Abstract
Recent evidence indicates differences in neural stem cell (NSC) biology in different brain regions. For example, we demonstrated that neurofibromatosis-1 (NF1) tumor suppressor gene inactivation leads to increased NSC proliferation and gliogenesis in the optic chiasm and brainstem, but not in the cerebral cortex. The differential effect of Nf1 inactivation in the optic nerve and brainstem (in which gliomas commonly form in children with NF1) vs. the cortex (in which gliomas rarely develop) suggests the existence of distinct ventricular zones for gliomagenesis in children and adults. Here, we characterized the third ventricle subventricular zone (tv-SVZ) in young and adult mice and human brains. In children but not adult humans the tv-SVZ contains nestin-positive, glial fibrillary acidic protein (GFAP)-positive, brain fatty acid binding protein-positive and sox2-positive cells with radial processes and prominent cilia. In contrast, the tv-SVZ in young mice contains sox2-positive progenitor cells and ciliated ependymal lining cells, but lacks GFAP-positive, nestin-positive radial glia. As in the lateral ventricle SVZ (lv-SVZ), proliferation in the human and murine tv-SVZ decreases with age. The tv-SVZ in adult mice lacks the hypocellular subventricular zone observed in adult human specimens. Collectively, these data indicate the existence of a subventricular zone relevant to our understanding of glioma formation in children and will assist interpretation of genetically engineered mouse glioma models.
Keywords: Germinal zone, Third ventricle
INTRODUCTION
With the emergence of the cancer stem cell hypothesis (1-4), considerable attention has focused on the lateral ventricle subventricular zone (lv-SVZ) as a potential germinal zone relevant to the genesis of high-grade gliomas in adults (5-7). Studies pioneered by Alvarez-Buylla et al have shown that neuroglial progenitor cells lining the lv-SVZ are authentic stem cells with the capability of producing all 3 major brain cell lineages (8-11). The existence of this specialized germinal zone in adults has prompted studies of the consequence of introducing glioma-associated genetic mutations into stem-like cells within the lv-SVZ. The ability to generate high-grade gliomas supports the hypothesis that gliomas arise from this specialized stem cell niche in adults (12-15).
In contrast to their adult counterparts, gliomas in children arise less commonly in the cerebral hemispheres and more often develop in the optic nerve, brainstem, and cerebellum (16). This spatial pattern of gliomagenesis is illustrated by World Health Organization grade I gliomas (pilocytic astrocytoma), which arise in the cerebellum (33%), optic pathway (27%), brainstem (16%), and cerebrum (13%) (17). Moreover, pilocytic astrocytomas arising in the context of the neurofibromatosis-1 (NF1) cancer predisposition syndrome are located predominantly in the optic pathway (67%), and less commonly form in other brain regions (18). The predilection for pediatric gliomas to form in these regions raises the possibility that additional germinal zones may contribute to the genesis of gliomas in children.
Because pediatric gliomas often arise in the optic pathway and hypothalamus, we sought to characterize an understudied potential germinal zone (i.e. the third ventricle) in human and mouse specimens. The location of the third ventricle (proximate to the hypothalamus and optic pathway) raises the possibility that optic nerve/hypothalamic gliomas arise from this unique ventricular zone. Support for regional heterogeneity in neuroglial cell function derives from our recent studies demonstrating that astrocytes and neural stem cells from different regions of the brain harbor region-specific transcriptomal signatures (19) and exhibit differential cell-autonomous responses to the pediatric brain cancer-causing genetic mutation (Nf1 gene inactivation) (20).
In this report, we characterize the third ventricle subventricular zone (tv-SVZ) in young and adult mouse and human brains
MATERIALS AND METHODS
Human Brain Samples
Samples were obtained at autopsy from the brains of 10 normal children (7 days to 4 years of age; mean = 9.6 months) and 10 adults (47-87 years of age; mean = 64.3 years) (Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A246). A midline section of the tv-SVZ was embedded in paraffin and 5-μm-thick consecutive sections were cut. The lv-SVZ was represented in 10 cases (5 from each group). Mouse and human specimens are denoted by “m” and “h”, respectively. The SVZ was the region that encompassed both the ependymal lining and an adjacent 1-3 mm subependymal zone (SEZ) along the ventricular wall. Autopsy specimens were obtained in accordance with an approved Human Studies protocol at the Washington University School of Medicine.
Animals
Brain tissues were obtained from wild-type C57BL/6 mice at postnatal day 2-3 (young) and at 4-6 months of age (adult) following intracardiac perfusion with PBS and 4% paraformaldehyde solution. After fixation the specimens were paraffin-embedded. The mice were used in accordance with an approved Animal Studies protocol at the Washington University School of Medicine.
Routine Histology and Immunohistochemistry
Hematoxylin and eosin and immunohistochemical staining using lineage-specific antibodies were performed following antigen retrieval (Table, Supplemental Digital Content 2, http://links.lww.com/NEN/A247). Immunohistochemistry was performed simultaneously on all sections with appropriate positive and negative controls. Double labeling was performed using separate antigen retrieval buffers when required. Three different peroxidase substrates were used: Vector- 3′3′ diaminobenzidine (SK-4100), Vector-NovaRED (SK-4800) and Vector-SG (SK-4700), all from Vector Laboratories, Burlingame, CA. The Ki-67 labeling index was defined as the number of Ki-67-positive cells divided by the total number of cells (including both Ki-67-positive and Ki-67-negative cells) counted. Only cells in the SVZ (both positive and negative for Ki-67) were counted. Appropriate Alexa Fluor-tagged secondary antibodies were used for fluorescence detection and the cells were counterstained with DAPI.
For ultrastructural analysis, a portion of the SVZ was fixed in 2% glutaraldehyde, post-fixed in phosphate-buffered 2% OsO4 for 2 hours, dehydrated in graded concentrations of ethanol and embedded in Embed-812 (Electron Microscopy Sciences, Hatfield, Pennsylvania) with propylene oxide as an intermediary solvent. One-μm-thick plastic sections were stained with toluidine blue and examined under light microscopy to define the orientation of the ependymal layer and SVZ. Ultrathin sections were subsequently stained with uranyl acetate and lead citrate and examined with a JEOL 1200 electron microscope equipped with an ABT digital camera.
Statistical Analysis
Statistical significance (p < 0.05) was determined using the Student t-test and GraphPad Prism 4.0 software (GraphPad, Inc., La Jolla, CA).
RESULTS
Human tv-SVZ
We first compared the ventricular lining (ependyma) and SEZ extending 1 to 3 mm beneath the ependyma in human specimens. In children less than 5 years of age (n = 10), the ependyma is a single layered tall columnar epithelium with prominent cilia and a conspicuous terminal bar (Fig. 1A). In adults (older than 45 years; n = 10), the ependyma appears as a single-layer of cuboidal cells with a marked reduction in cilia (Fig. 1B). The presence of cilia was confirmed using acetylated tubulin immunostaining (Fig. 1C, D) and electron microscopy (Fig. 1E, F; arrow). In addition, the subependymal zone is paucicellular in adults compared to children. On ultrastructural examination, the tv-SVZ in adults is also paucicellular and the ependymal cells contain lipofuscin.
Figure 1.
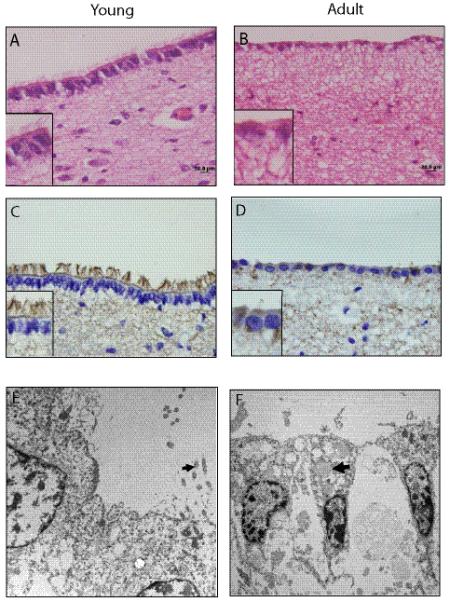
Age-dependent changes in the human third ventricle subventricular zone (tv-SVZ). (A, B) Section of the third ventricle from a child (A) reveals a single layered tall columnar epithelium with prominent cilia at the ventricular surface (inset). In an adult the ependymal layer is flattened and contains a single layer of cuboidal cells and there is marked reduction of lining cilia (B). (C, D) Acetylated tubulin immunostaining reveals abundant cilia in a pediatric (C) and few in an adult (D) tv-SVZ. Scale bars = 20 μm. (E, F) Electron microscopy demonstrates a relative abundance of cilia in a pediatric tv-SVZ (E; arrow), and the presence of lipopigment in lining ependymal cells in an adult tv-SVZ (F; arrow). A, B: Hematoxylin and eosin.
Immunohistochemistry using with antibodies that delineate neurons (Tuj1, polysialated neural cell adhesion molecule [PSA-NCAM], and NeuN), astrocytes (GFAP), and progenitor cells (nestin, vimentin, Sox2 and brain fatty acid binding protein [BLBP]) was performed to characterize the cellular composition of the human tv-SVZ. Several differences were noted between the pediatric and adult tv-SVZ specimens. First, GFAP-immunoreactive cells in the third ventricle SEZ of children had a radial appearance with prominent radial glial processes (Fig. 2A); however, in adults, GFAP immunostaining revealed a cobweb appearance with haphazard arrangements of glial processes (Fig. 2B). Second, numerous long perpendicularly oriented, nestin-immunoreactive processes arranged in parallel were found to extend from the ependymal layer into the SEZ in young children (Fig. 2C), whereas in adults there was only coarse punctate nestin staining in the subependymal region with more labeling at the base of the ependymal lining (Fig. 2D). In children, numerous cells with radial processes in the ependymal lining were immunopositive for both nestin and GFAP (Fig. 2E), and for BLBP (Fig. 2F); these data support their designation as radial glia. A small number of cells in the ependymal layer and SEZ also expressed the Sox2 progenitor marker (Fig. 2G). No differences in vimentin immunoreactivity were noted between the 2 age groups (data not shown). Third, the Tuj1- and PSA-NCAM-immunoreactive cell processes abut the base of the ependymal layer in young children (Fig. 3A, C). In contrast, there are few neurons beneath the ependymal layer in adults, resulting in a hypocellular zone (Fig. 3B, D). NeuN immunohistochemistry demonstrated staining of neurons in the regions much deeper to the SEZ, but was negative in the third ventricle SEZ of either age group (data not shown).
Figure 2.
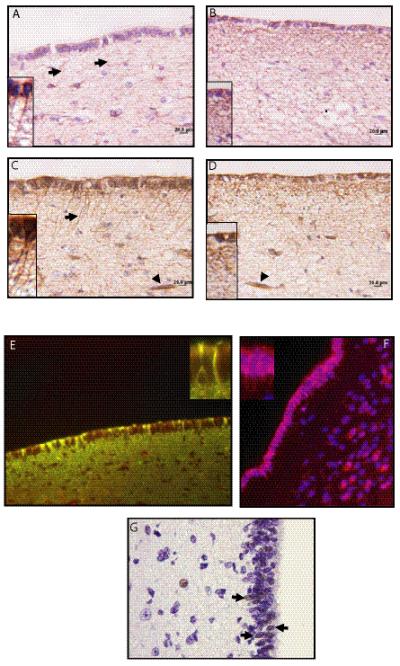
Radial glia in the third ventricle subventricular zone (tv-SVZ) of children. (A-D) Immunostaining for glial fibrillary acidic protein (GFAP) (A, B) and nestin (C, D) demonstrate moderate staining in the ependymal lining of tv-SVZ in a child (A, C) and an adult (B, D). Radial processes (inset, arrows) were only observed in the tv-SVZ of the child; there is a cobweb-like pattern of expression in the adult third ventricle subependymal zone (SEZ) (inset). Arrowheads in C and D indicate blood vessels as internal positive immunostaining controls. (E) Radial glia in a pediatric tv-SVZ co-label (yellow) for GFAP (green) and nestin (red). Inset shows radial processes double positive for GFAP and nestin. (F) The radial glia also express brain fatty acid binding protein (BLBP: red, DAPI: blue). BLBP expression is also seen in isolated cells within the SEZ. (G) A small number of cells within the lining ependyma and tv-SEZ are Sox2-immunoreactive (arrows). Scale bars = 20 μm.
Figure 3.
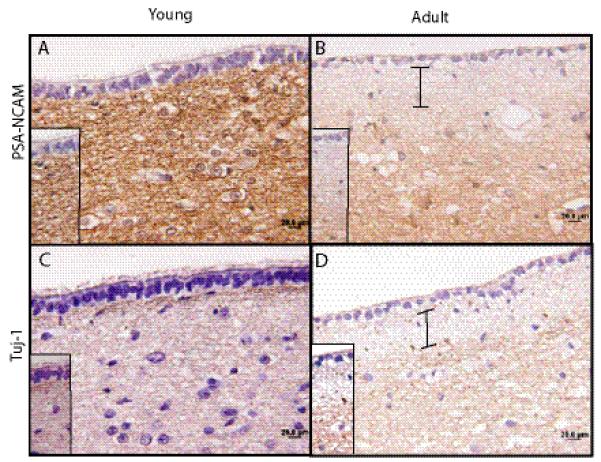
Age-dependent changes in neuronal architecture in the human third ventricle subventricular zone (tv-SVZ). (A-D) Immunostaining for (A, B) polysialated neural cell adhesion molecules (PSA-NCAM) (A, B) and Tuj-1 (C, D) shows intense staining of the processes abutting the third ventricle SEZ in a child (A, C), that is absent in the adult SEZ (B, D). The hypocellular region in the adult tv-SVZ is denoted by bars. Scale bar = 20 μm.
Ki67 immunolabeling was used to determine the proliferative indices within the tv-SVZ (MIB-1). In general, proliferating cells were predominantly located in the immediate SEZ, with a very few Ki67-positive ependymal cells. There was a 14-fold reduction in the Ki-67 labeling index in adults vs. children (n = 10 specimens, p = 0.04) (Fig. 4). To determine which cells in the SEZ were proliferating, Ki67 double labeling experiments were performed. The vast majority of the Ki67-positive cells did not co-label with GFAP (Fig. 5A, B), PSA-NCAM (data not shown), nestin (Fig. 5C), or Olig2 (data not shown). Only 10% of the Ki67-positive cells were GFAP-immunoreactive (glial progenitors or astrocytes), 5% to 7% were nestin-positive (glial progenitor or stem cells), 1% were Olig2-positive, and none were PSA-NCAM-immunoreactive.
Figure 4.
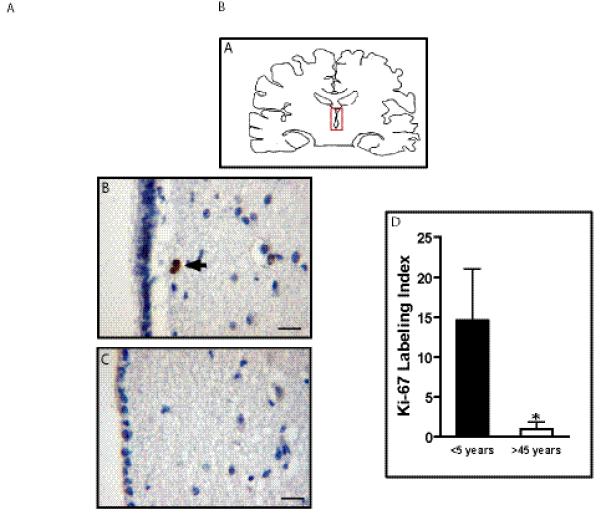
Proliferation in the human third ventricle subventricular zone (tv-SVZ) decreases with age. (A) Diagram of the region containing the human third ventricle (red box). (B, C) Ki67 immunolabeling of the tv-SVZ in representative specimens from a child (B) and an adult (C). (D) Mean and SD of Ki67 labeling indices (p < 0.005). Scale bar: B, C = 20 μm.
Figure 5.
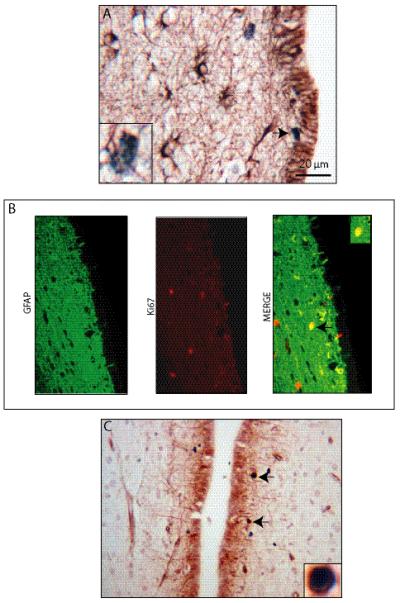
Proliferating cells in the human third ventricle subventricular zone (tv-SVZ) are glial fibrillary acidic protein (GFAP)- and nestin-immunoreactive. (A) A small population of GFAP-positive cells in the third ventricle subependymal zone (SEZ) co-labels with Ki67. Inset demonstrates a representative Ki67-positive, GFAP-positive cell. (B) Similar results are shown with GFAP/Ki67 immunofluorescence double labeling. The arrow denotes a representative GFAP-positive, Ki67-positive cell. (C) A small number of nestin-positive cells in the human third ventricle SEZ co-labels with Ki67. Inset demonstrates a representative nestin-positive, Ki67-positive cell. Scale bar = 20 μm.
Mouse tv-SVZ
Several age-dependent features were noted in the mice. The third ventricle of young mice lacked cells with GFAP expression, whereas there was diffuse GFAP immunoreactivity in the ependymal lining in adult mice (Fig. 6C, D). Small numbers of GFAP-positive cells were also found in the third ventricle SEZ in the adult mice. There was little nestin expression in the third ventricle of young mice, while widespread nestin immunoreactivity was found in the ependymal lining of adult mice (Fig. 6E, F). This absence of GFAP-positive, nestin-positive radial glia in the mouse TVZ sharply contrasts with the human specimens, both in the tv-SVZ (Fig. 2) and the lv-SVZ of children (Figure, Supplemental Digital Content 3, http://links.lww.com/NEN/A248). Third, as in human pediatric tv-SVZ specimens, sox2 immunostaining identified progenitor cells in the tv-SVZ of young mice (Figure, Supplemental Digital Content 4, http://links.lww.com/NEN/A249). There was also an age-dependent reduction in cilia, as evidenced by acetylated tubulin immunohistochemistry (Fig. 6G, H), but there were no differences in the pattern of neuronal (PSA-NCAM, Tuj1) staining between young and adult mice (Fig. 7). As in the human specimens (Fig. 4), Ki67 labeling index in the tv-SVZ was much lower (45-fold) in adult vs. early postnatal mice (n = 5 mice/group, p = 0.007) (Fig. 8). This age-dependent reduction in germinal zone proliferation within the human tv-SVZ was also observed in human (Fig. 9A) and mouse (Fig. 9C) lv-SVZ.
Figure 6.
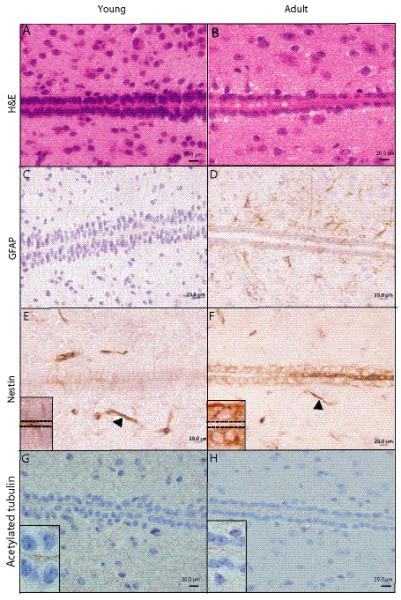
Age-dependent changes in the cellular composition of the mouse third ventricle subventricular zone (tv-SVZ). (A, B) Representative hematoxylin and eosin-stained sections of the third ventricle in young and adult mice. (C, D) Glial fibrillary acidic protein (GFAP)-positive cells are barely detected in the third ventricle in a young mouse (C) whereas an adult mouse exhibits focal GFAP immunoreactivity with diffuse expression in ependymal cells (D). (E, F) Rare nestin-positive cells in a young mouse (E); an adult moue ependymal layer contains numerous nestin-immunopositive cells (F). Arrowheads indicate blood vessels, which serve as internal positive controls. (G, H) Acetylated tubulin immunostaining illustrates age-dependent reduction in the number of cilia. The cilia appear relatively shorter than those in the human tv-SVZ.
Figure 7.
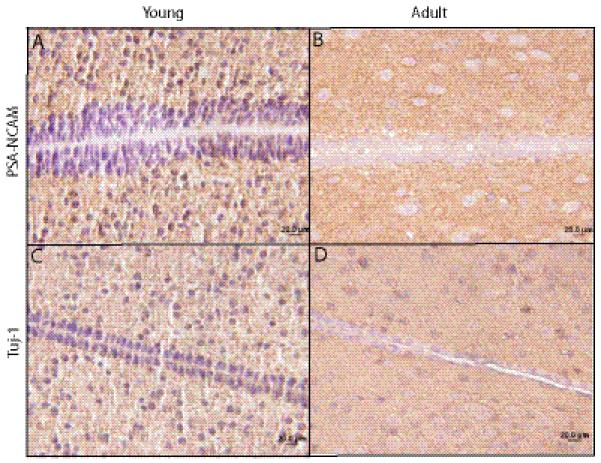
Age-dependent similarities in the cellular composition of the mouse third ventricle subventricular zone (tv-SVZ). (A-D) Similar patterns of polysialated neural cell adhesion molecule (PSA-NCAM) (A, B) and Tuj-1 in the tv-SEZ (C, D) are observed in young mice (A, C) and adult mice (B, D). No hypocellular zone is detected in the adult mouse tv-SVZ. Scale bar: 20 μm.
Figure 8.
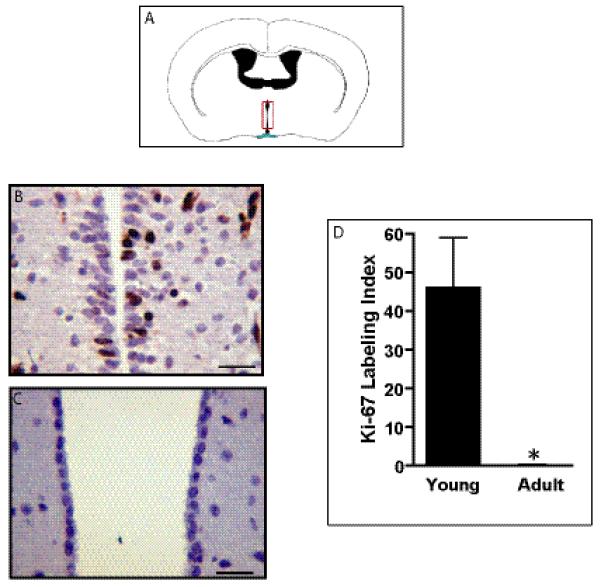
Proliferation in the mouse third ventricle subventricular zone (tv-SVZ) decreases with age. (A) Diagram of the region containing the mouse third ventricle (red box). (B, C) Ki67 immunolabeling of the tv-SVZ in a young mouse (B) and an adult mouse (C). Scale bar: 20 μm. (D) Mean and SD for the Ki67 labeling indices (p < 0.005).
Figure 9.
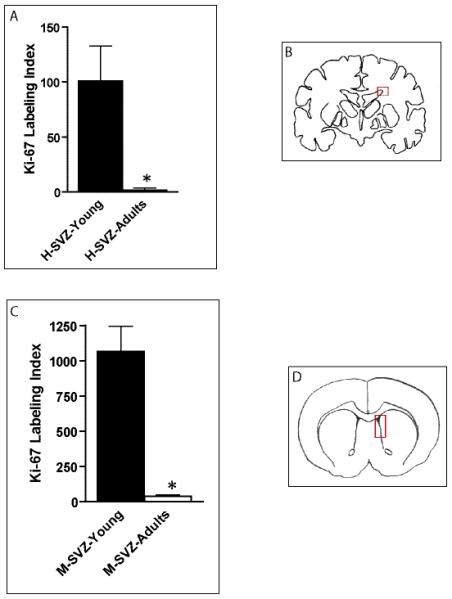
Age-dependent decreases in ventricular zone proliferation in the mouse and human third ventricle subventricular zone (tv-SVZ) and lateral ventricle SVZ (lv-SVZ). (A-D) The Ki67 labeling index is reduced in the human lv-SVZ as a function of age (p = 0.01) (A). The Ki67 labeling index is reduced in the murine lv-SVZ as a function of age (p = 0.0004) (C). Diagrams of the regions containing the human (B) and mouse (D) lv-SVZ (red boxes).
DISCUSSION
We used immunohistochemistry and electron microscopy to demonstrate the existence of an additional germinal zone within the third ventricle in humans. The designation of the tv-SVZ as a stem cell niche comparable to the better-studied lv-SVZ is supported by several observations. First, we identified cells with long radial processes that express both GFAP and nestin. While nestin-immunoreactive cells have been previously reported in the rat tv-SVZ, it is not known whether these nestin-positive cells co-labeled with GFAP (21), as seen in our study. GFAP-positive/nestin-positive radial glia-like cells most likely represent type B cells, as originally proposed for the lv-SVZ (9-11). Second, there is robust expression of BLBP (brain fatty acid binding protein; FABP7), a marker of both radial glia and neural stem cells. In this regard, radial glia in the lv-SVZ have been shown to give rise to adult neural stem cells (22). Additionally, we have previously used a BLBP-Cre transgenic mouse strain to demonstrate that BLBP-positive cells can self renew in vitro and give rise to oligodendrocytes, astrocytes, and neurons (multi-lineage differentiation) in vitro and in vivo, consistent with their role as neural stem cells (23). Third, the pediatric tv-SVZ also contains Olig2-positive cells, another marker of progenitor cells in the brain (24). In this regard, Olig2 is universally expressed in gliomas (25), and its targeted inactivation abrogates gliomagenesis in genetically engineered mice (26). Fourth, we identified sox2-positive cells in the tv-SVZ of children. Sox2 is a transcription factor critical for the maintenance of neural progenitor identity (27), such that alterations in sox2 expression in genetically engineered mice leads to defects in astrocyte and neuronal differentiation (28, 29). Fifth, we found that the cells lining the tv-SVZ in children contained cilia. Previous studies in adult rat (30) and primates (31) identified cilia along the lateral walls of the tv-SVZ, but not in the ventral parts. In contrast, we did not identify significant numbers of cilia in the adult human tv-SVZ, but could identify ciliated ependymal lining cells in the adult mouse tv-SVZ. The importance of cilia to neural stem cell function is highlighted by several studies demonstrating that primary cilia are required for the formation of adult neural stem cells (32), regulate hippocampal neurogenesis (33), and characterize B cells within the adult lv-SVZ (34).
In addition, we found an age-dependent development of a hypocellular region within the third ventricle SEZ. While the functional significance of this hypocellular region has not been fully elucidated, previous studies have demonstrated the existence of a similar paucicellular zone in the human lv-SVZ of adults (7, 10). Interestingly, this hypocellular region is very thin or not present in the lv-SVZ of other species (7, 35), similar to our findings in mice.
Next, we examined proliferation in the tv-SVZ. We describe an age-dependent decrease in the proliferative index in adults relative to their pediatric counterparts. This finding parallels a similar reduction in lv-SVZ proliferation in adults vs. children, and likely reflects an overall decrease in germinal zone capacity over time (36). To identify the proliferating cells within the pediatric tv-SVZ, we performed a series of double-labeling experiments. While there were small numbers of proliferating nestin-positive, Olig2-positive and GFAP-positive cells in the tv-SVZ, transient-amplifying type C cells could not be definitively identified. Similar to analogous studies in the human lv-SVZ, it is highly likely that some of the Ki67-positive cells contained within the tv-SVZ are neural stem cells. Current studies are focused on defining the identity of the proliferating cells in the tv-SVZ and on determining whether distinct subpopulations of progenitor cells exist in this pediatric germinal zone.
To inform future studies employing genetically engineered small animal glioma models, we compared the lv-SVZ and tv-SVZ in mice. As in humans, we found that proliferation decreased significantly with age in the murine tv-SVZ. In contrast to their human counterparts, we were unable to identify GFAP-positive, nestin-positive cells with clear radial processes in young mice. However, we could identify cells expressing the CD133 and BLBP progenitor markers in the young murine tv-SVZ (data not shown) as well as ciliated ependymal lining cells. The fact that BLBP-positive and CD133-positive cells can generate new neurons and astrocytes in the embryonic mammalian brain (37-40), as well as in the adult avian brain (41, 42), supports the presence of an analogous third ventricle germinal zone in mice. This contention is further strengthened by numerous studies examining a group of midline structures along the rodent third and fourth ventricles, termed circumventricular organs (CVOs). These CVO structures contain progenitor cells that express nestin, GFAP, sox2 and vimentin (43). Moreover, neurospheres generated from CVO tissues can proliferate and undergo multi-lineage differentiation in vitro and in vivo (44). Along these lines, preliminary results in our laboratory have established the existence of BLBP-positive neural stem cells (NSCs) in the postnatal day 1-2 murine tv-SVZ with the ability to sustain long-term self-renewal and undergo multi-lineage differentiation into oligodendrocytes, astrocytes and neurons in vitro (D.Y. Lee and D.H. Gutmann, manuscript in preparation). These preliminary findings parallel previous reports describing the presence of NSCs from the adult tv-SVZ with the capacity to self-renew and undergo multi-lineage differentiation (45, 46).
The observation that NSCs exist in the tv-SVZ is intriguing in light of mouse modeling experiments in which specific high-grade glioma-associated genetic changes introduced into lv-SVZ progenitor cells result in glioma formation. In these studies, coordinate loss of NF1, PTEN, and p53 function in mouse lv-SVZ cells is sufficient to generate high-grade gliomas in subcortical and cortical regions (13). Similarly, the expression of mutant p53 proteins (15, 47), platelet-derived growth factor (14) or oncogenic KRas in lv-SVZ neural stem cells initiates gliomagenesis (12). Although the relevance of the tv-SVZ to gliomagenesis is currently not clear, studies in rodents have demonstrated that ciliated ependymal cells in the rat third ventricle can give rise to differentiated progeny (48, 49), and that GFAP-positive cells can derive from neuroepithelial cells lining the third ventricle to populate the optic tract along retinal ganglion cell projections (50) and hypothalamic parenchyma (51).
Current studies are focused on determining the consequence of introducing specific pediatric glioma-associated genetic changes (Nf1 loss; BRAF activation) on third ventricle NSC function. Coupled with spatially distinct microenvironmental conditions, heterogeneity in NSC niches may contribute significantly to the pattern of gliomagenesis and may uncover new targets for therapeutic drug design specific for glioma-maintaining stem cells in children and adults.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the excellent technical support from members of the Gutmann laboratory, and Lisa Snipes and Karen Green from the Division of Neuropathology. We are also thankful to Dr. Robert E. Schmidt, MD, PhD for his expert advice in interpretation of our ultrastructural examination.
This work was partially supported by a grant from the National Institutes of Health (1R01NS65547 to DHG).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 2.Dirks PB. Brain tumor stem cells: the cancer stem cell hypothesis writ large. Mol Oncol. 2010;4:420–30. doi: 10.1016/j.molonc.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 4.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–36. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 5.Becher OJ, Holland EC. Evidence for and against regional differences in neural stem and progenitor cells of the CNS. Genes Dev. 2010;24:2233–8. doi: 10.1101/gad.1988010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–24. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 9.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 12.Abel TW, Clark C, Bierie B, et al. GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol Cancer Res. 2009;7:645–53. doi: 10.1158/1541-7786.MCR-08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llaguno S Alcantara, Chen J, Kwon CH, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–99. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Yang J, Zheng H, et al. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15:514–26. doi: 10.1016/j.ccr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–50. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 18.Guillamo JS, Creange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126:152–60. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- 19.Sharma MK, Mansur DB, Reifenberger G, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67:890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- 20.Lee da Y, Yeh TH, Emnett RJ, White CR, et al. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24:2317–29. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst C, Christie BR. Nestin-expressing cells and their relationship to mitotically active cells in the subventricular zones of the adult rat. Eur J Neurosci. 2005;22:3059–66. doi: 10.1111/j.1460-9568.2005.04499.x. [DOI] [PubMed] [Google Scholar]
- 22.Merkle FT, Tramontin AD, Garcia-Verdugo JM, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegedus B, Dasgupta B, Shin JE, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–57. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Setoguchi T, Kondo T. Nuclear export of OLIG2 in neural stem cells is essential for ciliary neurotrophic factor-induced astrocyte differentiation. J Cell Biol. 2004;166:963–8. doi: 10.1083/jcb.200404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ligon KL, Alberta JA, Kho AT, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 26.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham V, Khudyakov J, Ellis P, et al. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–65. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 28.Bani-Yaghoub M, Tremblay RG, Lei JX, et al. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Ferri AL, Cavallaro M, Braida D, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–19. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 30.Paull WK, Martin H, Scott DE. Scanning electron microscopy of the third ventricular floor of the rat. J Comp Neurol. 1977;175:301–10. doi: 10.1002/cne.901750305. [DOI] [PubMed] [Google Scholar]
- 31.Coates PW. The third ventricle of monkeys; Scanning electron microscopy of surface features in mature males and females. Cell Tissue Res. 1977;177:307–16. doi: 10.1007/BF00220306. [DOI] [PubMed] [Google Scholar]
- 32.Han YG, Spassky N, Romaguera-Ros M, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 33.Breunig JJ, Sarkisian MR, Arellano JI, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–32. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirzadeh Z, Merkle FT, Soriano-Navarro M, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawamoto K, Hirota Y, Alfaro-Cervello C, et al. Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J Comp Neurol. 2011;519:690–713. doi: 10.1002/cne.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mignone JL, Kukekov V, Chiang AS, et al. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–24. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 37.Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–63. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 38.Miyata T, Kawaguchi A, Okano H, et al. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–41. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 39.Noctor SC, Flint AC, Weissman TA, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 40.Tamamaki N, Nakamura K, Okamoto K, et al. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, et al. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–37. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–9. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- 43.Bennett L, Yang M, Enikolopov G, et al. Circumventricular organs: a novel site of neural stem cells in the adult brain. Mol Cell Neurosci. 2009;41:337–47. doi: 10.1016/j.mcn.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson CB, Svensson M, Wallstedt L, et al. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–6. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- 45.Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chouaf-Lakhdar L, Fevre-Montange M, Brisson C, et al. Proliferative activity and nestin expression in periventricular cells of the adult rat brain. Neuroreport. 2003;14:633–6. doi: 10.1097/00001756-200303240-00022. [DOI] [PubMed] [Google Scholar]
- 47.Gil-Perotin S, Marin-Husstege M, Li J, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–16. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao L, Miller RH. Specification of optic nerve oligodendrocyte precursors by retinal ganglion cell axons. J Neurosci. 2006;26:7619–28. doi: 10.1523/JNEUROSCI.0855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altman J, Bayer SA. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol. 1978;182:995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- 50.Seo JH, Chang JH, Song SH, et al. Spatiotemporal gradient of astrocyte development in the chick optic tectum: evidence for multiple origins and migratory paths of astrocytes. Neurochem Res. 2008;33:1346–55. doi: 10.1007/s11064-008-9590-3. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Tamamaki N, Noda T, et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–64. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


