Abstract
Clinical presentation with dural-based metastasis mimicking meningiomas is rare. We aimed to evaluate the role of frozen section in guiding surgery and histopathologic diagnosis in determining primary sites of dural-based metastatic carcinomas. Following the receipt of HAC approval, we retrospectively reviewed 7cases presenting with dural-based masses clinically suspected to be primary brain tumors (6 meningiomas and 1 superficial glioblastoma), but diagnosed to be metastatic carcinomas on subsequent resection. Pertinent clinical records and follow-up data were reviewed. Patient's age ranged from 59 to 80 years. Imaging showed extra-axial dural-based masses with contiguous but not primary brain involvement. On intra-operative frozen section (not performed in case 7), differential diagnoses included metastatic carcinoma in all cases, and surgery modified accordingly. Nesting, cribriform, and “picket-fence” like glands were among useful histologic diagnostic patterns. Immunoprofile supported histologic diagnosis in all cases. Subsequent clinical and radiologic evaluation confirmed coexistent sites of origin in all cases. The metastases were solitary in all cases; except multiple dural-based tumors in case 1, in which interestingly no systemic metastasis were identified. Dural-based metastatic carcinomas mimicking meningiomas may be solitary, of unknown primary, or without concomitant systemic spread on imaging. Frozen section evaluation is helpful in modifying surgery. Although high-grade, these are typically differentiated enough to allow accurate histopathologic diagnosis, and reasonable determination of primary tumor site, especially with a judicious panel of cytokeratins, transcription factors, hormone receptors and relatively organ-specific markers. Clinicians and pathologists need to be aware of the occurrence, spectrum, need for timely intervention, and accurate diagnosis of dural-based metastatic carcinomas.
Keywords: Dural, meningioma, metastatic carcinoma, radiology, immunohistochemistry, frozen section
Introduction
Clinical presentation with dural-based metastasis mimicking meningioma is very rare and described mostly as case reports from diverse primary locations. Historically, the most common primary sites in surgically resected dural metastases have been found to be breast and prostate, but may also be from a primary melanoma, sarcoma, or lymphoma. These often presented as single, cranial, and subdural lesions, as opposed to multiple lesions from diverse primary sites including unusual primary sites in autopsy series [1]. Clinically and/or radiologically these lesions are often misinterpreted as meningioma, which may delay surgery and consequently have a deleterious impact on patient care. A clinical diagnosis of “meningioma” may even mislead the pathologist at the time of in-traoperative consultation or the presurgical treatment algorithm. The importance of awareness among oncologists, neurosurgeons, and neuropathologists that carcinomas of diverse primary sites can present with dural-based masses and radiologically mimic meningiomas cannot be overstated. A correct diagnosis of metastasis on frozen section along with a detailed presurgical evaluation, should allow suitable modification to surgical planning, especially in elderly patients or those with known previous malignancies. This study was aimed at evaluating the role of intraoperative frozen section in establishing the diagnosis of dural-based metastatic carcinomas, and the role of histologic and immunohistochemical analysis on formalin-fixed paraffin-embedded (FFPE) sections in determining the primary tumor site to suitably guide oncologic therapy.
Materials and methods
Following the receipt of HAC approval, we retrospectively reviewed 7 cases presenting with dural-based masses clinically suspected to be primary brain tumors (6 meningiomas and 1 superficial glioblastoma), but diagnosed to be metastatic carcinomas on subsequent resection and examination. Their pathology slides and reports along with pertinent clinical records and follow-up data were reviewed.
Results
Case 1
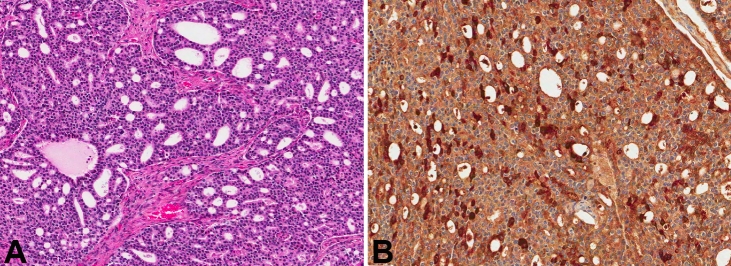
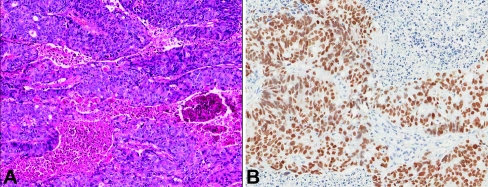
A 78-year-old African American (AA) male presented with complaints of altered mental status (Table 1). The patient had been developing confusion since falling and hitting his head three days prior to admission. A computed tomography (CT) scan was performed, which showed multiple extra-axial, dural-based masses in the bilateral frontal, temporal, and parietal convexities. Radiologic impression was multiple meningiomatosis. The patient was brought to the operating room for resection and an intraoperative consultation was sought. A smear and frozen section revealed a neoplasm with cribriform architecture, suggestive of high-grade prostatic adenocarcinoma (Figure 1A). Subsequent review of the patient's chart revealed a history of prostate carcinoma, diagnosed at an outside facility. A PET (positron emission tomography)-CT did not show evidence of any other metastatic focus. Immunohistochemistry on FFPE tissue showed the neoplastic cells to be prostate specific antigen (PSA) positive (Figure 1B) and cytokeratin (CK) 7 and 20 negative, confirming the histologic diagnosis. The patient was lost to follow-up immediately after surgery and therefore his treatment is unknown.
Table 1.
Clinicopathologic features of the examined cases with dural-based metastases
| Case number | Age/Sex | Location | Radiology Impression | Immunoprofile | Path Proposed Primary | Confirmed Primary | Mode of Primary Confirmation | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 78/M | Frontal, temporal, and parietal | Multiple meningiomatosis | PSA+, CK7-, CK20- | Prostate | Prostate | Chart review; Previous outside diagnosis | Lost to follow-up immediately after surgery |
| 2 | 75/M | L parietal | Meningioma | Pankeratin+, PSA+, CK7-, CK20-, TTF1-, PR- | Prostate | Prostate | CT showing enlarged prostate with bladder invasion | Alive 2 months+after surgery |
| 3 | 59/F | L frontal | Meningioma | Pankeratin+, CK7+, CK20-, TTF1-, CDX2-, ER-, PR- | Breast | Breast | Subsequent breast biopsy showing invasive ductal carcinoma | Alive 9 months+after surgery |
| 4 | 80/M | R parietooccipital | Meningioma | Pankeratin+, CK7+, TTF1+, CK20-, CDX2- | Lung | Lung | CT showing lung mass with hepatic and adrenal metastases | Alive 3 years+after surgery |
| 5 | 71/F | L frontal | Meningioma or metastasis | CK20+, CK19+, CDX2+, CK7-, TTF1- | Colorectal | Colon | Outside hospital colon resection | Alive 2 years+after surgery |
| 6 | 59/M | R parietal | GBM involving dura | CK7+, CK19+, CK20-, TTF1-, CDX2- | Lung, upper Gastrointestinal, pancreaticobiliary, or head & neck | Lung | PET showing lung mass | Alive 2.5 years+after surgery |
| 7 | 64/M | R frontotemporoparietal | En-plaque meningioma | PSA+ | Prostate | Prostate | History of prostatic carcinoma | Alive a few days after surgery |
M: male; F: female; R: right; L: left; GBM: glioblastoma multiforme; PSA: Prostate specific antigen; CK: cytokeratin; TTF: thyroid transcription factor; PR: progesterone receptor; CDX: caudal-related homeobox; ER: estrogen receptor; CT: computed tomography; PET: positron emission tomography
Figure 1.
A. H&E stained slide from patient 1 showing cribriform architecture with bland cells (magnification, ×7). B. Cells in (A) stained positive with antibodies to PSA (magnification, ×10).
Case 2
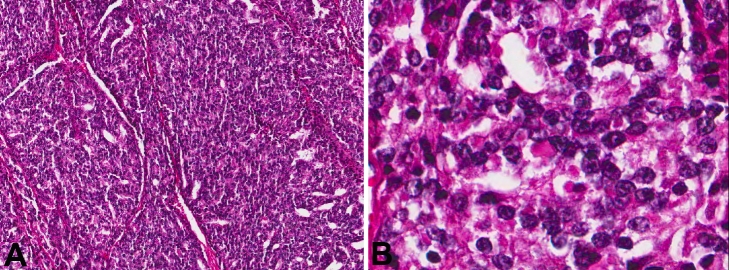
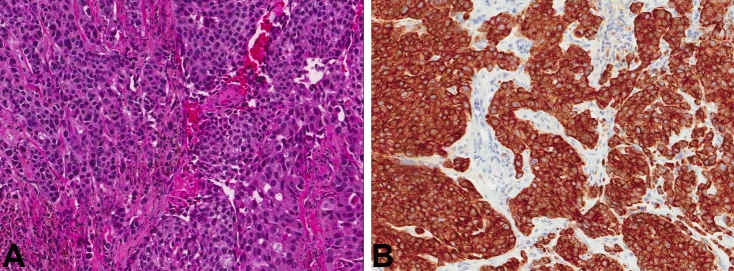
A 75-year-old Caucasian male presented with complaints of right facial drop, aphasia, and mild headaches for 2 to 3 weeks. The patient denied any nausea or vomiting, blurred vision. He reported weight loss, but denied any prior history of malignancy (Table 1). A CT scan showed an area of vasogenic edema surrounding an enhancing 4.3 × 2.1 × 4.3 cm extra-axial mass in the left parietal lobe. This vasogenic edema was causing a small amount of effacement of the posterior horn of the left lateral ventricle without evidence of midline shift. Radiologic impression was meningioma. He was initially treated conservatively for 2 weeks with observation, however, his headaches were worsening, and he subsequently developed decreased grip strength and leg weakness. Subsequent MRI of brain showed an extra axial lesion measuring 3.7 × 2.2 × 3.9 cm, involving the left parietal bone, with significant mass effect. With advancing symptoms and deficit, the patient was sent to operating room for resection. On intraoperative smear and frozen section, the differential diagnosis included metastasis versus atypical meningioma. The FFPE sections showed a poorly differentiated carcinoma with a random admixture of acinar, cribriform, nested, and solid/syncytial growth patterns (Figure 2A). The neoplastic cells were uniform with rounded nuclei, prominent nucleoli, and moderate amounts of cytoplasm (Figure 2C). Immunohistochemistry showed the neoplastic cells to be positive for pankeratin, PSA, prostate specific acid phosphatase (PSAP) and PIN-4 (peptidyl-prolyl cistrans isomerase NIMA-interacting 4). CK7, CK20, TTF1 (thyroid transcription factor 1), and progesterone receptor (PR) were negative. A follow-up CT scan revealed an enlarged, heterogeneous prostate with possible invasion of the bladder floor and diffuse bony metastases with pelvic lymphadenopathy. He was also found to have T2 to T10 metastases with partial spinal cord compression. A serum PSA was elevated at 82.08 ng/ml. The patient initially received targeted radiation therapy to his spine to palliate the cord compression. Meanwhile, he was concurrently treated with androgen deprivation therapy. However, during his radiation therapy, he developed several necrotic skin lesions, deemed to be secondary to tumor emboli phenomena. After completion of radiation therapy, he declined the further chemotherapy or hormonal therapy and was discharged to home with hospice care on comfort measures only two months after craniotomy.
Figure 2.
A. H&E stained slide from patient 2 showing cribriform architecture (magnification, ×8). B. High-power view of cells in figure 2a showing bland cells with nucieoii (H&E; magnification, ×40).
Case 3
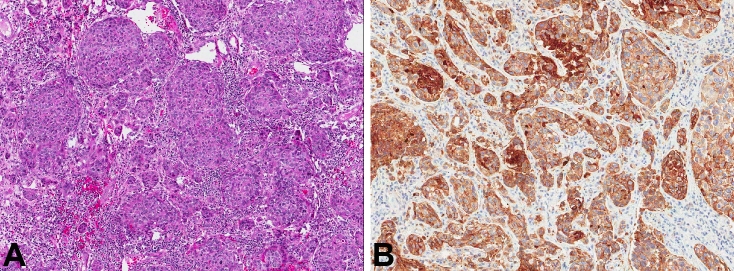
A 59-year-old AA female presented with weakness of the right side for 2 weeks, agraphia, and aphasia for 2 to 3 days. The patient denied any nausea, vomiting, headaches, other symptoms, or prior history of malignancy (Table 1). A CT scan revealed a left frontal lobe mass, measuring approximately 3 cm with associated significant vasogenic edema producing some mass effect, particularly producing partial effacement of the frontal horn of the left lateral ventricle. There was no evidence of midline shift with initial radiographic impression of this mass as a meningioma versus metastatic disease. A follow-up magnetic resonance imaging (MRI) favored meningioma. On intraoperative frozen section, the differential diagnosis included metastasis versus anaplastic meningioma. Hematoxylin and eosin (H&E) stained FFPE sections demonstrated an epithelial neoplasm with nesting and desmoplasia, morphologically consistent with metastatic ductal adenocarcinoma (Figure 3A). Pankeratin and CK7 (Figure 3B) were positive. CK20, TTF1, CDX2 (caudal-related homeobox 2), as well as estrogen receptor (ER) and PR were negative. A subsequent CT scan showed multiple large breast masses with axillary lymphadenopathy and probable metastatic disease to the lung. A breast biopsy revealed invasive ductal adenocarcinoma, which was ER and PR negative. The patient underwent whole brain radiation as well as four cycles of single-agent trastuzumab-based therapy. However, due to her poor performance status, further systemic therapy was not pursued. She was referred to hospice for palliative care and lost to follow-up 9 months after craniotomy.
Figure 3.
A. H&E stained slide from patient 3 with ductal-like growth of neoplastic cells (magnification, ×5). B. CK7 immunohistochemical stain was positive in patient 3 (magnification, ×7).
Case 4
An 80-year-old Caucasian male with a history of prostate cancer status post radiation therapy 10 years ago presented to his primary physician's office with chief complaints of fatigue, mild headache, and blurred vision for 3 month, without any nausea, vomiting, or focal weak-ness was referred to our institution for further evaluation (Table 1). A CT scan revealed a bi-lobed, dural-based mass, centered on the pos terior interhemispheric falx, most consistent with a meningioma. A subsequent MRI of the brain showed two parafalcine heterogeneous, centrally necrotic lesions which heterogeneously enhanced and had evidence for hemorrhage. Radiologic considerations again included hemorrhagic metastases versus atypical meningioma. The patient was referred to neurosurgery for diagnosis. On intraoperative smear and frozen section, the differential diagnosis in cluded high-grade meningioma versus metastasis. H&E stained FFPE sections demonstrated an epithelial neoplasm arranged in nests, cords, and ribbons surrounded by thin-walled capillary channels (Figure 4A). Patchy necrosis was also identified. The individual tumor cells had stippled chromatin and prominent nucleoli. Immunohistochemical stains on FFPE sections showed positivity for pankeratin, CK7, chromogranin, and TTF1 (Figure 4B). CK 20, CDX2, PR, and calcitonin were negative. This morphology and immunophenotype (CK7, chromogranin and TTF1 positivity) are consistent with a metastatic large cell neuroendocrine carcinoma of lung primary. The lack of marked pleomorphism, relatively low mitotic activity and low Ki-67 labeling raised the close differential diagnosis of an atypical carcinoid tumor of lung origin. A negative calcitonin stain excluded a medullary thyroid carcinoma. A follow-up whole body CT scan revealed a 1.6 cm rounded soft tissue density in the left lower lobe of lung (likely primary site), with multiple hepatic lesions, and bilateral adrenal masses likely representing additional metastases. He was referred for physical therapy and occupational therapy. Thereafter, he obtained chemotherapy and hormonal therapy. The patient was treated with Gamma knife radiation to residual tumor, and was alive almost three years after his resection. Follow-up brain MRIs have been negative for recurrent or new intrac-ranial lesions.
Figure 4.
A. Medium-power view of cellular neopiastic infiltrate from patient 4 (H&E; magnification, ×10). B. The infiltrate in figure 4a showed positive nuclear staining with TTF1 (magnification, ×10).
Case 5
A 71-year-old Caucasian female with a history of heavy tobacco use (>50 years) and a prior hysterectomy for fibroids, but otherwise no other significant medical history presented with new onset of seizures (Table 1). A large dural-based mass in the left frontal region, measuring 4.7 × 4 cm, was noted on MRI. There was associated vasogenic edema seen surrounding this mass with 4-5 mm midline shift toward the right side.
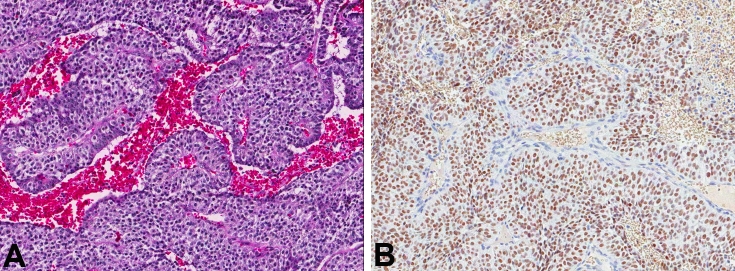
Radiologic impression was that of meningioma versus dural-based metastasis. The patient underwent a stereotactic volumetric computer-assisted targeted craniotomy for assessment of this supratentorial intraparenchymal brain tumor. On intraoperative smear and frozen section, the diagnosis of metastatic adenocarcinoma was rendered. H&E stained FFPE sections demonstrated a moderate to poorly-differentiated adenocarcinoma with “picket-fence”-like architecture with necrosis (Figure 5A). The tumor was immunopositive for CK 20, 19, and CDX2 (Figure 5B) and negative for CK7 and TTF1. These histopathologic features were most consistent with colorectal primary. Although a metastatic work-up at that time did not reveal a primary malignancy, colon carcinoma was diagnosed and treated with resection 2-months later at an outside institution per follow-up clinical records. The patient received whole-brain radiation therapy, and was alive almost two years after his resection. Follow up MRI 2-years later demonstrated previous craniotomy and a cortical-subcortical enhancing lesion at the junction of the left middle frontal and inferior frontal gyri, measuring 20 × 18 mm lesion in keeping with metastatic disease, which was targeted for Gamma knife. No additional parenchymal lesions were identified.
Figure 5.
A. Adenocarcinoma with abundant necrosis from patient 5 (H&E; magnification, ×6). B. These cells in 5a were positive with antibodies to CDX-2 (magnification, ×10).
Case 6
A 59-year-old AA male was transferred from an outside facility because of new onset seizures.
He had no history of nausea, vomiting, focal weakness. MRI of the brain revealed a hetero-geneously enhancing mass (3.2 × 3.1 × 2.2 cm) in the right parietal lobe in the parasagittal location with areas of necrosis/cystic degeneration. The mass extended peripherally in the midline apparently involving the dura (Table 1). Radiologic impression was that of a superficial glioblastoma multiforme (GBM) extending to involve the dura. On intraoperative smear and frozen section, the diagnosis of metastatic carcinoma, non-small cell type was rendered (Figure 6A). The immunoprofile of neoplastic cells on FFPE sections for CK7 (Figure 6B), and CK19, along with lack of staining for CK20, TTF1, and CDX2 suggested a lung primary. Follow-up metastatic work-up showed a lung primary, with PET active lung nodules and medi-astinal lymphadenopathy. The patient was treated with whole brain radiation therapy, two cycles of adjuvant chemotherapy with cisplatin and paclitaxel, followed by 17-doses of single agent docetaxel and more recently erlotinib. About one-year post-surgery, a follow-up PET scan showed an active intracranial focus for which he received a total of 20 gray of gamma knife radiation. He is alive almost two and a half years following surgery, with activity in lung and lymph nodes but without evidence of recurrent or new intracranial lesions.
Figure 6.
A. H&E stained slide from patient 6 showing metastatic adenocarcinoma (magnification, ×10). B. CK7 immunohistochemical stain was positive in patient 6 (magnification, ×10).
Case 7
64-year-old white male, who had prior prostatectomy for prostatic carcinoma 4 years prior, was placed on chemotherapy after detection of metastatic disease to the spine, but was unresponsive to three clinical trials with evidence of disease spread. Recent post-gadolinium T1-weighted MRI showed an extensive 10.5 × 2.3 × 5.5 cm predominantly extra-axial dural-based, right lateral convexity fronto-temporo-parietal, irregular, enhancing solitary mass. Associated vasogenic edema with 13 mm subfalcine herniation, and effacement of right lateral ventricle was noted. The appearance was of en plaque meningioma with invasion to the brain, but was consistent with dural metastasis by history. Craniotomy with tumor resection was performed. Gross examination showed nine fragments of tan/brown soft tissue, with focal yellow areas measuring 7.0 × 5.7 × 1.5 cm in aggregate. Histologic examination showed a metastatic adenocarcinoma, with prominent cribriform architecture (similar to case 1), high-grade nuclear features, and immunopositivity for PSA, consistent with prostatic primary. Whole body bone scan showed increased uptake in the right frontoparietal and focal uptake in the left parietal skull. Increased uptake was also noted in the diaphysis of the left humerus, right third and tenth rib, left scapula or lateral left 5th rib, T11 and T12 vertebral bodies, superior left acetabulum, inferior right acetabulum, and right sacroiliac joint region.
Discussion
Classification of diseases presenting as dural-based masses
Both neoplastic and non-neoplastic lesions can present as dural-based masses, radiologically mimic meningiomas, and yet require different therapeutic approaches. The non-neoplastic lesions include infectious and non-infectious etiologies such as tuberculomas, neurosarcoidosis, plasma cell granulomas, Rosai-Dorfman disease, xanthomas, Castleman disease, and rheumatoid nodules [2, 3]. A thorough review of the patient's clinical history as well as the use of special stains to highlight infectious organisms is necessary to establish the diagnosis of these entities. Neoplastic lesions involving the dura are nearly always primary dural tumors mainly meningiomas, occasionally primary superficial brain tumors, and rarely metastatic lesions. Rare primary dural lesions include solitary fibrous tumors (SFT), hemangiopericytomas, and leiomyosarcomas [3]. Primary brain tumors, which are superficially located, such as gliosarcoma or pleomorphic xanthoastrocytoma may mimic a meningioma, particularly if associated with desmoplastic response from involved meninges.
Carcinoma of diverse primary sites and histology can uncommonly present with solitary dural metastases in elderly, especially in the parasagittal convexity, and radiologically as well as clinically mimic meningioma. For instance, meningiomas have a clear female predominance and are associated with several disease states such as breast carcinoma. Therefore it is not improbable that a dural-based metastatic breast carcinoma in a female patient with a previous diagnosis of ductal adenocarcinoma can be easily mistaken clinically for a meningioma Furthermore, radiologically, dural-based metastatic carcinoma can be indistinguishable from meningioma as they may have the telltale sign of a “dural-tail”, which has been traditionally regarded as a typical feature of meningioma [4]. Therefore, relying on clinical and radiologic findings may be misleading.
Metastatic lesions to the dura are more varied than previously thought in both sites of origin and post-operative outcome, with some patients surviving in excess of 2 years, including a subset of our cases [1,5]. Surgical cases have shown a greater spectrum of tumor types than autopsy series [1]. Carcinomas of breast and lung origin in females and prostate and lung origin in males have been the most commonly reported dural based metastases. A recent series from Memorial Sloan-Kettering Cancer Center, identified breast carcinomas as being the most common malignancy to cause dural-based metastasis with 41 of their 122 cases (34%) being from breast metastases as compared to 17% from prostate [4]. However, tumors from other uncommon primary sites with dural-based metastases are being increasingly cited. These include cervical, endometrial, gastric, and renal cell carcinomas [1]. Dural-based metastases have also been reported from primary sarcomas, skin cancers, and lymphomas. Dural involvement of these metastatic lesions was most commonly due to direct extension of metastatic skull metastases [4]. Hematogenous spread and possible surgical seeding were also noted in 33% and 6% of cases, respectively. Contiguous malignant disease in underlying brain was particularly frequent with non-ocular melanoma, whereas contiguous skull without brain involvement has been reported more often in metastatic breast carcinoma [1]. Most cases of dural metastatic disease in the autopsy series were associated with lung involvement, especially lymphangitic and intravascular pulmonary tumor spread, suggesting heavy vascular tumor burden [1]. One of our cases (case 7) had coexistent overlying bone metastases, however none had other brain parenchymal, dural venous sinus involvement or subdural hematoma. No coexistent meningiomas were identified. Moreover, no lymphangitic or intravascular tumor spread was documented. Hematogenous route appears to be the most likely mode of tumor spread in our series.
Of note, overall survival and progression free survival improved with resection [1,4]. In a study of 22 patients, median patient survival of 11 months following resection of the dural tumor, was comparable to metastatic brain tumors without dural extension of 10 months [6], but suggested that unlike most brain metastases, patients with metastatic brain tumors with dural extension did not necessarily have more end-stage disease and could potentially be suitable surgical candidates. Tumor survival appears to have varied considerably depending primarily on histologic tumor type and the extent of systemic tumor involvement [1]. Primary sites in our series were represented by breast (1 case), lung (2 cases), prostate (3 cases) and colorectal primary (1 case). Of the 3 cases of prostatic carcinoma in our series, 1 did not have any other sites of metastasis on subsequent PET-CT. Cases 4,5, and 6 have been alive and free of dural disease at least 2-years following resection of dural-based tumor.
Appropriate imaging studies, such as CT scan or MRI, can be helpful to differentiate the malignant tumor from a benign process, but there have been somewhat conflicting reports published in the past as to their efficacy. The so-called “dural tail sign” or “flare sign” in MRI examination is almost specific to meningioma, however, after further assessment by surgery, there are findings of a wide spectrum of disorders, such as neuromas, chloromas, metastases, lymphoma, gliomas, pituitary diseases, granulomatous disorders, and also cerebral Erdheim-Chester disease. Current guidelines suggest that the dural tail sign is not specific to a particular pathological process. In second case of this report, the MRI of brain showed a dural tail sign, both anterior and posteriorly to the lesion, that lead to the radiologic impression of meningioma, but the final pathology report indicated a prostate cancer with dural metastasis. Based on such cases and previously published studies, the final diagnosis must be based on the cerebrospinal fluid study and histological study. In addition, a thorough clinical history and examination is also very vital to overall treatment planning.
Role of intraoperative pathologic consultation (frozen section)
When a dural-based lesion is received for intraoperative consultation with the clinical history of “meningioma”, the presumptive clinical diagnosis must be understood to have a wide range of pathologic differential diagnoses, and accordingly met with appropriate skepticism by the pathologist. Solitary deposits of metastatic carcinoma represent one such possibility, and when poorly differentiated, can occasionally range in the histologic differential diagnosis of anaplastic meningioma or melanoma, at the time of frozen section, and become a source of diagnostic pitfall. Therefore, when morphologic differential diagnosis on frozen section includes an anaplastic meningioma, poorly differentiated carcinoma, and melanoma, it is best to relay this differential diagnosis to the neuro-surgeon and oncologist, thereby allowing them to do a systemic PET-CT imaging work up to evaluate for other possible primary sites, pending final histopathologic diagnosis on FFPE sections.
In our series, differential diagnosis of or favoring metastatic carcinoma was correctly offered on all cases at the time of frozen section (excluding case 7, in which no frozen section was requested) based on morphology, subsequently confirmed on final pathologic evaluation and corroborated by review of clinical records and/or follow up. Diagnostic features that were particularly helpful included cribriform architecture and prominent nucleoli for prostate carcinoma (cases 1, 2 and 7), ductal growth with desmoplasia for breast carcinoma (case 3), and extensive necrosis with” picket-fence” architecture favoring a colorectal primary (case 5). Frozen section can also help adequately triage other dural-based lesions. For example, if an infectious etiology is considered at the time of frozen section, it is wise to request fresh tissue for culture with realization that some organisms, namely Mycobacterium tuberculosis, are more fastidious in nature and may need special culture media.
Histopathologic and immunohistochemical approach to differential diagnosis
Histopathologic evaluation combined with judiciously selected immunohistochemical stains can resolve nearly all differential diagnostic considerations on FFPE sections. Our study clearly shows that histopathologic evaluation combined with a judicious immunohistochemical panel on FFPE sections including transcription factors and hormone receptors can reliably differentiate metastatic carcinomas from anaplastic meningioma, a known close differential diagnostic consideration [7], and also determine the primary tumor site of latter cases with reasonable precision to guide oncologic therapy. It may be possible to suggest the primary site on morphologic basis alone in some cases such as those of breast (ductal growth with desmoplasia), high-grade prostate (cribriform), or colorectal carcinoma (adenocarcinoma with “picket-fence” architecture and geographic necrosis with nuclear debris) [8], patterns which to the best of our knowledge have not been described in either melanoma or anaplastic meningioma [9,10]. However, it is prudent to perform immunohistochemical studies to provide a more comprehensive evaluation.
Strong diffuse pankeratin with negative EMA (epithelial membrane antigen) supports the histopathologic diagnosis of metastatic carcinoma, whereas the reverse pattern favors meningioma. Depending on the morphology and proposed possible primary sites, an initial panel of CK7, CK20, mucicarmine, CK5/6, and p63 can support morphologic differentiation between a poorly differentiated adenocarcinoma and squamous cell carcinoma. Cytokeratin expression has been shown to remain relatively stable as malignancy evolves and metastasizes [11]. Further work-up of an adenocarcinoma is best directed by The CK7/CK20 profile [12,13]. However, transcription factors and hormone receptors play a key role in this evaluation. For instance, TTF1 positivity in a CK7 positive, CK20 negative carcinoma confirms lung primary, with the only exception of a thyroid primary which has been exceptionally reported to cause dural-based metastasis [4]. Positivity for CDX2 (a transcription factor of intestinal differentiation) in a CK 7 negative CK20 positive adenocarcinoma supports a colo-rectal primary, whereas in a CK 7 positive CK20 negative tumor indicates an upper GI primary, but may also be positive in mucin-secreting carcinomas of ovary and lung. In a tumor with acinar or cribriform architecture with prominent nucleoli in a male patient, the possibility of metastatic prostate carcinoma must be excluded using PSA, PSAP and PIN-4.
Finally, if morphology suggests a ductal carcinoma of breast in a female, ER and PR stains as well as Her-2 neu analysis (by immunohistochemical staining or FISH analysis) can further support such a possibility in clinically unsuspected cases. It is imperative to remember that meningiomas are often PR positive, and therefore, its presence is not diagnostic of a breast carcinoma or other gynecologic malignancy. However, it is unlikely that a breast or gynecologic malignancy would show only PR positivity with negative ER expression as ER positivity is typically more sensitive [13]. Furthermore, it is important to remember that antigenicity may be lost or gained as a tumor metastasizes so it is best to use more than one marker per suspected primary site when possible. Ultimately, the use of a panel of immunohistochemical stains on paraffin sections before committing to a diagnosis is often the most reliable approach.
Immunohistochemistry can also help exclude other uncommon dural based primary tumors. SFT can be especially difficult to distinguish from a fibrous meningioma; however, the presence of fibroblastic cells with intercellular collagen, CD34 (cluster of differentiation) positivity, and EMA negativity distinguish SFT from meningioma [14]. Hemangiopericytomas show compactly arranged short spindle cells with prominent staghorn vascular channels, CD34 positivity, and EMA negativity thus distinguishing it from angiomatous meningioma. A leiomyosarcoma should have spindle neoplastic cells with blunt ends, and show positivity for smooth muscle actin and desmin. In addition, melanocytic neoplasms, either metastatic from a systemic (usually cutaneous) primary or uncommonly a primary arising from meningeal melanocytes, can present as dural-based masses. This tumor with markedly heterogeneous morphology must be evaluated with the aid of melan A and HMB-45 (Human Melanoma Black-45). Of note, both meningiomas and melanomas may show S100 positivity, and therefore, this stain is not useful in distinguishing these two entities [9]. In monomorphic lymphoid or plasma cell rich lesion, evaluation of light chain expression for polytypic nature of the infiltrate versus clonality is vital to rule out a lymphoma or plasmacytoma.
In conclusion, carcinomas of diverse primary sites and histology can rarely present with dural metastases in elderly, especially in parasagittal convexity, thereby clinically and radiologically mimic meningiomas. The metastasis may be solitary simulating a primary brain neoplasm (noted in all, except case 1). It may present with unknown primary. Moreover, even in cases of identifiable coexistent primary, no other systemic sites of metastasis may be recognized on whole body imaging (case 1). If correct diagnosis of metastasis is not established, surgery and oncologic therapy may be delayed often under the mistaken impression of meningioma, causing deleterious effects to patient care necessary for high-stage carcinomas of visceral origin. Frozen section can reliably render a diagnosis or at least raise a differential diagnosis of metastatic carcinoma, leading to timely performance of whole-body imaging. A judicious combination of cytokeratins, transcription factors especially TTF1 and CDX2, hormone receptors and relatively organ-specific markers (PSA) as well as thorough history-taking and physical examination in a multidisciplinary setting are helpful in determining the primary site with reasonable precision.
References
- 1.Kleinschmidt-DeMasters BK. Dural metastases. A retrospective surgical and autopsy series. Arch Pathol Lab Med. 2001;125:880–887. doi: 10.5858/2001-125-0880-DM. [DOI] [PubMed] [Google Scholar]
- 2.Savage NM, Shah H, Alleyne CH, Switzer JA, Lee JR, Steele J, Sharma S. Neurosarcoidosis with Necrotizing Sarcoid Granulomatosis Mimicking Meningiomatosis Cerebri: Case Report and Literature Search. British Medical Journal Case Reports. 2009 doi: 10.1136/bcr.11.2008.1187. [doi: 10.1136/bcr.11.2008.1187] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33:1211–1226. doi: 10.1053/hupa.2002.129200. [DOI] [PubMed] [Google Scholar]
- 4.Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer. 2009;115:1947–1953. doi: 10.1002/cncr.24203. [DOI] [PubMed] [Google Scholar]
- 5.Lyons MK, Drazkowski JF, Wong WW, Fitch TR, Nelson KD. Metastatic prostate carcinoma mimicking meningioma: case report and review of the literature. Neurologist. 2006;12:48–52. doi: 10.1097/01.nrl.0000186809.04283.17. [DOI] [PubMed] [Google Scholar]
- 6.Rumana CS, Hess KR, Shi WM, Sawaya R. Metastatic brain tumors with dural extension. J Neurosurg. 1998;89:552–558. doi: 10.3171/jns.1998.89.4.0552. [DOI] [PubMed] [Google Scholar]
- 7.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–91. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 8.Marchevsky AM, Gupta R, Balzer B. Diagnosis of Metastatic Neoplasms. A Clinicopathologic and Morphologic Approach. Arch Pathol Lab Med. 2010;134:194–206. doi: 10.5858/134.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. In: Meningiomas. Louis, Ohgaki, Wiestler, Cavenee, editors. Vol. 2007. IARC, Lyon: WHO Classification of Tumors of the Central Nervous System; pp. 164–172. [Google Scholar]
- 10.Brat DJ, Perry A. In: Melanocytic lesions. Louis, Ohgaki, Wiestler, Cavenee, editors. Vol. 2007. IARC, Lyon: WHO Classification of Tumors of the Central Nervous System; pp. 164–172. [Google Scholar]
- 11.Wang NP, Zee S, Zarbo RJ. Coordinate expression of cytokeratins 7 and 20, defines unique subsets of carcinomas. Appl Immunohistochem. 1995;3:99–107. [Google Scholar]
- 12.Bahrami A, Truong LD, Ro JY. Undifferentiated Tumor: True Identity by Immunohistochemistry. Arch Pathol Lab Med. 2008;132:326–348. doi: 10.5858/2008-132-326-UTTIBI. [DOI] [PubMed] [Google Scholar]
- 13.Krishna M. Diagnosis of Metastatic Neoplasms. An Immunohistochemical Approach. Arch Pathol Lab Med. 2010;134:207–215. doi: 10.5858/134.2.207. [DOI] [PubMed] [Google Scholar]
- 14.Mekni A, Kourda J, Hammouda KB, Tangour M, Kchir N, Zitouna M, Haouet S. Solitary fibrous tumour of the central nervous system: pathological study of eight cases and review of the literature. Pathology. 2009;41:649–54. doi: 10.3109/00313020903071439. [DOI] [PubMed] [Google Scholar]