Abstract
Pre-mRNA splicing in trypanosomes requires the SMN-mediated assembly of small nuclear ribonucleoproteins (snRNPs). In contrast to higher eukaryotes, the cellular localization of snRNP biogenesis and the involvement of nuclear-cytoplasmic trafficking in trypanosomes are controversial. By using RNAi knockdown of SMN in T. brucei to investigate its functional role in snRNP assembly, we found dramatic changes in the steady-state levels of snRNAs and snRNPs: The SL RNA accumulates, whereas U1, U4 and U5 snRNA levels decrease, and Sm core assembly in particular of the SL RNA is strongly reduced. In addition, SMN depletion blocks U4/U6 di-snRNP formation; the variant Sm core of the U2 snRNP, however, still forms efficiently after SMN knockdown. Concerning the longstanding question, whether nuclear-cytoplasmic trafficking is involved in trypanosomal snRNP biogenesis, fluorescence in situ hybridization (FISH) and immunofluorescence assays revealed that the SL RNA genes and transcripts colocalize with SMN. Remarkably, SMN silencing leads to a nucleoplasmic accumulation of both SL RNA and the Sm proteins. In sum, our data demonstrate an essential and snRNA-selective role of SMN in snRNP biogenesis in vivo and strongly argue for a nucleoplasmic Sm core assembly of the SL RNP.
Key words: snRNAs, snRNP biogenesis, SMN, SL RNA, trypanosomes
Introduction
Small nuclear ribonucleoproteins (snRNPs) are important and essential cofactors in many processes of eukaryotic gene expression. For example, pre-mRNA splicing requires the U1, U2, U4/U6 and U5 snRNPs, and the U7 snRNP plays a special role during histone 3′-end processing (reviewed in ref. 1). Studying snRNP biogenesis in various systems helped to establish many principles of the assembly of RNA-protein complexes, regarding both the biochemical functions of RNA-binding proteins involved and the cell biology of these complex processes. In particular the studies of the assembly of the spliceosomal snRNPs served as a paradigm. The classical model, based primarily on the Xenopus oocyte and mammalian cell systems, describes the assembly of the U1, U2, U4 and U5 snRNPs as a multistage process, from snRNA transcription, m7G capping and nuclear export, to cytoplasmic Sm protein binding and m3G cap trimethylation back to nuclear import. Dependent on the particular small nuclear RNA (snRNA), binding of specific proteins and additional nucleotide modifications occur along this pathway. The U6 snRNA deviates from this nuclear-cytoplasmic pathway, in that it remains intranuclear during its maturation, but also undergoes multiple RNA modifications (reviewed in ref. 1 and 2).
During the cytoplasmic phase of snRNP biogenesis, the SMN (survival of motor neuron) protein complex serves as an assembly factor for the efficient and specific binding of the heteroheptameric Sm complex to a conserved binding site of the spliceosomal U1, U2, U4 and U5 snRNAs. As characterized particularly well in the mammalian system, SMN acts within the SMN-Gemin protein complex, which consists of at least 8 core and seven substrate (Sm/LSm) polypeptides, collaborating with additional factors, such as the methyltransferase PRMT5.3,4 In contrast, in Drosophila a simpler SMN core complex, composed of SMN and Gemin2, was shown to be sufficient to mediate snRNP assembly.5
Trypanosomes often deviate from the gene expression pathways known in other eukaryotes. For example, pre-mRNAs are generally polycistronic and only a very small fraction of trypanosome protein-coding regions contain classical internal introns that are removed by the spliceosome;6–8 however, all protein-coding genes require trans splicing at their 5′ ends, thereby adding the same 39-nucleotide spliced leader (SL) miniexon to each of the protein-coding units.9,10 This SL miniexon with its unique cap4 structure is derived from the SL RNA, which is part of the very abundant SL RNP. The SL RNA is stoichiometrically consumed during trans splicing, which contributes to its high turnover;11,12 in addition, trans splicing requires the snRNPs U2, U4/U6 and U5.13,14 In contrast to cis splicing, U1 is not required for trans splicing, as shown in the nematode system.15 Except for U6, each of these snRNPs contains an Sm core of 7 Sm polypeptides, and additional, snRNA-specific protein components, only some of which have been identified.
Regarding the Sm core structure of the spliceosomal snRNPs, we have recently discovered that the T. brucei U2 snRNP contains two Sm subunits that differ from the canonical Sm heptamer core;16,17 this variation provides functions in U2-specific snRNA binding and protein-protein interactions.18 A similar case was subsequently found in the trypanosome U4 snRNP.16,19 These variations impose an additional complication on the specificity of Sm core assembly in trypanosomes. Searching for potential specificity factors involved, we could identify a very divergent SMN-homologous protein in T. brucei, which strongly deviates in its domain organization from higher eukaryotes; in particular, it lacks the central Tudor domain.20 Based entirely on in vitro reconstitution assays, we could indeed demonstrate a direct chaperone role of the trypanosome SMN, in that it confers specificity to canonical Sm core assembly and discriminates against binding of the canonical Sm core to nonspecific RNA or mutant Sm sites. In the same study, initial immunofluorescence assays revealed that trypanosomal SMN localizes to the nucleus, another unusual feature and in contrast to the predominantly cytoplasmic SMN in mammalian cells.20 Note that in another simple eukaryotic system, S. pombe, the SMN complex has also been localized predominantly in nuclear domains.21–23
Focussing on the trans-splicing specific SL RNA, it is surprising that besides the canonical Sm core, no specific protein components have been identified so far. Several SL-specific RNA modifications, including the responsible enzymatic activities, have been biochemically characterized: the unique cap4 modifications at the 5′ end, 3′-end processing and an internal pseudouridine modification at U28, guided by the spliced-leader-associated (SLA1) RNA.24–31 Some of these enzymes and modification reactions have been localized: The cap4 modifications are introduced cotranscriptionally in the nucleus and the SLA1-guided internal pseudouridylation of the SL RNA is also nuclear.32,33 The multistep process of 3′-end formation involves both initial cytoplasmic and final nuclear 3′ trimming.30 However, where SL Sm core assembly takes place, whether it includes a cytoplasmic phase, and in which order these SL RNA modifications and protein binding events occur, have remained controversial issues. Some studies argued for cytoplasmic Sm core assembly, with the cap4 modifications acting as a trafficking signal;28,34,35 other studies also presented evidence for nuclear Sm core assembly.20,36
Here we have used RNAi knockdown of SMN expression in trypanosomes to interfere with the assembly of canonical Sm cores in vivo. Thereby we have investigated the role of SMN in discriminating between the assembly of canonical versus non-canonical Sm cores. In addition, SMN knockdown also provided a unique opportunity to dissect the assembly pathway of the spliceosomal snRNPs in vivo, and to address the longstanding question of whether nuclear-cytoplasmic shuttling may be involved in snRNP biogenesis in trypanosomes.
In sum, we found that SMN plays an snRNA-selective role during snRNP assembly in trypanosomes. In this context, it is essential for the biogenesis of the canonical Sm cores in the SL, U1, U5 snRNPs, but also for assembly of the U4-specific Sm core and of the U4/U6 di-snRNP. In contrast, the variant Sm core in the U2 snRNP still formed efficiently after SMN knockdown. Based on FISH and immunofluorescence assays, we conclude that the SL RNA genes as well as the SL RNA transcripts colocalize with SMN in distinct nuclear domains. Silencing of the Sm core assembly factor SMN clearly leads to a significant nuclear accumulation of SL RNA and Sm proteins. Taken together, our data give new insights into the controversy of trypanosomal snRNP biogenesis, strongly arguing for a nucleoplasmic Sm core assembly of the SL RNP.
Results
SMN knockdown in T. brucei differentially affects steady-state levels of snRNAs and SL RNA cap4 formation.
Our initial study on the functional role of SMN protein in snRNP assembly had shown that SMN mediates the assembly of the canonical Sm cores, interacting directly with the SmB subunit. Since this had relied on in vitro assembly assays, we used here RNAi knockdown of SMN expression to analyze in detail how snRNA steady-state levels and snRNP assembly depend on SMN in vivo. SMN expression was silenced by RNAi (Fig. 1A) and steadystate levels of the SL RNA and the snRNAs U1, U2, U4, U5 and U6 were analyzed by northern blot hybridization over the time period of three days (Fig. 1B left part; for quantitation, see Fig. 1C). Compared to ribosomal RNAs, which served as an input control (see right part of Fig. 1B), the SL RNA was affected most dramatically, increasing nearly four-fold after three days of SMN depletion. Both U2 and U6 snRNAs also increased, although less strongly (three- and two-fold, respectively); in contrast, U4, U1 and U5 snRNAs decreased to levels of between 30% and 35%.
Figure 1.
SMN knockdown in T. brucei differentially affects steady-state levels of snRNAs and SL RNA capping. (A) RNAi downregulation of SMN expression. SMN mRNA was measured by semiquantitative RT-PCR from uninduced cells (t0) and after 1, 2 or 3 days of RNAi induction (t1–t3). As a control, 7SL RNA was analyzed from the same RNA samples. M, markers (100, 200 and 300 bp). (B and C) Effects of SMN knockdown on snRNA steady-state levels. From uninduced cells (t0) and after 1, 2 and 3 days of RNAi silencing of SMN (t1–t3) equal amounts of T. brucei total RNA were analyzed by denaturing polyacrylamide gel electrophoresis and northern blotting, detecting U2, SL, U4, U6, U1 and U5 snRNAs (left part; U1 and U5 snRNAs from a longer exposure of the same blot). As loading control, equal amounts of total RNA were analyzed for each time point (as indicated) by silver staining (right part). Positions of ribosomal RNAs (sRNA1, 2, 4, 5.8S and 5S) are marked on the right. M, markers in nucleotides. (C) shows quantitations of the steady-state levels of the snRNAs; the fold changes of each snRNA during SMN knockdown are diagrammed, relative to the signal intensity of uninduced cells (t0). (D) SMN knockdown blocks SL RNA cap4 modification. Total RNA was prepared from T. brucei cells after RNAi knockdown of SmD1 (as a control; lanes 1–4) or SMN (lanes 5–8), comparing corresponding samples from uninduced cells (lanes −) and after two days of induction (lanes +). U2 snRNA (lanes 1, 2, 5 and 6) and SL RNA (lanes 3, 4, 7 and 8) were detected by primer-extension assays with 32P-labeled specific primers, using a denaturing 6% polyacrylamide gel that resolves unmodified (cap0), partially and fully modified cap4 structures (cap1, 2 and cap4).
Other studies had linked Sm core assembly of the SL RNA with the SL-specific cap4 modification.34 Therefore we assayed the formation of the SL RNA cap4 after SMN knockdown (Fig. 1D). Primer-extension assays for SL RNA and, as a control, U2 snRNA were done on total RNA from uninduced T. brucei cells (lanes −) and after knockdown (lanes +) of SmD1, one of the canonical Sm proteins or SMN (as indicated in D). SmD1 knockdown resulted in a strong reduction of U2 snRNA levels and an increase of SL RNA, specifically in unmodified and partially modified cap structures (cap0, cap1 and cap2; see lanes 1–4), consistent with previous studies.34 Strikingly, SMN depletion affected SL RNA levels and its various cap4 intermediates in a very similar manner as SmD1 depletion did: highly increased steady-state levels and accumulation of partially modified cap4 structures (compare lanes 7–8 with lanes 3–4). In contrast to the effect after SmD1 knockdown, U2 snRNA levels increased moderately (compare lanes 5–6 with lanes 1–2).
SMN knockdown results in snRNA-specific effects on snRNP assembly.
To assess the snRNA-specific effects more comprehensively, we next investigated whether and to what extent the SL RNA and the snRNAs U1, U2, U4, U5 and U6 assemble to RNPs under conditions of in vivo depletion of SMN, using both anti-Sm immunoprecipitation (Fig. 2A and B) and CsCl density gradient centrifugation (Fig. 2C). SMN was downregulated by RNAi in T. brucei cells and lysates were prepared from uninduced cells (t0) and after one to three days of SMN knockdown (t1, t2 and t3), followed by anti-Sm immunoprecipitation and northern blot analysis for U2, SL, U4, U6, U1 and U5 RNAs (Fig. 2A). The immunoprecipitation efficiencies during SMN knockdown were also quantitated (Fig. 2B). Sm immunoprecipitation measures the assembly of snRNAs into Sm core complexes, since our anti-Sm antibodies are directed against Sm polypeptides present in all Sm cores.37 In uninduced cells, the absolute anti-Sm immunoprecipitation efficiencies for U2, SL and U4 snRNAs ranged between 40% and 70%, which probably reflects different accessibilities of the individual Sm cores. Regarding U6 snRNA, anti-Sm co-immunoprecipitation, which was around 40%, assesses the assembly of U4/U6 di-snRNPs: U6 snRNA associates with the LSm set of proteins and can be detected only when coprecipitated with U4 snRNA, which binds the common Sm proteins.38
Figure 2.
SMN knockdown results in snRNA-specific effects on snRNP assembly. (A and B) Anti-Sm immunoprecipitation analysis. From uninduced cells (t0) and after 1, 2 and 3 days of RNAi silencing (t1–t3) lysates were prepared and subjected to anti-Sm immunoprecipitation. To assess immunoprecipitation efficiencies, RNA from 15% input (lanes 1–4) and total anti-Sm immunoprecipitates (lanes 5–8) were analyzed by northern blotting for U2, SL, U4, U6, U1 and U5 snRNAs (the latter two from a longer exposure of the same blot; (A); see (B) for quantifications; U1 and U5 snRNAs could not be quantitated because very long exposure times were necessary). (C) Analysis by CsCl density gradient fractionation. T. brucei total cell extracts from uninduced cells (t0) and after 3 days (t3) of SMN knockdown were fractionated by isopycnic CsCl gradient centrifugation, followed by RNA preparation and northern blot analysis of gradient fractions #1–10 (from top to bottom), detecting U2, SL, U4, U6, U1 and U5 snRNAs (positions marked on the left). Note that fractions #10 include pellet material and cannot be quantitatively compared with the other fractions. For comparison, 10% of the inputs are shown.
Over the three-day time course of SMN knockdown, we consistently observed the following effects (Fig. 2A and B): First, the anti-Sm immunoprecipitation efficiency of U2 snRNA did not greatly change and remained around 40%, in contrast to that of the SL RNA, which drastically decreased after three days (from 90% to 30%). U4 snRNA showed a small increase in its immunoprecipitation efficiency (from 55% to 75%), in parallel to a strong decrease of coprecipitated U6 snRNA (from 40% to 10%).
Finally, these quantitative immunoprecipitation assays were complemented by CsCl density gradient centrifugation (Fig. 2C): Extracts from uninduced control cells (t0) and cells after three days of SMN knockdown (t3) were fractionated on CsCl density gradients, followed by RNA purification from fractions #1–10 and northern blot detection of U2, SL, U4, U6, U1 and U5 snRNAs. The Sm core particles of the SL, U4/U6, U1 and U5 snRNAs are characteristically stable under these conditions and distribute within gradient fractions #1–8, depending on their RNA-protein ratios. In contrast, free RNA sediments in the bottom fractions #9 and 10. The U2 snRNPs, as well as U6 snRNPs not associated with U4 are unstable under these stringent conditions.19,39–41 Although SL RNA total levels increased several-fold after SMN knockdown (compare input lanes), only a minor portion of SL RNA remained in the form of stable core complexes (fractions #2–3) and most of the SL RNA sedimented at the bottom of the CsCl gradient, consistent with the strongly decreased anti-Sm immunoprecipitability (Fig. 2A and B). Regarding U4 and U6, we observed a dramatic change after SMN knockdown: The CsCl-stable U4/U6 Sm core complex (fractions #3–4) completely disappeared; only some U4 snRNA remained, most likely as a U4 Sm core complex (fraction #3) and U6 snRNA completely shifted to the bottom, reflecting free U6 snRNP or snRNA (fraction #9). Finally, U1 and U5 snRNAs, although strongly reduced in their levels, showed no significant change in their RNP status (fractions #1–2).
Combining the results from analyzing the snRNA steadystate levels with those on the Sm core assembly after SMN knockdown, we conclude that in the trypanosome system the SMN protein plays an snRNA-specific and essential role in snRNA and snRNP metabolism in vivo.
The SL RNA gene loci colocalize with a nuclear domain of high SMN concentration.
The cellular localization of snRNP biogenesis in trypanosomes and the involvement of nuclear-cytoplasmic trafficking have been controversially discussed for many years, in particular for the most abundant spliceosomal factor, the SL RNP (see introduction for references). Our results described above clearly demonstrated in vivo the snRNA-specific role of SMN as a snRNP assembly factor; moreover, as the surprising nuclear localization of SMN suggested, Sm core assembly may be a nuclear event in trypanosomes.20 To demonstrate this conclusively, we carefully localized the SL RNA gene locus, the SL RNA itself and the SMN protein, using FISH and immunofluorescence assays in a T. brucei cell line, which stably and exclusively expresses PTP-tagged SMN, as well as after RNAi knockdown of SMN expression. Initially, we established optimal conditions for the specific in situ detection of the SL RNA gene locus and the SL RNA, respectively. These assays also included localization of the SMN protein, thereby combining immunofluorescence and in situ hybridization (Figs. 3 and 4).
Figure 3.
The SL RNA gene loci reside within a nuclear domain of high SMN concentration. (A) T. brucei cells expressing PTP-tagged SMN exclusively were fixed, permeabilized (brightfield), stained with DAPI (DAPI) and submitted to in situ hybridization, using a biotin-labeled SL RNA sense riboprobe and Alexa Fluor 555 Streptavidin conjugate (SL RNA genes). Then slides were processed for immunofluorescence, detecting the PTP-tagged SMN by anti-protein A antibodies (SMN-PTP). In addition, superimposition of all three signals is shown (merge). The fluorescence images represent a single layer of a deconvoluted z-stack of the cell. The insets show a magnified view of the nucleus. The arrows point to colocalizing foci of the SL RNA genes and nuclear domains of high SMN concentration. (B) Three-dimensional representation of SMN distribution and SL RNA gene cluster. Fluorescence image stacks of the cell shown in (A) were deconvoluted and rendered as an isosurface model showing overlapping signals for SMN and the SL RNA genes (left part). In addition, their location in the cell is represented (middle part), as well as their location in the nucleus, by including the 3D-representation of DAPI staining (right part).
Figure 4.
SMN protein largely colocalizes with the SL RNA transcripts in the nucleus. (A and B) T. brucei cells stably expressing SMN-PTP were fixed, permeabilized and treated with RQ1-DNase to degrade cellular DNA (brightfield); for control, see DAPI staining (DAPI). Subsequently cells were subjected to fluorescence in situ hybridization, using either the biotin-labeled SL RNA sense (as negative control: A) or antisense probe (B). In both cases, cells were further processed for immunofluorescence (SMN-PTP); the images on the right show a merge of the in situ hybridization and immunofluorescence signals (merge). The arrows point to colocalizing SL RNA and SMN foci. (C) Three-dimensional representation of SMN and SL RNA distribution. Fluorescence image stacks of the cell shown in (B) were deconvoluted and rendered as an isosurface model showing overlapping SMN and SL RNA signals (left part). In addition, their location in the cell is represented by merging the image with a brightfield view (right part).
First, cells were probed with a T7-transcribed sense riboprobe specific for the SL RNA gene locus; second, the same cells were subjected to indirect immunofluorescence, using the protein A epitope of the PTP tag (Fig. 3A). The SL-sense riboprobe clearly detected the SL RNA gene locus as one bright nuclear spot (see arrows in Fig. 3A), or in some cases two spots (data not shown). In contrast, SMN exhibited a non-homogenous distribution over several nuclear subdomains. Strikingly, in all cells analyzed, at least one of the foci of the SL RNA genes was embedded within an SMN domain, suggesting that SL RNA transcription and biogenesis take place in the same nuclear location. To further support this conclusion, we studied the spatial distribution of both signals. As shown in a three-dimensional isosurface model, generated from the deconvoluted image stack of the cell shown in Figure 3A, the SL RNA genes are indeed embedded within a nuclear domain enriched in SMN protein (Fig. 3B). To confirm specificity of the in situ signals, a probe derived from a non-specific sequence, was used: Under those conditions, no specific signal was detectable (data not shown).
SMN protein largely colocalizes with the SL RNA transcripts.
Next we asked whether SMN protein also colocalizes with the SL RNA (Fig. 4). Cells expressing PTP-tagged SMN exclusively were used for combined indirect immunofluorescence (for SMN protein) and in situ hybridization (for SL RNA). However, in contrast to the assays shown in Figure 3, a T7-transcribed antisense SL RNA riboprobe was used. Since this antisense probe can detect both the SL RNA transcript and the SL RNA gene repeats, cells were first subjected to RQ1-DNase treatment. Degradation of DNA was controlled by DAPI staining and by in situ hybridization using the SL RNA sense probe, which is specific for the SL RNA gene locus (Fig. 4A). After RQ1-DNase treatment no signals could be observed for DAPI staining nor with the SL RNA sense probe. Furthermore, indirect immunofluorescence of the PTP-tagged SMN showed that the nuclear compartment was still intact.
When we used the SL RNA antisense probe (identical in length and GC content to the sense probe), under conditions of RQ1-DNase treatment, the SL RNA distributed in nucleoplasmic domains, similar to the distribution of SMN (Fig. 4B). Strikingly, we found that the SL RNA and SMN signals largely overlapped (Fig. 4B merge). Moreover, we could discern foci of particularly high SMN concentration colocalizing with high-intensity SL RNA spots, which are reminiscent of SL RNA-specific transcription sites (Fig. 4B see arrows). Again, we verified the colocalization by recording z-stacks of the cells and constructing three-dimensional models after deconvolution (Fig. 4C). Taken together, these results strongly argue for a tight coupling of SL RNA transcription and SL Sm core formation.
Nuclear SMN-mediated Sm core biogenesis of the SL RNP in T. brucei.
To address the question, whether nuclear-cytoplasmic trafficking is involved in trypanosomal snRNP biogenesis, we next combined FISH and indirect immunofluorescence assays with SMN silencing: Thereby we followed the distribution of the SL RNA (by FISH) and of the Sm proteins (by immunofluorescence) during a three-day time course of SMN silencing (Fig. 5).
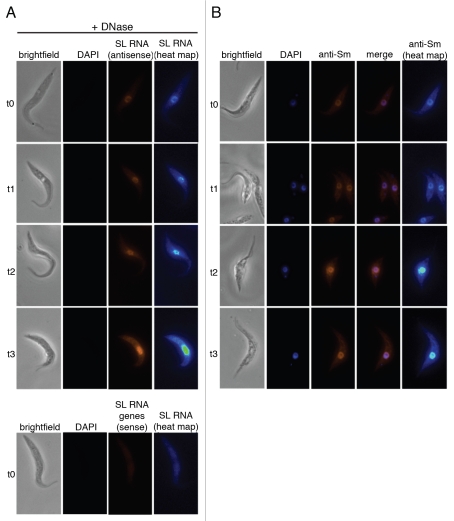
Figure 5.
Nuclear accumulation of SL RNA and Sm proteins during SMN knockdown. (A) T. brucei cells without (t0) and after one, two and three days of RNAi-mediated SMN knockdown (t1–t3) were fixed, treated with RQ1-DNase (brightfield) and stained with DAPI (DAPI). SL RNA was detected by a biotin-labeled antisense riboprobe (SL RNA antisense), shown also in pseudocolors (SL RNA heat map) to indicate the degree of SL RNA accumulation more clearly. As an additional control, cells without RNAi induction were probed with the SL RNA sense riboprobe, which can only detect the gene loci (lower parts). (B) Cells from the same SMN knockdown were fixed (brightfield) and stained with DAPI (DAPI). The distribution of Sm proteins was visualized by indirect immunofluorescence, using anti-Sm antibodies (anti-Sm) and by merging both signals (merge). Sm fluorescence images were additionally pseudocolored (anti-Sm heat map) in order to show the degree of Sm protein accumulation more clearly.
For FISH, uninduced cells (t0) and cells after one, two and three days of silencing (t1, t2 and t3) were hybridized with the SL RNA antisense probe, excluding hybridization with the gene loci by RQ1-DNase treatment (Fig. 5A): SMN silencing resulted in a strong nuclear accumulation of SL RNA over the three-day time course. In parallel, a minor increase of the cytoplasmic SL RNA-specific signal was also observed. To better visualize this, signals are additionally represented as a pseudocolored heat map, reflecting the distribution of the SL RNA by a blue-to-yellow scale. The possibility of incorrect hybridization with cellular DNA was ruled out by using the SL-sense probe as a negative control (Fig. 5A bottom part): Under these conditions, no signals for DAPI nor for the SL RNA gene locus were detected. A similar nuclear accumulation of SL RNA as shown in Figure 5 was also observed when the same SL-antisense probe was used without RQ1-DNase pre-incubation (Sup. Fig. S1): Under these conditions, the probe detected both the SL RNA and the gene loci and probably as a result of these competitive conditions, the nuclear SL RNA accumulation is slightly less pronounced. Additionally, cells from the same knockdown were processed for immunofluorescence, detecting the Sm proteins (Fig. 5B): In parallel to the dramatic increase in the SL RNA levels, the Sm proteins strongly accumulated in the nucleus over the three-day time course of SMN knockdown. In sum these data strongly argue for an SMN-mediated, exclusively nuclear Sm core assembly on the SL RNA.
Discussion
Only recently we identified the trypanosomal SMN ortholog, uncovering its unusual chaperone function in the snRNP-selective assembly of canonical Sm cores.20 This was primarily based on biochemical evidence, in particular on in vitro reconstitution of various Sm cores from recombinant Sm protein subunits. Here we have combined several experimental approaches to study the in vivo role of the SMN protein in trypanosomes: We have investigated thereby not only the unusual snRNA-specific assembly function of SMN, but addressed the controversial issue of where in the trypanosome cell the biogenesis of the SL RNP takes place, and whether nuclear-cytoplasmic trafficking is involved (see introduction for references).
Regarding the snRNA-specific function of SMN during in vivo snRNP assembly, we analyzed how steady-state levels and Sm core formation of the SL RNA and the spliceosomal snRNAs U1, U2, U4, U5 and U6 are affected during SMN knockdown.
First, the SL RNA is affected most dramatically by the SMN depletion: It accumulates in a form not stably associated with the Sm core proteins, in agreement with our previous findings that in vitro reconstitution of the canonical Sm core on the SL RNA strongly depends on SMN.20 We also observed that upon SMN knockdown SL RNA with a partially processed cap4 structure accumulates (Fig. 1D), very similar to the phenotype resulting from SmD1 knockdown.34 In conclusion, the appearance of the same cap4-defective SL RNA after knockdown of either a canonical Sm core protein or the assembly factor SMN argues that correct Sm core formation is a prerequisite for full cap4 modification. The strong accumulation of SL RNA in the absence of Sm core assembly may be surprising, given that other snRNAs decrease in their levels (Fig. 2). Perhaps this reflects the extremely high turnover and short half-life of the SL RNA, in particular, since SMN is essential for splicing in trypanosome cells, the general block in splicing after SMN depletion is expected to contribute to the accumulation of SL RNA.11,12,20
Second, and in contrast to the SL RNA, the U2 snRNA is not significantly affected in its Sm complex formation during SMN downregulation. This is consistent with our previous study, which had shown that the in vitro assembly of the variant Sm core of the U2 snRNP does not require SMN as a cofactor.20 The moderate accumulation of U2 snRNA during SMN knockdown may be explained by the general splicing block and the lack of U2 snRNPs engaged in spliceosome cycles.
Third, U1, U4 and U5 snRNAs strongly decrease in their steady-state concentrations, arguing for SMN being required for their stability, most likely for their Sm core assembly. For U1 and U5 snRNAs, which associate with canonical Sm cores, this is consistent with our in vitro assembly assays.17,20 For U4 snRNA, however, this was surprising, since it associates with an Sm core variant, in which SmD3 is replaced by SSm4.16,19 Most likely this reflects a requirement of SMN for the in vivo assembly of the U4-specific Sm core. We note that both the U4-specific and the canonical Sm cores share the SmB subunit, which as shown for the canonical Sm core-interacts directly with SMN.20 Finally, after RNAi knockdown of SMN no U4/U6 di-snRNPs could be detected (Fig. 2C), consistent with the strongly decreased Sm co-immunoprecipation of U6 (Fig. 2A and B). Taken together, these results suggest that efficient U4-U6 interaction requires the prior Sm core assembly of the U4 snRNA.
Regarding the cellular location and dynamics of snRNP biogenesis, we have focussed on the SL RNA. Initial experiments revealed that the other small nuclear RNAs, whose abundance is far below that of the SL RNA, could not be reliably detected by our current FISH technology. By combining silencing of SMN expression, SL-specific in situ hybridization and immunofluorescence of SMN and Sm proteins, our analysis included also the SL Sm core assembly, where SMN participates as a transiently acting chaperone.
Our data argue for an exclusively nucleoplasmic Sm core assembly of the SL RNP. In this context, it was previously shown that RNA polymerase II-mediated SL RNA transcription takes place in a defined nuclear region, which is separate from pre-mRNA transcription sites.42 By in situ hybridization against the SL RNA gene repeat combined with SMN immunofluorescence, we colocalized both signals, which was also confirmed by 3D modeling, suggesting a close link between SL RNA transcription and SMN-mediated Sm core formation. Furthermore, the signals for the SL RNA gene repeat were displayed as one to two specific dots, which may be explained by the accessibility of the two alleles, depending on nuclear organization during cell stage development. In all cases observed, at least one of the two detectable alleles colocalized with SMN, reflecting different transcriptional activities on one side, but also the need for continuous SL RNA transcription and Sm core assembly on the other side. Outside of these areas of high SMN protein concentration, colocalizing with SL RNA, there are additional SMN signals in the nucleus. These may correspond to SMN being engaged in the biogenesis of the other snRNPs, which are much less abundant than the SL RNP; the participation of SMN in other activities also cannot be ruled out.
A more direct evidence for a link between SL RNA transcription and Sm core assembly was obtained by using an SL-antisense probe, targeting specifically the SL RNA: The majority of SL RNA clearly colocalized with SMN, and distinct foci of high levels of SMN protein and SL RNA were found. These results strongly argue for a close arrangement of the SL RNA transcripts and the Sm core assembly factor SMN. Functionally, this may reflect the need for a highly efficient commitment of SL RNA transcripts to the SL RNP assembly process, in particular the formation of the heteroheptameric Sm core structure. This is further supported by a gradual nuclear accumulation of SL RNA after SMN silencing. Increasing amounts of SL RNA inside the nucleus had previously been observed when cells were depleted of canonical Sm proteins.36 In parallel to the increasing SL RNA signals, we observed a gradual nuclear accumulation of Sm proteins during SMN knockdown. We assume that the Sm subcomplexes are imported into the nucleus by themselves, due to their own nuclear localization signals, as shown for the T. brucei SmB protein.43
Taken together, our data argue that the SMN-mediated assembly of heptameric Sm complexes proceeds in the nuclear compartment. Considering our result that SL Sm core assembly is strongly reduced upon SMN depletion, the observed nuclear accumulation of SL RNA and Sm proteins most likely reflects this assembly defect. Moreover, SMN depletion results in a general decrease in trans-splicing activity, further contributing to the nuclear accumulation of unassembled SL RNPs.20
Materials and Methods
Cell culture.
Cell culture of the procyclic forms of T. brucei strains 427 and 29-13, as well as of the stably transfected cell line, exclusively expressing PTP-tagged SMN, was done as described previously in reference 20, 39 and 44.
RNA interference (RNAi) silencing of SMN expression and reverse transcription (RT)-PCR.
The doxycyclin-inducible RNAi cell line, RNAi mediated knockdown of SMN expression and RT-PCR conditions were described recently in reference 20.
In vitro transcription of biotinylated sense and antisense probes for FISH.
PCR products from the SL-RNA coding region were made from trypanosome genomic DNA, using oligonucleotides containing a T7 promoter at the forward or reverse primer to generate sense and antisense transcription templates, respectively. In vitro transcription reactions were carried out, using the MEGAshortscript T7 kit (Ambion) and according to the manufacturer's instructions. For labeling the RNA probes, biotin-16-UTP (Roche) was added to the nucleotide mixture at a final concentration of 3.75 mM (at an equimolar ratio of modified and unmodified nucleotides). Oligonucleotide sequences are available upon request.
Steady-state RNA analysis, northern blotting and primer-extension analysis.
For the analysis of steady-state snRNA levels, cells were silenced for 1 to 3 days (t1 to t3) and total RNA was prepared by using TRIzol reagent (Invitrogen). As a control, RNA from uninduced cells (t0) was prepared. 5 µg of total RNA from each time point was treated with 5 U of RQ1-DNase (Promega) for 30 min at 37°C, phenolized and ethanol precipitated. Equal amounts of RNA were separated on a 10% denaturing polyacrylamide gel and analyzed by northern blotting with a mixture of snRNA-specific, digoxigenin (DIG)-labeled probes or by silver staining.45
For primer-extension reactions, 5 µg of total RNA and 100,000 cpm 32P-labeled oligonucleotides were used per reaction. Annealing was done at 60°C for 15 min, followed by chilling on ice for 2 min. Next 1 U of reverse transcriptase (Expand RT, Roche) and 1 U of RNase inhibitor (Promega) were added, followed by extension at 42°C for 90 min. After ethanol precipitation, extension products were analyzed on a denaturing 6% polyacrylamide gel.
Immunoprecipitation and cesium chloride density gradient centrifugation.
50 µl of a rabbit serum containing antibodies against the common Sm proteins of T. brucei (anti-CP37) and the corresponding non-immune serum were coupled to 35 µl pre-swollen protein A-Sepharose CL-4B beads (GE Healthcare) in 450 µl NET-2 buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% NP40, 0.5 mM DTT) at 4°C overnight. The pre-coated beads were washed five times with 1 ml of NET-2 buffer and resuspended in 200 µl of the same buffer. For immunoprecipitation, 300 µl of cleared T. brucei lysate, prepared in NET-2 buffer, were incubated with the protein A-Sepharose bound antibodies for 4 h at 4°C. After washing, bound material was released from the beads by incubating in 1x proteinase K (PK) buffer (100 mM Tris-HCl, pH 7.5, 12.5 mM EDTA, 150 mM NaCl, 1% SDS) for 10 min at 50°C, followed by PK treatment of the supernatant. Coprecipitated RNAs were prepared by phenol/chloroform extraction, ethanol precipitated and analyzed by denaturing polyacrylamide gel electrophoresis and northern blotting; signals were quantitated, using Quantity One software (Bio-Rad).
For snRNP analysis, cleared T. brucei cell lysates were prepared after three days of silencing (t3) and from uninduced cells (t0) in buffer D [20 mM HEPES-KOH, pH 8.0, 100 mM KCl, 15 mM MgCl2, 0.2 mM EDTA, 20% glycerol, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), EDTA-free protease inhibitor (Roche)] by disrupting the cells with a PT 3100 cell homogenizer (Kinematica) and subsequent sonication. A CsCl stock solution (1.55 g/ml), supplemented with 0.5 mM DTT and 0.5 mM PMSF, was mixed with the cell lysate at a ratio of 4:1. After centrifugation (Beckman TLX tabletop ultracentrifuge; TLA 120.2 rotor; 90,000 rpm for 20 h at 4°C), the gradient was fractionated from top to bottom (10–100 µl fractions). Each fraction was adjusted to 1% SDS and RNA was prepared by phenolization and ethanol precipitation, followed by denaturing polyacrylamide gel electrophoresis and northern blotting for the detection of snRNAs.
Immunofluorescence and fluorescence in situ hybridization.
For FISH, approximately 2 × 107 to 4 × 107 T. brucei cells were harvested, washed and resuspended in 1 ml phosphate-buffered saline (PBS). 25 µl of the suspension were fixed onto slides with 8% formaldehyde in PBS for 20 min at room temperature and permeabilized in PBS with 1% Triton X-100 for 5 min. Optional RQ1-DNase treatment was done, using 6 U of RQ1 RNase-free DNase (Promega) for 1 h at 37°C to exclude the possibility of hybridization with DNA. The fixed and permeabilized cells were washed with PBS and the slides were prehybridized with hybridization buffer (60% deionized formamide, 50 mM sodium phosphate pH 7.2, 0.5 mg/ml salmon sperm DNA, 1 µg/µl yeast tRNA and 5x Denhardt's solution in 2x SSC) for 3–4 h at 72°C in a humid chamber.
Bio-UTP labeled SL probes were diluted to 40 ng/µl in 50% formamide and denatured for 5 min at 85°C. Then probes were added to the slides to a final concentration of 4 ng/µl in hybridization buffer. The slides were sealed with Fixogum (Marabu), heated for 5 min at 80°C and then immediately transferred to 50.5°C for incubation overnight. The seal was removed and the slides were washed five times with 2x SSC for 5 min at room temperature, with 0.2x SSC for 30 min at 50.5°C and then five times with 0.2x SSC for 5 min. The slides were incubated with Alexa Fluor 555 Streptavidin conjugate (Invitrogen), diluted 1:1,000 in 2x SSC for 1 h at room temperature and washed five times with 2x SSC for 5 min. Finally, 1x PBS containing 4,6-diamidino-2-phenylindol (DAPI; 1 mg/ml) was added and incubated for 10 min. After washing, slides were mounted in Immu-Mount (Thermo Scientific).
For combined immunofluorescence and in situ hybridization, cells were—after the final wash with 2x SSC—blocked with 1% cold-water fish gelatin (Sigma-Aldrich) in PBS and incubated with a 1:80,000 dilution of rabbit anti-protein A primary anti-body (Sigma-Aldrich), or for single immunofluorescence with rabbit antisera developed against T. brucei common Sm proteins (anti-CP37), at a dilution of 1:5,000 for 1 h at room temperature. Slides were washed with PBS containing 0.05% Tween 20 and goat anti-rabbit Alexa Fluor 594 or goat anti-rabbit Alexa Fluor 488 secondary antibody (1:800 dilution, Invitrogen) was added for 45 min at room temperature. After extensive washing, slides were mounted in Immu-Mount (Thermo-Scientific). A Zeiss Axioscop 20 microscope and AxioVision software were used for imaging.
Image stacks were recorded with an iMIC digital microscope (Till photonics) equipped with a 100x, NA 1.4 Oil immersion objective (Olympus) and a 12 bit sensicam qe CCD camera (pco. imaging). For each stack, 50 images were recorded in 100 nm steps in z-direction. The stacks were deconvolved using Huygens Software (Scientific Volume Imaging) and PSFs experimentally recorded under the same conditions, utilizing the PS-Speck Microscopic Point Source Kit (Molecular Probes). Three-dimensional isosurface models of the deconvoluted image stacks were rendered using Amira (Visage Imaging).
Acknowledgements
We thank members of our laboratories for advice and discussions. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG Bi 316/13-1; IRTG1384; German-Israeli Project Cooperation Grant; to A.B.), an International Research grant from the Howard Hughes Medical Institute (to S.M.) and the European-Commission-funded Network of Excellence EURASNET (to A.B.).
Supplementary Material
References
- 1.Tycowski KT, Kolev NG, Conrad NK, Fok V, Steitz JA. Spliceosome structure and function. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. 3rd Ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2006. pp. 327–368. [Google Scholar]
- 2.Will CL, Lührmann R. Spliceosomal UsnRNP biogenesis, structure and function. Curr Opin Cell Biol. 2001;13:290–301. doi: 10.1016/s0955-0674(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 3.Otter S, Grimmler M, Neuenkirchen N, Chari A, Sickmann A, Fischer U. A comprehensive interaction map of the human survival of motor neuron (SMN) complex. J Biol Chem. 2007;282:5825–5833. doi: 10.1074/jbc.M608528200. [DOI] [PubMed] [Google Scholar]
- 4.Neuenkirchen N, Chari A, Fischer U. Deciphering the assembly pathway of Sm-class U snRNPs. FEBS Lett. 2008;582:1997–2003. doi: 10.1016/j.febslet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Kroiss M, Schultz J, Wiesner J, Chari A, Sickmann A, Fischer U. Evolution of an RNP assembly system: a minimal SMN complex facilitates formation of UsnRNPs in Drosophila melanogaster. Proc Natl Acad Sci USA. 2008;105:10045–10050. doi: 10.1073/pnas.0802287105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 7.Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol Biochem Parasitol. 2007;156:93–101. doi: 10.1016/j.molbiopara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Mair G, Shi H, Li H, Djikeng A, Aviles HO, Bishop JR, et al. A new twist in trypanosome RNA metabolism: cis-splicing of pre-mRNA. RNA. 2000;6:163–169. doi: 10.1017/s135583820099229x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Günzl A. The pre-mRNA splicing machinery of trypanosomes: complex or simplified? Eukaryot Cell. 2010;9:1159–1170. doi: 10.1128/EC.00113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang XH, Haritan A, Uliel S, Michaeli S. Trans and cis splicing in trypanosomatids: mechanism, factors and regulation. Eukaryot Cell. 2003;2:830–840. doi: 10.1128/EC.2.5.830-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laird PW, ten Asbroek AL, Borst P. Controlled turnover and 3′ trimming of the trans splicing precursor of Trypanosoma brucei. Nucleic Acids Res. 1987;15:10087–10103. doi: 10.1093/nar/15.24.10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullu E, Tschudi C. Trans splicing in trypanosomes requires methylation of the 5′ end of the spliced leader RNA. Proc Natl Acad Sci USA. 1991;88:10074–10078. doi: 10.1073/pnas.88.22.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dungan JM, Watkins KP, Agabian N. Evidence for the presence of a small U5-like RNA in active trans-spliceosomes of Trypanosoma brucei. EMBO J. 1996;15:4016–4029. [PMC free article] [PubMed] [Google Scholar]
- 14.Tschudi C, Ullu E. Destruction of U2, U4 or U6 small nuclear RNA blocks trans splicing in trypanosome cells. Cell. 1990;61:459–466. doi: 10.1016/0092-8674(90)90527-l. [DOI] [PubMed] [Google Scholar]
- 15.Hannon GJ, Maroney PA, Nilsen TW. U small nuclear ribonucleoprotein requirements for nematode cis- and trans-splicing in vitro. J Biol Chem. 1991;266:22792–22795. [PubMed] [Google Scholar]
- 16.Tkacz ID, Lustig Y, Stern MZ, Biton M, Salmon-Divon M, Das A, et al. Identification of novel snRNA-specific Sm proteins that bind selectively to U2 and U4 snRNAs in Trypanosoma brucei. RNA. 2007;13:30–43. doi: 10.1261/rna.174307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Palfi Z, Preusser C, Lücke S, Lane WS, Kambach C, Bindereif A. Sm core variation in spliceosomal small nuclear ribonucleoproteins from Trypanosoma brucei. EMBO J. 2006;25:4513–4523. doi: 10.1038/sj.emboj.7601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preußer C, Palfi Z, Bindereif A. Special Sm core complex functions in assembly of the U2 small nuclear ribonucleoprotein of Trypanosoma brucei. Eukaryot Cell. 2009;8:1228–1234. doi: 10.1128/EC.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaé N, Wang P, Gu T, Hühn M, Palfi Z, Urlaub H, Bindereif A. Essential role of a trypanosome U4-specific Sm core protein in small nuclear ribonucleoprotein assembly and splicing. Eukaryot Cell. 2010;9:379–386. doi: 10.1128/EC.00353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palfi Z, Jaé N, Preußer C, Kaminska KH, Bujnicki JM, Lee JH, et al. SMN-assisted assembly of snRNP-specific Sm cores in trypanosomes. Genes Dev. 2009;23:1650–1664. doi: 10.1101/gad.526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannus S, Bühler D, Romano M, Seraphin B, Fischer U. The Schizosaccharomyces pombe protein Yab8p and a novel factor, Yip1p, share structural and functional similarity with the spinal muscular atrophy-associated proteins SMN and SIP1. Hum Mol Genet. 2000;9:663–674. doi: 10.1093/hmg/9.5.663. [DOI] [PubMed] [Google Scholar]
- 22.Owen N, Doe CL, Mellor J, Davies KE. Characterization of the Schizosaccharomyces pombe orthologue of the human survival motor neuron (SMN) protein. Hum Mol Genet. 2000;9:675–684. doi: 10.1093/hmg/9.5.675. [DOI] [PubMed] [Google Scholar]
- 23.Paushkin S, Charroux B, Abel L, Perkinson RA, Pellizzoni L, Dreyfuss G. The survival motor neuron protein of Schizosacharomyces pombe. Conservation of survival motor neuron interaction domains in divergent organisms. J Biol Chem. 2000;275:23841–23846. doi: 10.1074/jbc.M001441200. [DOI] [PubMed] [Google Scholar]
- 24.Arhin GK, Li H, Ullu E, Tschudi C. A protein related to the vaccinia virus cap-specific methyltransferase VP39 is involved in cap 4 modification in Trypanosoma brucei. RNA. 2006;12:53–62. doi: 10.1261/rna.2223406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangs JD, Crain PF, Hashizume T, McCloskey JA, Boothroyd JC. Mass spectrometry of mRNA cap 4 from trypanosomatids reveals two novel nucleosides. J Biol Chem. 1992;267:9805–9815. [PubMed] [Google Scholar]
- 26.Mittra B, Zamudio JR, Bujnicki JM, Stepinski J, Darzynkiewicz E, Campbell DA, Sturm NR. The TbMTr1 spliced leader RNA cap 1 2′-O-ribose methyltransferase from Trypanosoma brucei acts with substrate specificity. J Biol Chem. 2008;283:3161–3172. doi: 10.1074/jbc.M707367200. [DOI] [PubMed] [Google Scholar]
- 27.Zamudio JR, Mittra B, Foldynová-Trantírková S, Zeiner GM, Lukes J, Bujnick JM, et al. The 2′-O-ribose methyltransferase for cap 1 of spliced leader RNA and U1 small nuclear RNA in Trypanosoma brucei. Mol Cell Biol. 2007;27:6084–6092. doi: 10.1128/MCB.00647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamudio JR, Mittra B, Chattopadhyay A, Wohlschlegel JA, Sturm NR, Campbell DA. Trypanosoma brucei spliced leader RNA maturation by the cap 1 2′-O-ribose methyltransferase and SLA1 H/ACA snoRNA pseudouridine synthase complex. Mol Cell Biol. 2009;29:1202–1211. doi: 10.1128/MCB.01496-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturm NR, Yu MC, Campbell DA. Transcription termination and 3′-end processing of the spliced leader RNA in kinetoplastids. Mol Cell Biol. 1999;19:1595–1604. doi: 10.1128/mcb.19.2.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeiner GM, Hitchcock RA, Sturm NR, Campbell DA. 3′-End polishing of the kinetoplastid spliced leader RNA is performed by SNIP, a 3′→5′ exonuclease with a motley assortment of small RNA substrates. Mol Cell Biol. 2004;24:10390–10396. doi: 10.1128/MCB.24.23.10390-10396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang XH, Xu YX, Michaeli S. The spliced leader-associated RNA is a trypanosome-specific sn(o)RNA that has the potential to guide pseudouridine formation on the SL RNA. RNA. 2002;8:237–246. doi: 10.1017/s1355838202018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mair G, Ullu E, Tschudi C. Co-transcriptional cap 4 formation on the Trypanosoma brucei spliced leader RNA. J Biol Chem. 2000;275:28994–28999. doi: 10.1074/jbc.M004193200. [DOI] [PubMed] [Google Scholar]
- 33.Hury A, Goldshmidt H, Tkacz ID, Michaeli S. Trypanosome spliced-leader-associated RNA (SLA1) localization and implications for spliced-leader RNA biogenesis. Eukaryot Cell. 2009;8:56–68. doi: 10.1128/EC.00322-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandelboim M, Barth S, Biton M, Liang XH, Michaeli S. Silencing of Sm proteins in Trypanosoma brucei by RNA interference captured a novel cytoplasmic intermediate in spliced leader RNA biogenesis. J Biol Chem. 2003;51:51469–51478. doi: 10.1074/jbc.M308997200. [DOI] [PubMed] [Google Scholar]
- 35.Zeiner GM, Sturm NR, Campbell DA. Exportin 1 mediates nuclear export of the kinetoplastid spliced leader RNA. Eukaryot Cell. 2003;2:222–230. doi: 10.1128/EC.2.2.222-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biton M, Mandelboim M, Arvatz G, Michaeli S. RNAi interference of XPO1 and Sm genes and their effect on the spliced leader RNA in Trypanosoma brucei. Mol Biochem Parasitol. 2006;150:132–143. doi: 10.1016/j.molbiopara.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Palfi Z, Bindereif A. Immunological characterization and intracellular localization of trans-spliceosomal small nuclear ribonucleoproteins in Trypanosoma brucei. J Biol Chem. 1992;267:20159–20163. [PubMed] [Google Scholar]
- 38.Tkacz ID, Cohen S, Salmon-Divon M, Michaeli S. Identification of the heptameric Lsm complex that binds U6 snRNA in Trypanosoma brucei. Mol Biochem Parasitol. 2008;160:22–31. doi: 10.1016/j.molbiopara.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Cross M, Günzl A, Palfi Z, Bindereif A. Analysis of small nuclear ribonucleoproteins (RNPs) in Trypanosoma brucei: structural organization and protein components of the spliced leader RNP. Mol Cell Biol. 1991;11:5516–5526. doi: 10.1128/mcb.11.11.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Günzl A, Cross M, Bindereif A. Domain structure of U2 and U4/U6 small nuclear ribonucleoprotein particles from Trypanosoma brucei: identification of trans-spliceosomal specific RNA-protein interactions. Mol Cell Biol. 1992;12:468–479. doi: 10.1128/mcb.12.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palfi Z, Xu GL, Bindereif A. Spliced leader-associated RNA of trypanosomes. Sequence conservation and association with protein components common to transspliceosomal ribonucleoproteins. J Biol Chem. 1994;269:30620–30625. [PubMed] [Google Scholar]
- 42.Dossin FdeM, Schenkman S. Actively transcribing RNA polymerase II concentrates on spliced leader genes in the nucleus of Trypanosoma cruzi. Eukaryot Cell. 2005;4:960–970. doi: 10.1128/EC.4.5.960-970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girard C, Mouaikel J, Neel H, Bertrand E, Bordonné R. Nuclear localization properties of a conserved protuberance in the Sm core complex. Exp Cell Res. 2004;299:199–208. doi: 10.1016/j.yexcr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Schimanski B, Laufer G, Gontcharova L, Günzl A. The Trypanosoma brucei spliced leader RNA and rRNA gene promoters have interchangeable TbSNAP50-binding elements. Nucleic Acids Res. 2004;32:700–709. doi: 10.1093/nar/gkh231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell M, Bindereif A. Cloning and mutational analysis of the Leptomonas seymouri U5 snRNA gene: function of the Sm site in core RNP formation and nuclear localization. Nucleic Acids Res. 1999;27:3986–3994. doi: 10.1093/nar/27.20.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.