Abstract
Hypoxia is a common environmental stress factor and is associated with fibrogenesis. Matrix metalloproteinase-2 (MMP-2), produced by hepatic stellate cells (HSCs), plays an important role in liver fibrogenesis. However, inconsistent results have been reported on the impact of hypoxia on MMP-2 expression and activity in HSCs. We speculated that cell–cell interaction is involved in the regulation of MMP-2 expression and activity at low oxygen level in vivo. Therefore, in this report we investigated the mechanism by which hypoxic hepatocytes regulates MMP-2 expression in HSCs. Our results showed that the conditioned medium from hypoxia-treated rat hepatocytes strongly induced the expression of MMP-2 mRNA and protein in rat HSC-T6 cells. Reduced glutathione neutralized ROS released from hypoxic hepatocytes, leading to reduced MMP-2 expression in HSC-T6 cells. In addition, phospho-IκB-α protein level was increased in HSC-T6 cells treated with hypoxia conditioned medium, and NF-κB signaling inhibitor inhibited MMP-2 expression in HSC-T6 cells. Taken together, our data suggest that ROS is an important factor released by hypoxic hepatocytes to regulate MMP-2 expression in HSCs, and NF-κB signaling is crucially involved in ROS-induced MMP-2 expression in HSCs. Our findings suggest that strategies aimed at antagonizing the generation of ROS in hypoxic hepatocytes and inhibiting NF-κB signaling in HSCs may represent novel therapeutic options for liver fibrosis.
Keywords: hypoxia, hepatocyte, hepatic stellate cells, liver fibrosis, reactive oxygen species
1. Introduction
It is estimated that over 100 million people suffer from liver fibrosis in the world. As the main complication of chronic liver damage, liver fibrosis is a wound healing process characterized by the accumulation of extracellular matrix (ECM) proteins in the liver. Although liver fibrosis is caused by a variety of etiologic agents, including chronic viral hepatitis, alcohol toxicity, autoimmune disease, and hereditary metabolic disorders, it is now generally accepted that a central pathologic mechanism underlying liver fibrosis is the generation and proliferation of smooth muscle α-actin (α-SMA)-positive myofibroblasts of periportal and perisinusoidal origin that arise as a consequence of the activation of hepatic stellate cells (HSCs) [1–3,4]. HSCs play a critical role in the excessive production and secretion of ECM, resulting in the generation of fibrous tissue and scar formation [5]. The activation of HSCs is considered to be the key factor responsible for liver fibrosis [1]. In addition to cytokines and other soluble factors released by Kupffer cells, inflammatory cells and damaged hepatocytes, changes of ECM composition have been suggested to be implicated in the activation of HSCs [6].
Enzymes known to degrade type IV collagen include matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), both of which are members of zinc-dependent matrix metalloproteinase family. MMP-2 is involved in ECM remodeling during tissue and organ development, wound repair, tumor invasion and metastasis. It has been shown that activated HSCs proliferate quickly and produce a large amount of ECM and MMP-2 [7,8]. Therefore, MMP-2 and HSCs activation are interdependent. During the progression of liver fibrosis, deposition of type IV collagen increases significantly, which might be due to the inability of MMP-2 to degrade type IV collagen. If the expression and activity of MMP-2 were interfered at different stages, the progression of liver fibrosis may be changed. Thus, further characterization of HSCs is crucial for the understanding of the pathogenesis of hepatic fibrosis.
Many factors have been shown to affect MMP-2 expression and activity in HSCs, such as reactive oxygen species (ROS) generated during oxidative stress, transforming growth factor β1 (TGF-β1), p53, AP-1, membrane-type matrix metalloproteinase (MT1-MMP), tissue inhibitor of matrix metalloproteinase-2 (TIMP-2), plasmin, thrombin, type I collagen, Zn2+, reversion-inducing-cysteine-rich protein with Kazal motifs (RECK), and the interaction between hepatocytes and hepatic stellate cells [9–11]. Oxygen is necessary for cellular metabolic process and acts as a modulator of gene expression [12]. One of the most prominently pathological significance of hypoxic mammals is an increase in ECM synthesis. The steady-state concentration of the collagens depends on both the rate of their synthesis and their degradation. Hypoxia is involved in the pathogenic process of liver fibrosis and may activate HSCs [13–15]. In addition, our previous studies have shown that hypoxia stimulated MMP-2 synthesis in HSCs in vitro, and the expression of hypoxia inducible factor-1α was increased in hepatocytes in the rat liver fibrosis tissues [16–18]. However, it is unknown whether and how hepatocytes modulate MMP-2 expression in HSCs under hypoxic conditions. Therefore, in this study we investigated the mechanism by which hypoxic hepatocytes regulates MMP-2 expression in HSCs. Our results suggest that ROS is an important factor released by hypoxic hepatocyte to regulate MMP-2 expression in HSCs and NF-κB signaling is crucially involved in these processes.
2. Materials and Methods
2.1. Cell Culture and Preparation of Conditioned Media
Rat hepatocyte BRL-3A cells and hepatic stellate cell HSC-T6 cells were obtained from Type Culture Collection of Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen, USA) containing 10% fetal calf serum (FBS, Gibco, USA) and 100 units/mL penicillin/streptomycin at 37 °C in a humidified atmosphere with 5% CO2. Cells were plated in 6-well plates at a density of 2 × 105 cells/well. At 80% confluence, BRL-3A cells were treated with hypoxia. The cells were replaced with serum-free DMEM and then incubated in the chambers flushed with air mixture containing 1% O2, 5% CO2 and 94% N2 for 30 min. The ultimate oxygen tension was 5%. The chambers were sealed and placed at 37 °C and incubated for 12 h. Controls included parallel cultures in which cells were exposed to ambient air (e.g., normaxia/21% oxygen tension). The supernatant was collected from the cultured cells at 12 h and passed through a 0.22 μm filter. The filtrate was defined as hypoxia conditioned medium and normoxia conditioned medium. The conditioned medium was used immediately for following experiments.
To induce HSC-T6 cell differentiation and MMP-2 expression by hypoxia conditioned medium, HSC-T6 cells were grown to 80% confluence and then washed twice with PBS. Then HSC-T6 cells were cultured in hypoxia conditioned medium and collected after 6 h, 12 h and 24 h, respectively. As controls, HSC-T6 cells were cultured in DMEM medium or normoxia conditioned medium. All the cells were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
2.2. Real-Time RT-PCR
Total RNA was extracted from HSC-T6 cells with Trizol reagent (Invitrogen, USA) following the manufacturer’s instructions. Total RNA (1 μg) was used for cDNA synthesis using PrimeScript RT reagent kit (TaKaRa, Dalian, China). Aliquot of diluted first-strand cDNA was amplified with a Real-Time PCR Detection System (ABI7300, USA) using SYBR PrimeScript RT-PCR Kit according to the manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The following primers were used: MMP-2, 5-AGGGCACCTCCTACAACAGC-3 and 5-CAGTGGACATAGCGGTCTCG-3 (126 bp) [19]; GAPDH, 5-GAAGGGCTCATGACCACAGT-3 and 5-GGATGCAGGGATGATGTTCT-3 (117 bp) [20]. RT-PCR was performed three times in triplicate. The relative mRNA level of MMP-2 was compared to that of GAPDH and was calculated by the 2−ΔΔCt method. Each Ct value used for these calculations was the mean of the triplicate for each reaction.
2.3. Western Blot
HSC-T6 cells were lysed with RIPA lysis buffer (Beyotime, China). After centrifugation, the lysates were determined by the Bradford assay (Beyotime, China) for protein concentration. Total protein for each sample (50 μg) was fractionated on 10% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk at room temperature for 1 h and incubated with the rabbit polyclonal anti-MMP-2 antibody (Bioworld, China, 1:500 dilution), phospho-IκB-α antibody (Bioworld, China, 1:500 dilution), IκB-α antibody (Bioworld, China, 1:500 dilution), or mouse monoclonal anti-β-actin antibody (Santa Cruz, USA, 1:1000 dilution) at 4 °C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated anti-goat or anti-mouse antibody (Santa Cruz, USA, 1:10,000 dilution) at room temperature for 1 h. After washing, the bound antibody was visualized with Super ECL Detection Reagent (Applygen, China).
2.4. Gelatin Zymography for MMP-2
MMP-2 activity was determined by gelatin zymography. Supernatants were collected from HSC-T6 cells and centrifuged. Protein content of the supernatants was determined by the Bradford assay. Total protein for each sample (15 μg) was fractionated on 10% SDS-PAGE gel containing 0.1% gelatin under non-reducing conditions. Gelatin zymography was performed using a MMP Zymography assay kit (Applygen Technologies Inc.) according to the manufacturer’s instructions. Gelatinolytic bands were observed as clear zones against the blue background and the intensity of the bands was estimated using the ScnImage Software.
2.5. Quantitative Assay for Reactive Oxygen Species
BRL-3A cells were cultured and treated with hypoxia as previously described in 96-well plates. The final oxygen tensions in the chambers were 5% and 10%, respectively. Controls included parallel cultures in which cells were exposed to normoxia (21% oxygen tension). 12 h later, the supernatants were collected and put in another 96-well cell culture plates. The ROS in the supernatants was measured by ROS assay kit (Genmed, China). Briefly, the samples were incubated with 10 μL 3,3′,5,5′ tetramethylbenzidine (TMB Substrate) at 37 °C for 1 h. This would result in blue color development proportional to the amount of ROS and the level of ROS in each sample was measured with the spectrophotometer at a wavelength of 650 nm.
2.6. Treatment of BRL-3A Cells with Reduced Glutathione
BRL-3A cells were cultured in 6-well plates. At 80% confluency, the medium was changed to serum-free DMEM medium containing reduced glutathione (0, 0.5, 2.5, 10 mmol/L). After 30 min, the plates were incubated in a chamber flushed with air mixture containing 1% O2, 5% CO2 and 94% N2 for 30 min. The chambers were sealed and placed at 37 °C and incubated for 12 h. The level of ROS in each sample was measured according to the method above. Meanwhile, the supernatants of BRL-3A were collected from the cells and passed through a 0.22 μm filter. The filtrate was designated as ROS-reduced-hepatocyte-conditioned medium and stored at −20 °C for later use. The ROS-reduced-hepatocyte-conditioned medium was used to culture HSC-T6 cell as previously described for 6 h, 12 h, and 24 h. Then the supernatants were collected, and total protein and RNA were extracted from HSC-T6 cells.
2.7. Statistical Analysis
Experiments were repeated at least three times. The results were presented as means ± SEM. Comparison of multiple parameters was performed with one-way ANOVA test (SPSS 11.5), followed by Tukey-Kramer’s post hoc test. p < 0.05 was considered as statistically significant.
3. Results
3.1. Hepatocyte Conditioned Medium Upregulates MMP-2 Expression in HSCs
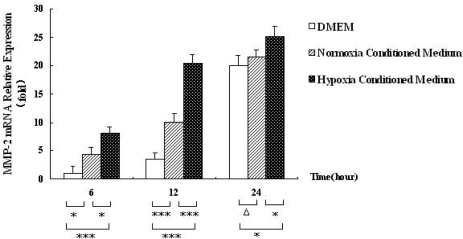
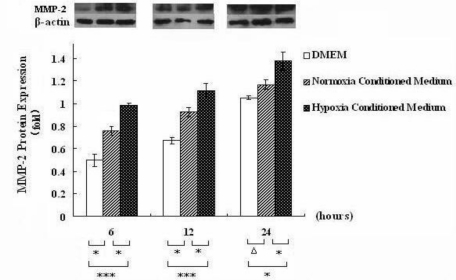
Our prior study has shown that hypoxia could regulate the expression of MMP-2 mRNA in HSC-T6 cell [16,17,21]. To investigate whether hypoxic hepatocytes affect the expression of MMP-2 mRNA in HSC-T6 cells, we measured MMP-2 mRNA level in rat HSC-T6 cells cultured in hepatocyte-conditioned medium for 6, 12 and 24 h, respectively. As shown in Figure 1, real-time RT-PCR analysis showed that MMP-2 mRNA expression was increased in HSC-T6 cells treated with hepatocyte-conditioned medium. Time factor analysis by one-way ANOVA showed that MMP-2 mRNA level in HSC-T6 cells treated with the conditioned medium was significantly higher than that in the controls (p < 0.001). These results indicate that hepatocyte conditioned medium upregulates MMP-2 expression in HSCs. To provide further evidence for this, we performed Western blot analysis to examine MMP2 expression at the protein level in HSC-T6 cells cultured with hepatocyte-conditioned medium at 6, 12 and 24 h, respectively. The results showed that MMP-2 protein was induced in HSC-T6 cells treated with the hepatocyte-conditioned medium. The protein level of MMP-2 at 12 h was higher than that at 6 h and 24 h (Figure 2). Taken together, these data suggest that MMP-2 expression in HSC-T6 cells exhibited a slow response to hepatocyte-conditioned medium.
Figure 1.
Hepatocyte conditioned medium upregulates MMP-2 mRNA expression in HSCs. Relative MMP-2 mRNA level was determined by Real-time RT-PCR in HSC-T6 cells treated with hepatocyte conditioned medium, serum-free DMEM medium and hepatocyte-conditioned control medium (normoxia conditioned medium) for 6, 12 and 24 h. Data were expressed as means ± SEM from 3 independent experiemnts. * p < 0.05, ** p < 0.01, *** p < 0.001, and Δp > 0.05.
Figure 2.
Hepatocyte conditioned medium upregulates MMP-2 protein expression in HSCs. Western blot analysis showing MMP-2 protein level in HSC-T6 cells cultured in hepatocyte conditioned medium, serum-free DMEM medium and hepatocyte-conditioned control medium (normoxia conditioned medium) for 6, 12 and 24 h. The specificity of MMP-2 antibody was demonstrated by the detection of MMP2 as a 72 KD protein. Shown were representative blots from three independent experiments with similar results. Data were expressed as means ± SEM from 3 independent experiments. * p < 0.05, and Δp > 0.05.
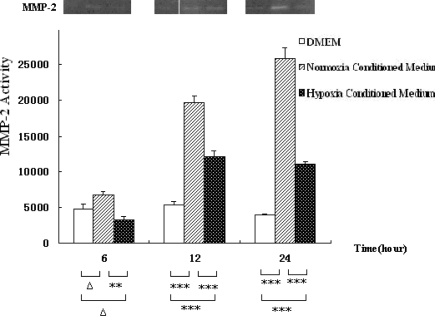
To find out whether anoxic hepatocytes affect the activity of MMP-2 in HSC-T6 cells, gelatin zymography was conducted. The results showed that MMP-2 activity was inhibited by hepatocyte-conditioned medium. The effect within 24 h was more intensive. Time factor analysis by one-way ANOVA test showed that MMP-2 activity in HSC-T6 cells in hepatocyte-conditioned medium and in the controls had significant differences (p = 0.024). Group factor analysis (p < 0.001) was shown in Figure 3, indicating that hepatocyte-conditioned medium inhibits MMP-2 activity in rat HSCs.
Figure 3.
MMP-2 activity measured by gelatin zymography in HSC-T6 cells cultured with hepatocyte conditioned medium (hepatocyte conditioned culture), serum-free DMEM medium, and hepatocyte-conditioned control medium at 6, 12 and 24 h. Typical results were shown from independent experiments performed at least three times. Data were expressed as means ±SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 and Δp > 0.05.
3.2. Reduced Glutathione Antagonizes the Generation of ROS in the Supernatants of Hepatocytes
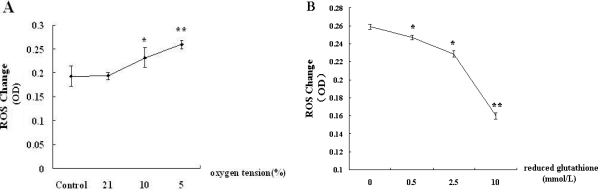
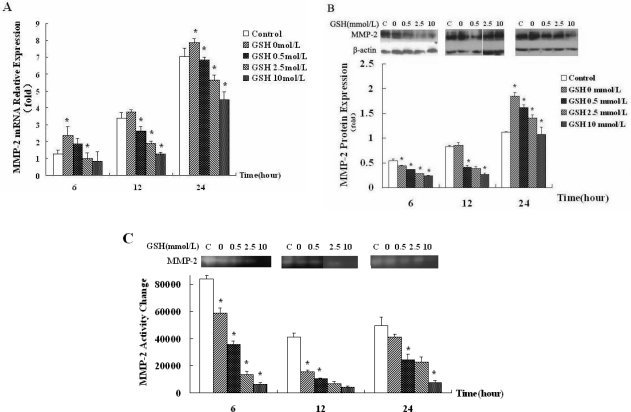
Because ROS could regulate MMP-2 expression, we determined the level of ROS in the supernatant of hepatocytes treated with hypoxia. BRL-3A cells were treated with different levels of oxygen (5% O2, 10% O2, and 21% O2) for 12 h and supernatants were collected for quantitative colorimetric assay. The results showed that ROS level gradually increased with the decrease of oxygen tension (Figure 4A). To investigate whether reduced glutathione (GSH) could eliminate the release of ROS to the supernatant of hepatocytes treated with hypoxia, we treated BRL-3A cells with GSH (0, 0.5, 2.5, 10 mmol/L), and examined ROS level in the conditioned medium. The results showed that the level of ROS in hypoxia conditioned medium decreased gradually with the increase of reduced glutathione (Figure 4B).
Figure 4.
GSH antagonizes the generation of ROS in the supernatants of hepatocytes. (A) BRL-3A cells were treated with different levels of oxygen (5% O2, 10% O2 and 21% O2) for 12 h. ROS in the supernatants was quantified using a colorimetric assay. Data were expressed as means ± SEM from 3 independent experiments. * p < 0.05, ** p < 0.01 vs. Control; (B) BRL-3A cells were treated with GSH (0, 0.5, 2.5, 10 mmol/L) and then exposed to 5% oxygen for 12 h. The ROS level in the hypoxia conditioned medium was analyzed. Data were expressed as means ± SEM from 3 independent experiments. * p < 0.05, *** p < 0.001 vs. group treated with 0 mmol/L GSH.
3.3. ROS Generated by Hypoxic Hepatocyte Contributes to the Downregulation of MMP-2 Expression and Activity in HSCs
To confirm that ROS generated by hypoxic hepatocyte is one of the main factors that contribute to increased expression of MMP-2 in HSCs, we examined MMP-2 expression at both mRNA and protein levels in HSC-T6 cells cultured in ROS-neutralized-hepatocyte conditioned medium. Real-time RT-PCR analysis showed that with the increase of GSH concentration, MMP-2 mRNA expression in HSC-T6 cells was significantly inhibited (Figure 5A, p < 0.001). In addition, Western blot analysis showed that MMP-2 protein expression in HSC-T6 cells was reduced corresponding to increased GSH concentration (Figure 5B, p < 0.001). The result of gelatin zymography (Figure 5C) was consistent with Real-time RT-PCR and Western blot (p < 0.001).
Figure 5.
ROS-neutralized hepatocyte conditioned medium downregulates MMP-2 expression and activity in HSCs. BRL-3A cells were treated with reduced glutathione (0, 0.5, 2.5, 10 mmol/L) and exposed to 5% oxygen for 12 h. Then the supernatant was collected and HSC-T6 cells were cultured with ROS-neutralized-hepatocyte-conditioned medium for 6, 12 and 24 h. As a control, HSC-T6 cells were cultured in serum-free DMEM medium. (A) relative MMP-2 mRNA level in HSC-T6 cells were determined by Real-time RT-PCR. Data were expressed as means ± SEM from 3 independent experiments. * p < 0.05 vs. the former group; (B) MMP-2 protein level in HSC-T6 cells was detected by Western blot. Shown were representative blots from three independent experiments with similar results; (C) MMP-2 activity measured by gelatin zymography.
3.4. Hepatocyte Conditioned Medium Upregulates MMP-2 Expression in HSCs via NF-κB Signaling
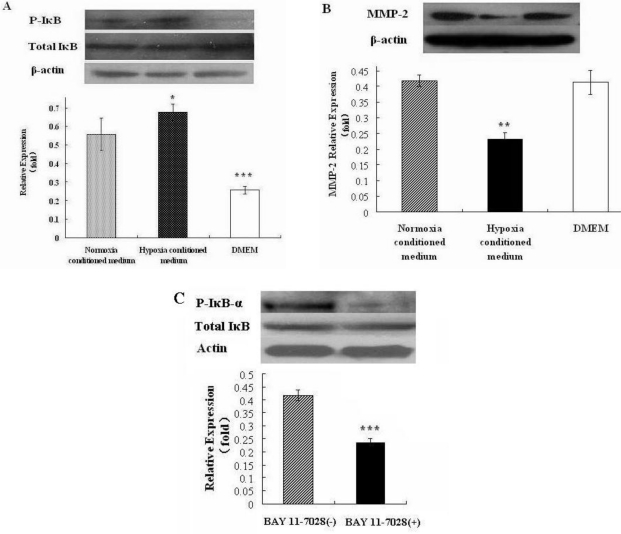
To investigate whether ROS in hepatocyte conditioned medium modulates MMP2 expression via NF-κB signaling, we measured the level of phospho-IκB-α protein in HSC-T6 cells cultured with serum-free DMEM, normoxia conditioned medium, hypoxia conditioned medium, respectively, for 12 h. Western blot analysis showed that phospho-IκB-α protein level in HSC-T6 cells treated with the hypoxia conditioned medium was increased compared to that in cells cultured in normoxia conditioned medium (Figure 6A, p < 0.05). BAY 11-7082 is a commonly used NF-κB inhibitor. When we treated HSC-T6 cells with BAY 11-7082, we found that MMP2 expression in HSC-T6 cells was significantly inhibited compared to cells treated with vehicle control (Figure 6B, p < 0.05). As expected, BAY 11-7082 inhibited the activation of NF-κB in HSC-T6 cells (Figure 6C). Collectively, these data suggest that NF-κB signaling mediates ROS-induced MMP-2 expression in HSCs.
Figure 6.
NF-κB signaling mediates ROS-induced MMP-2 expression in HSCs. (A) Western blot analysis showing MMP-2 and phosphor-IκB-α levels in HSC-T6 cells cultured in hepatocyte conditioned medium, serum-free DMEM medium and hepatocyte-conditioned control medium (normoxia conditioned medium). Data were expressed as means ± SEM from 3 independent experiments. * p < 0.05, Δp > 0.05; (B) HSC-T6 cells were treated by BAY 11-7082 (100 μM) or vehicle control and MMP-2 protein level was detected by Western blot. Data were expressed as means ± SEM from 3 independent experiments. * p < 0.05 vs. vehicle control; (C) HSC-T6 cells were treated by BAY 11-7082 (100 μM) or vehicle control, total and phosphor IκB-α levels were detected by Western blot. Data were expressed as means ± SEM from 3 independent experiments. *** p < 0.01 vs. vehicle control.
4. Discussion
Hypoxia is crucially involved in acute and chronic liver injury. As a repair process in response to a variety of chronic injury stimuli, the progression of liver fibrosis is accompanied by hypoxia. Following a fibrogenic stimulus, HSCs are activated which then synthesize and deposit a large amount of ECM in the liver [22–24]. The activation of HSCs is a key event of fibrogenesis [4,7]. We have found that hypoxia induced the expression of MMP-2 at both mRNA and protein levels, but inhibited its activity in the in vitro assay [16–18]. In vivo experiment suggested that at the beginning of liver fibrosis, MMP-2 expression and activity were decreased, and fewer HSCs were activated. However, during the development of fibrosis, MMP-2 activity was increased, which degrades collagen IV [17,25–27]. Given these inconsistent results between in vivo and in vitro studies, we believe that cell–cell interaction is involved in the regulation of MMP-2 expression and activity at low oxygen level in vivo. Therefore, in this report we investigated the mechanism by which hypoxic hepatocytes regulates MMP-2 expression in HSCs.
First, we examined whether hypoxic hepatocytes affect MMP-2 expression in HSC-T6 cells. Real-time RT-PCR analysis showed that hypoxic hepatocytes may secrete some factor(s) to affect MMP-2 expression at mRNA level in HSC-T6 cells. Consistent with RT-PCR analysis, Western blot analysis further showed that hepatocyte-conditioned medium induced the expression of MMP-2 in HSC-6T cells at protein level. Taken together, these data indicate that hypoxic hepatocytes release some factor(s) to upregulate the expression of MMP-2 in HSC-T6 cells.
Next, a serial of experiments was conducted to identify the factor(s) released from hypoxic hepatocytes responsible for the upregulation of MMP-2 expression in HSCs. It is known that ROS serves as a signal molecule that regulates many important cellular events, such as transcription factor activation, gene expression, cell differentiation and proliferation. ROS is also involved in the regulation of MMP-2 expression and activation, mainly through Ras and mitogen activated protein kinase (MAPK) signaling cascades [21,28]. Therefore, we detected ROS level in the supernatant of BRL-3A cells treated with different levels of oxygen and found that with the decrease of oxygen tension, ROS was gradually increased. Reduced glutathione (GSH) is a non-protein antioxidant and can protect cells from ROS damage. After treatment with GSH, we detected ROS in the supernatants of hypoxia conditioned medium to determine whether ROS can be scavenged by reduced glutathione. The results evidently demonstrated that as the concentration of GSH increased, the level of ROS in the supernatants of hypoxia conditioned medium was gradually decreased, proving that ROS generated in hepatocytes was scavenged efficiently by reduced glutathione.
To provide further evidence that ROS generated in hepatocytes contributes to the upregulation of MMP-2 expression in HSC-T6. Last, we characterized the potential mechanism by which ROS regulates MMP-2 expression in HSCs. By probing phospho-IκB-α protein level as an indication of the activation of NF-κB signaling, we found that hypoxia conditioned medium induced increased phospho-IκB-α protein level in HSC-T6 cells, corresponding to increased MMP-2 protein level. Furthermore, inhibition of NF-κB signaling by specific inhibitor BAY 11-7082 led to reduced MMP2 protein level in HSC-T6 cells. Based on these data we speculate that NF-κB signaling is an important, although not exclusive, signaling pathway that mediates ROS-induced MMP-2 expression in HSCs.
Gelatin zymography analysis (Figure 5C) showed that hypoxic hepatocytes released ROS to persistently inhibit the activity of MMP-2 in HSC-T6 cell within 24 h. This resutl was consistent with that of Real-time RT-PCR and Western blot. However, in contrast to the result showed in Figure 3, we found that MMP-2 activity was inhibited by hepatocyte-conditioned medium. These results suggest that ROS may be the main factor released by anoxic hepatocyte to regulate MMP-2 mRNA and protein expression, but may not be the main factor that regulates MMP-2 activity.
MMP-2 is secreted as a latent form of zymogen and can be activated when the pro-domain is cleaved and bound to TIMP-2 and MT1-MMP. Many factors are involved in regulating MMP-2 activity. As the main inhibitor, tissue inhibitor of metalloproteinase (TIMP) is the most important regulator of MMP-2 activity. It is possible that the factor released by anoxic hepatocyte regulates MMP-2 activity by means of disturbing the balance between MMP-2 and its endogenous inhibitors TIMPs [29]. As a novel membrane-anchored matrix metalloproteinase (MMP) inhibitor, reversion-inducing cysteine-rich protein with Kazal motifs (RECK) also plays a role in the regulation of MMP activity. RECK inhibits MMP-2, MMP-9, and membrane type-1 MMP (MMP-14) secretion and activity [30]. MMP-2 is Zn-dependent enzyme. Therefore, Zn2+ is necessary for MMP-2 activity [31]. In addition, MMP-2 is also directly activated by oxidizing the sulphydril bond between a cysteine residue of the prodomain and the Zn2+ catalytic center, resulting in partial enzyme activation followed by an intramolecular cleavage of the propeptide [32]. In our future study we will identify the factors generated by anoxic hepatocytes that inhibit MMP2 activity.
MMP-2 has been shown to promote the formation and development of liver fibrosis, mainly through the regulation of the activation, proliferation, and migration of HSCs. In vitro studies found that MMP-2 could degrade type IV collagen-rich matrix around the HSCs, which could activate HSCs. The activated HSCs proliferate actively, produce large amounts of extracellular matrix, and are main source of MMP-2 in the liver [33]. In addition, oxidative stress induced by some pathogenic factors promoted the proliferation and migration of HSCs through MMP-2 [34]. MMP-2 also promoted the proliferation of HSCs through release of growth factors stored in the matrix [35]. Furthermore, MMP-2 has been shown to induce the release of VEGF [36], which is involved in hepatic sinusoidal capillarization and promotes the development of liver fibrosis [7,37–39]. Further study is important to investigate the mechanism underling the regulation of the expression and activity of MMP-2 in liver fibrosis.
5. Conclusions
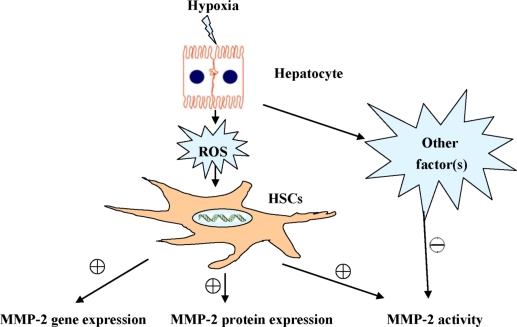
In summary, in this study we provide several lines of evidence that ROS is an important factor released by hypoxic hepatocyte to regulate MMP-2 expression in HSCs, and NF-κB signaling is crucially involved in ROS-induced MMP-2 expression in HSCs. Our findings suggest that strategies aimed at antagonizing the generation of ROS in hypoxic hepatocytes and inhibiting NF-κB signaling in HSCs may represent novel therapeutic options for liver fibrosis.
Figure 7.
Hypoxic hepatocytes regulate MMP-2 expression and activity in Hepatic Stellate Cells.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China. (No. 30470780).
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senoo H. Structure and function of hepatic stellate cells. Med. Electron. Microsc. 2004;37:3–15. doi: 10.1007/s00795-003-0230-3. [DOI] [PubMed] [Google Scholar]
- 3.Agnieszka SC, Krzysztof P, Jadwiga D, Martyna KS. Zinc supplementation attenuates ethanol-and acetaldehyde-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS) production and by influencing intracellular signaling. Biochem. Pharmacol. 2009;78:301–314. doi: 10.1016/j.bcp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K. Liver fibrosis: Insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 5.Parola M, Marra F, Pinzani M. Myofibroblast-like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol. Aspects Med. 2008;29:58–66. doi: 10.1016/j.mam.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 7.Arthur MJP. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:245–249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 8.McCrudden R, Iredale JP. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol. Histopathol. 2000;15:1159–1168. doi: 10.14670/HH-15.1159. [DOI] [PubMed] [Google Scholar]
- 9.Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J. Gastroenterol. 2008;43:419–428. doi: 10.1007/s00535-008-2180-y. [DOI] [PubMed] [Google Scholar]
- 10.Gardi C, Arezzini B, Fortino V, Comporti M. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem. Pharmacol. 2002;64:1139–1145. doi: 10.1016/s0006-2952(02)01257-1. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored-glycoprotein RECK. Proc. Natl. Acad. Sci. USA. 1998;95:13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Life with Oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 13.Hao XJ, Li XJ, Du L. Observation of shenqirougan decection on hepatic fibrosis. J Emerg Traditional Chin Med. 2010;19:583–584. 591. [Google Scholar]
- 14.Guo L, Wang Z. Effects of ganxinning capsule on super micro-structure of fibrotic liver rats. J Emerg Traditional Chin Med. 2010;19:638–639. 654. [Google Scholar]
- 15.Shi YF, Fong CC, Zhang Q, Cheung PY, Tzang CH, Wu RS. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. Febs. Lett. 2007;581:203–210. doi: 10.1016/j.febslet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Fan RH, Chen PS, Zhao D, Zhang WD. Hypoxia induced by CoCl2 influencing the expression and the activity of matrix metalloproteinase-2 in rat hepatic stellate cells. Chin. J. Hepatol. 2007;15:654–657. [PubMed] [Google Scholar]
- 17.Chen PS, Zhai WR, Zhou XM, Zhang JS, Zhang YE, Ling YQ. Effects of hypoxia on the regulation of expression and activity of matrix metalloproteinase-2 in hepatic stellate cell. World J. Gastroenterol. 2001;7:647–651. doi: 10.3748/wjg.v7.i5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao D, Fan RH, Chen PS. Effects of hypoxia on expression and activity of matrix metalloproteinase-2 and the mechanism of regulation by hypoxia-inducible factor1α in hepatic stellate cells. Pathol. Int. 2007;57:A24. [Google Scholar]
- 19.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 20.Scheid A, Wenger RH, Christina H, Camenisch I, Ferenc A, Stauffer UG. Hypoxia-regulated gene expression in fetal wound regeneration and adult wound repair. Pediatr. Surg. Int. 2000;16:232–236. doi: 10.1007/s003830050735. [DOI] [PubMed] [Google Scholar]
- 21.Zhang AF, Chen PS. Effects of hyperbaric oxygen with free-radical antagonists on the expression and activity of matrix metalloproteinase-2 in rat livers. Chin. J. Hepatol. 2005;6:545–546. [PubMed] [Google Scholar]
- 22.Friedman SL. Cytokines and fibrogenesis. Semin. Liver Dis. 1999;19:129–140. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 23.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Basalyga DM, Simionescu A, Isenburg JC, Simionescu DT, Vyavahare NR. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am. J. Pathol. 2006;168:490–498. doi: 10.2353/ajpath.2006.050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason HR, Grove SD, Rubin BS, Nowak RA, Castellot JJ., Jr Estrogen induces CCN5 expression in the rat uterus in vivo. Endocrinology. 2004;145:976–982. doi: 10.1210/en.2003-0823. [DOI] [PubMed] [Google Scholar]
- 26.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K. Liver fibrosis: Insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 28.Nelson KK, Melendez JA. The powerhouse takes control of the cell: The role of mitochondria in signal transduction. Free Radic. Biol. Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 30.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 31.Newsome AL, Johnson JP, Seipelt RL, Thompson MW. Apolactoferrin inhibits the catalytic domain of matrix metalloproteinase-2 by zinc chelation. Biochem. Cell Biol. 2007;85:563–572. doi: 10.1139/o07-073. [DOI] [PubMed] [Google Scholar]
- 32.Han YH, Park WH. The changes of reactive oxygen species and glutathione by MG132, a proteasome inhibitor affect As4.1 juxtaglomerular cell growth and death. Chem. Biol. Interact. 2010;184:319–332. doi: 10.1016/j.cbi.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin. Chim. Acta. 2006;364:33–60. doi: 10.1016/j.cca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, et al. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP-2 mediated mechanism. Hepatology. 2005;41:1074–1084. doi: 10.1002/hep.20683. [DOI] [PubMed] [Google Scholar]
- 35.Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulzet R. Regulation of matrix metalloproteinase-2 activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 36.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 37.Kumar M, Sarin SK. Is cirrhosis of the liver reversible? Indian J. Pediatr. 2007;74:393–399. doi: 10.1007/s12098-007-0067-1. [DOI] [PubMed] [Google Scholar]
- 38.Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J. Biol. Chem. 2007;282:30485–30496. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- 39.Chen PS, Feng Z, Zhang XM, Liu DF. Expression of HIF-1α and its downstream molecules in rat liver fibrosis tissue. J. Gastroenterol. Hepatol. 2006;21:A144. [Google Scholar]