ZAP-70
Introduction
Subcorneal pustular dermatosis (SPD; Sneddon Wilkinson disease), is an uncommon, chronic, relapsing sterile pustular eruption typically involving the trunk and flexoral proximal extremities [1,2]. It most commonly affects women aged 40 years or older, and is occasionally classified with the neutrophilic dermatoses. The neutrophilic dermatoses represent non-infectious disorders, histopathologically characterized by a neutrophil predominant infiltrate and clinically rapidly responsive to corticosteroids or dapsone [1,2]. Conditions with a predominant neutrophilic vasculitis are excluded from this group. In our case report, we intend to briefly outline the dermnatopathology of neutrophilic dermatoses. Potential classification of other, selected dermatoses within this group, i.e., rheumatoid arthritis neutrophilic dermatosis and bowel associated-dermatosis syndrome has also been previously addressed [1,2].
Associations of SPD with other disorders have been documented, including IgG and IgA gam-mopathies or myelomas [3,4]. However, the exact pathophysiology of SPD is unknown. Earlier authors tested a patient with SPD for autoantibodies to desmogleins 1 and 3, and obtained negative results [5]. Other studies have recognized a subgroup of SPD with a presence of a utoanti bodies to IgA; however, further immunoblotting studies documented an autore-activity to desmocollin 1, and intercellular deposits of IgA as detected by direct immunofluo-resence (DIF). Some experts have considered this subgroup to be a rare variant of pemphigus, in contradistinction to a SPD subgroup [6].
Case Report
A 54-year-old female presented with a history of a relapsing pustular eruption involving the trunk and flexoral proximal extremities. The classic lesion presented as a “half and half” blister, in which purulent fluid seemed to accumulate in the lower half of the blister. The patient reported that some blisters had arisen within a few hours. Physical examination revealed individual pustular lesions in the flexoal areas of the extremities. Laboratory data included a normal complete blood count, and a normal erythrocyte sedimentation rate. Serum electrolytes, blood urea nitrogen, creatinine, liver function tests, urinalysis and chest radiographs were within normal limits. Biopsies of the lesions were performed, and submitted for hematoxylin and eosin (H&E) staining, multicolor direct im-munofluorescence (DIF) and immunohistochemistry (IHC); technical procedures were followed as previously outlined [7,8]. We utilized the following Dako antibodies: HLADPDQDR, mast cell tryptase, CD68, ZAP-70, anti-ribosomal protein S6-pS240 and myeloperoxidase.
Microscopic examination
H&E: Examination of the tissue sections demonstrated a subcorneal blistering disorder. Individual blisters were tense, and included serum scale crust. Within the blister lumens, numerous neutrophils were appreciated; lymphocytes and eosinophils were rare. Focal intraepidermal acantholysis was noted. No suprabasilar blistering was appreciated. Within the papillary dermis, a mild, superficial, perivascular infiltrate of lymphocytes, histiocytes, neutrophils and rare eosinophils was noted. No definitive evidence of an infectious, or a neoplastic process was seen. A PAS special stain was also reviewed; the positive control stained appropriately. The PAS special stain revealed no fungal organisms. Direct immunofluorescence (DIF) findings were as follows: IgG was seen as a sub-basement membrane zone(BMZ) reinforcement; specifically, a thin, linear band was appreciated under the BMZ of the dermal-epidermal junction. The pattern was distinctively different from the DIF patterns observed in bullous pemphigoid, dermatitis herpetiformis, epidermolysis bullosa acquisita and other subepidermal autoimmune blistering disorders. FITC conjugated IgE, IgA and fibrinogen were also seen in a pericytoplasmic and perinuclear pattern, in several patches within the epidermal stratum corneum (++). Other findings included IgM (+, intercellular epidermal stratum spinosum); IgD (+/-, focal BMZ cytoid bodies; complement/C1q (-); complement/C3 (+, roof of subcorneal pustules); albumin (+, intercellular epidermal stratum spinosum); and fibrinogen (++, focally within papillary dermal tip areas, focally within the superficial corneal layer, and surrounding some upper and intermediate dermal blood vessels). The blister lumens were positive for IgG, IgA, IgM, IgE and fibrinogen (Figure 1). A Gram stain of the blister was negative for bacterial organisms. Immuno-histochemisty (IHC) corroborated the DIF findings, and also revealed a strong positivity to mast cell tryptase (MCT), CD68 and HLADPDQDR surrounding upper dermal blood vessels under the inflammatory process (Figure 1). Myeloperoxidase was positive in the entire blister and in some of the corneal layers. We observed positive staining with HLA-DPDQDR, mast cell tryptase, CD68, and ZAP-70, anti-ribosomal protein S6-pS240 and myeloperoxidase Negative staining was noted with LAT, BAX, CD34, CD35, survivin and topoisomerase II alpha.
Figure 1.
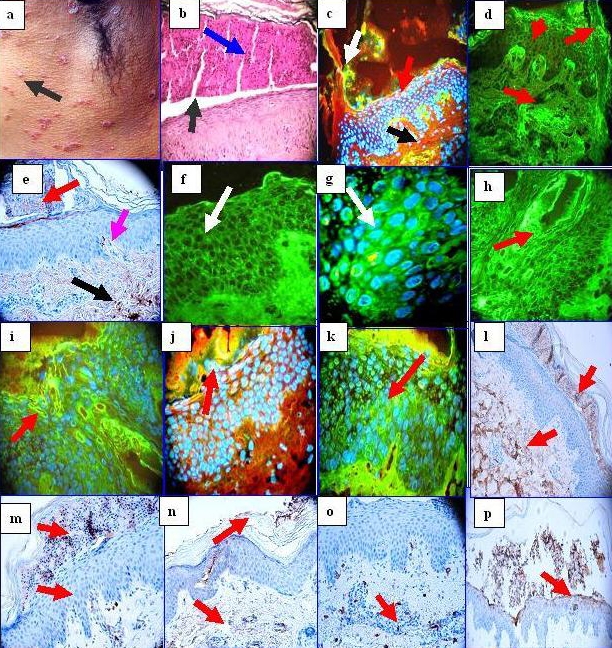
a. A group of clinical pustules near the axilla (black arrow). b. H & E stain, showing a subcorneal accumulation of neutrophils (blue arrow), within a subcorneal blister (black arrow). c. DIF showing localized deposits of FITC conjugated anti-human fibrinogen in multiple foci, including within a subcorneal blister (green staining; white arrow), within a dermal papillary tip (green staining; red arrow) and around upper dermal vessels (green staining; black arrow). Epidermal keratinocyte nuclei of are highlighted with 4',6-diamidino-2-phenylindole (Dapi) in blue d. Utilizing DIF and FITC conjugated fibrinogen in isolation, we were able to detect an identical reactivity pattern as observed in c (red arrows). e. IHC with anti-human fibrinogen antibody that corroborates our DIF findings of fibrinogen in a subcorneal blister (red arrow), within a dermal papillary tip (purple arrow) and around upper dermal blood vessels (black arrow). f. DIF showing staining between epidermal keratinocytes utilizing FITC conjugated anti-human IgG. The staining pattern resembles the intercellular staining pattern of pemphigus; however, the staining pattern observed in our patient appears more pericytoplasmic, and predominates in upper layers of the epidermis (white arrow). g. Similar to f, at higher magnification(white arrow) with epidermal keratinocyte nuclei counterstained with DAPI (light blue staining), h, i. Show similar DIF staining as f and g, but using FITC conjugated anti-human albumin. Please note enhanced staining in areas close to the epidermal corneal layer (red arrows). j and k, Show similar DIF staining as f and g, but using FITC conjugated anti-human-IgA. Please note enhanced staining in areas close to the epidermal corneal layer and the blister red arrows). l. Positive IHC staining with anti-human IgA within a subcorneal blister, and at multiple foci within the dermal extracellular matrix (brown staining, red arrows). m and n, IHCs demonstrating positive staining within a subcorneal blister and around upper dermal blood vessels with IgE in m, and with IgM in n (brown staining; red arrows). o. IHC, demonstrating strongly positive mast cell tryptase staining around the upper dermal blood vessels (brown staining). p, Strong IHC staining with anti-ribosomal protein S6-pS240.
Discussion
Direct and indirect immunofluorescence results have previously been reported as negative in subcorneal pustular dermatosis. However, previous investigators utilized single antibody, single fluorochrome DIF techniques. In contradistinction, we have utilized simultaneous multiple antibody, multiple fluorochome techniques. IHC was further utilized to corroborate our DIF findings, which could have been interpreted in earlier reports as non-specific in nature. By IHC, we also found strong positivity to mast cell tryptase (MCT), CD68 and HLA-DPDQDR associated with upper dermal blood vessels subjacent to the blistering process. Myeloperoxidase was positive throughout the blister lumens, and focally within the corneal layer by IHC staining.
Of interest, the upper corneal layer in proximity to the SPD blisters seems to be positive for IgG, IgM, IgA, IgE and fibrinogen. These findings suggest that SPD may display a delicate autoanti-body response to localized “dysregulated cells” in the corneal layer. Very few studies have addressed the immunotypification of SPD. One of the few studies suggested that the accumulation of neutrophils in the subcorneal pustules indicated that the upper epidermis released chemoattractants.12 Further, the neutrophilic chemoattractants interleukin (IL)-1 beta, IL-6, IL-8, IL-10, leukotriene B4, and complement components C5a and C5a were found at increased levels in scale extracts from patients with SPD, compared with those of controls [9]. Also, tumor necrosis factor-alpha levels have been found to be significantly elevated in the serum and blister fluid of patients with SPD. Some authors have described the presence of epidermal IgA by DIF in what were considered SPD cases. The authors evaluated cases with clinical SPD-like disease from 29 patients, and intraepidermal IgA detected by DIF [9]. The disease pustules were considered to be subcorneal; intraepidermal. IgA deposits were usually found in the intercellular areas of the epidermis, although a subcorneal linear pattern was also described. The disease usually responded to dapsone. In six cases, a monoclonal IgA gammopathy was present [3,4].
Our positive findings with HLA DPDQDR, mast cell tryptase, CD68, and ZAP-70, anti-ribosomal protein S6-pS240, and myeloperoxidase may indicate a restricted immune response, including the presence of T cell activation markers. In addition, the presence of ribosomal protein S6 kinase (which plays a critical role in the regulation of cell growth and energy metabolism) may indicate alterations within the family of serine/threonine kinases, downstream effectors of the mTOR and PI3K signalling pathways [10, 11]. Protein kinases are important regulators of intracellular signal transduction pathways, and thus play critical roles in diverse cellular functions. Previous reports have noted that steady-state levels of the S6K isoforms S6K1 and S6K2 are regulated by ubiquitination-mediated proteasomal degradation [10, 11]. Moreover, accumulating evidence indicates that the activation of protein kinases, in turn, commonly initiates their downregulation via the ubiquitin/proteasome pathway. Failure to regulate protein kinase activity or expression levels can elicit human disease. Thus, our findings documenting the presence of this antibody exclusively directed to focal areas of the hair follicle and sebaceous glands warrants further investigation.
Acknowledgments
We would like to thank Jonathan S. Jones, HT (ASCP) for excellent technical work. All work was performed with funds from Georgia Dermatopathology Associates (GDA).
References
- 1.Sneddon IB, Wilkinson DS. Subcorneal pustular dermatoses. Br J Dermatol. 1956;68:385–394. doi: 10.1111/j.1365-2133.1956.tb12774.x. [DOI] [PubMed] [Google Scholar]
- 2.Nischal KC, Khopkar U. An approach to the diagnosis of neutrophilic dermatoses: A histo-pathological perspective. Indian J Dermatol Venereol Leprol. 2007;73:222–230. doi: 10.4103/0378-6323.33634. [DOI] [PubMed] [Google Scholar]
- 3.Takata M, Inaoki M, Shodo M, Hirone T, Kaya H. Subcorneal pustular dermatosis associated with IgA myeloma and intraepidermal IgA deposits. Dermatology. 1994;189:111–114. doi: 10.1159/000246947. [DOI] [PubMed] [Google Scholar]
- 4.Scerri L, Zaki I, Allen BR. Pyoderma gangrenosum and subcorneal pustular dermatosis, without monoclonal gammopathy. Br J Dermatol. Mar. 1994;130:398–399. doi: 10.1111/j.1365-2133.1994.tb02941.x. [DOI] [PubMed] [Google Scholar]
- 5.Bordignon M, Zattra E, Montesco MC, Alaibac M. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) with absence of desmoglein 1 and 3 antibodies: case report and literature review. Am J Clin Dermatol. 2008;9:51–55. doi: 10.2165/00128071-200809010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Gruss C, Zillikens D, Hashinoto T, et al. Rapid response of IgA pemphigus of subcorneal pustular dermatosis type to treatment with isotretinoin. J Am Acad Dermatol. 2000;43:923–926. doi: 10.1067/mjd.2000.104002. [DOI] [PubMed] [Google Scholar]
- 7.Abreu Velez AM, Howard MS, Brown VM. Antibodies to piloerector (arrector pili) muscle in a case of lupus/lichen planus overlap syndrome. North Am J Med Sci. 2010;2:276–280. [PMC free article] [PubMed] [Google Scholar]
- 8.Abreu Velez AM, Klein AD, Howard MS. Autore-activity to sweat glands and nerves in clinical scabies infection. North Am J Med Sci. 2010;2:422–425. [PMC free article] [PubMed] [Google Scholar]
- 9.Grob JJ, Mege JL, Capo C, et al. Role of tumor necrosis factor-alpha in Sneddon-Wilkinson subcorneal pustular dermatosis. A model of neutrophil priming in vivo. J Am Acad Dermatol. 1991;25:944–947. doi: 10.1016/0190-9622(91)70290-i. [DOI] [PubMed] [Google Scholar]
- 10.Gwalter J, Wang ML, Gout I. The ubiquitination of ribosomal S6 kinases is independent from the mitogen-induced phosphorylation/activation of the kinase. Int J Biochem Cell Biol. 2009;4:828–833. doi: 10.1016/j.biocel.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Hunter T. Degradation of activated protein kinases by ubiquitination. Annu Rev Bio chem. 2009;78:435–75. doi: 10.1146/annurev.biochem.013008.092711. [DOI] [PMC free article] [PubMed] [Google Scholar]


