Abstract
Most colorectal cancers have mutations in the tumor suppressor APC. The best-understood function of APC is its participation in a protein complex that regulates the availability of β-catenin. Solid tumors are characterized by the presence of hypoxia as well as inflammation, which leads to the upregulation of Hypoxia Inducible Factors like HIF-1α. We recently demonstrated a novel antagonistic link between APC and HIF-1α. We found that hypoxia results in reduced levels of APC mRNA and protein via a direct HIF-1α-dependent mechanism. Similarly, APC mediates the repression of HIF-1α. However, this requires wildtype APC, low levels of β-catenin and NFκB activity. These results reveal the downregulation of APC as a novel mechanism that contributes to the survival advantage induced by hypoxia and cytokines such as TNFα. Our data indicate that loss-of-function mutations in APC result in the engagement of the hypoxia response. Importantly, this suggests that other stimuli that induce HIF, such as inflammatory cytokines and oncogenes, alter APC function.
Key words: HIF-1, APC, hypoxia, NFκB
Adenomatous polyposis coli (APC) is a tumor suppressor mutated in most colorectal cancers.1 APC is also mutated in the human syndrome Familial Adenomatous polyposis (FAP). FAP patients are heterozygous for APC. They develop hundreds of polyps in their gut,2,3 and progression to malignancy involves the presence of inflammation and hypoxia.4,5 The APC protein is involved in many of the fundamental processes that govern normal gut epithelium. It is best known for controlling the Wnt/β-catenin pathway, where it regulates β-catenin levels, thus regulating the transcriptional activity of TCF/ LEF transcription factors.6 APC also contributes to the regulation of cytoskeletal proteins.7
Hypoxia is a common feature of solid tumors and regulates tumor angiogenesis and growth.4,8 Hypoxia leads to the induction of the transcription factor Hypoxia Inducible Factor (HIF),9 a heterodimeric transcription factor composed of α and β subunits. While HIF-1β is constitutively expressed, HIF-α subunits are extremely labile at normal oxygen levels. Oxygen controls HIF-α levels through post-translational hydroxylation, catalyzed by a class of 2-oxoglutarate dioxygenases called prolyl-hydroxylases (PHDs). Hydroxylation of HIF-α signals for the ubiquitin recognition complex containing the von Hippel Lindau tumor suppressor and subsequent degradation by the proteasome.10,11 When oxygen levels are reduced or cofactors such as iron ions are not available, PHD activity is inhibited resulting in increased HIF-α levels. Under these conditions, HIF-α translocates to the nucleus and transactivates its target genes.
In addition to hypoxia, other stimuli also result in the induction of HIF-α.12–14 Specifically, the HIF-1α gene is under the control of NFκB13,15,16 and the chromatin remodelling complex SWI/SNF.17 Furthermore, NFκB also controls HIF-1β directly and HIF-2α indirectly,14 making NFκB a key regulator of the HIF system. NFκB is the collective name for a family of important transcription factors that control many cellular processes such as apoptosis and proliferation (reviewed in ref. 18).
We recently reported functional crosstalk between HIF-1α and APC at the transcriptional level;19 depletion of HIF-1α results in increased APC mRNA and protein, just as depletion of APC results in increased HIF-1α. The former is the result of direct transcriptional repression of APC by HIF-1α. We discovered a hypoxia-responsive element (HRE) in the APC promoter and demonstrated that hypoxia induces HIF-1α binding to this site. Importantly, hypoxia promotes a reduction in APC mRNA and protein in a variety of cells, suggesting that suppression of APC by hypoxia can contribute to increased survival in hypoxic conditions in tumors with wild-type APC.
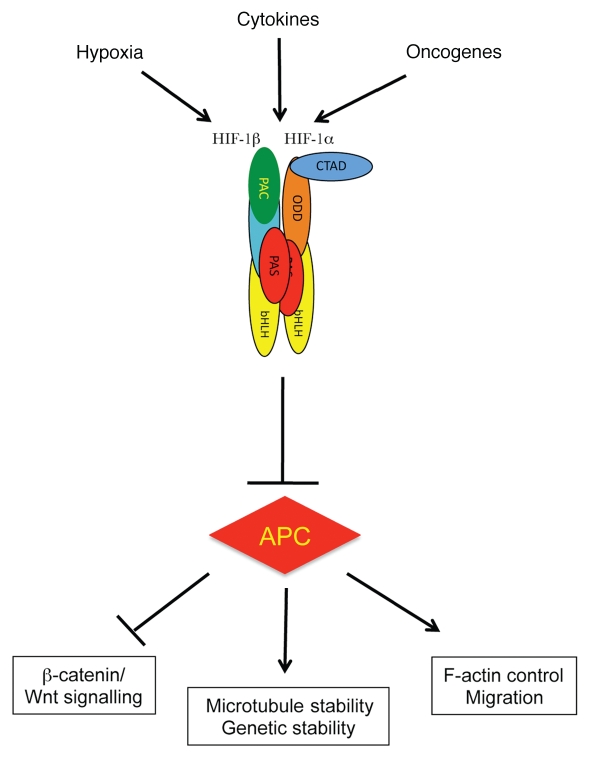
Cytokines and oncogenes can induce HIF levels and activity12,14,20 suggesting that these stimuli can modulate APC levels via HIF-dependent mechanisms (Fig. 1). Cytokine- and oncogene-induced repression of APC is predicted to increase β-catenin and Wnt signaling, allowing cells to progress to a more proliferative phenotype (Fig. 1).
Figure 1.
HIF-1α represses APC. Schematic diagram depicting how hypoxia, cytokines and oncogenes induce HIF to produce transcriptional repression of APC and hence deregulation of APC function.
Our study demonstrated that HIF-1 represses the APC promoter directly and does not discriminate between wild-type and mutant APC. Consistently, hypoxia results in decreased levels of mutant (truncated) APC protein in cancer cells. The significance of this observation is not clear. Truncated N-terminal fragments of APC can interact with a number of proteins.7 The activity of these fragments may be different in isolation than in the context of the full-length molecule. For instance, N-terminal fragments are more active in stimulating the GEF activity of ASEF than full-length APC.21,22 Furthermore, N-terminal domains of APC bind to C-terminal regions, which are lacking in tumor cells.23 This interaction can regulate protein interactions of the N-terminal domain.23 Thus in tumor cells, when the C-terminal region is missing, such regulation is not available, leaving the isolated N-terminal fragments unregulated.23,24 The exact nature of the functions or activities carried out by N-terminal fragments is not clear at all. However, expression of N-terminal APC fragments in diverse cells and tissues has strong dominant effects on cell migration (unpublished observations). At this time, the direct consequences of the loss of truncated APC fragments induced by elevated HIF-1α are not understood and require further studies.
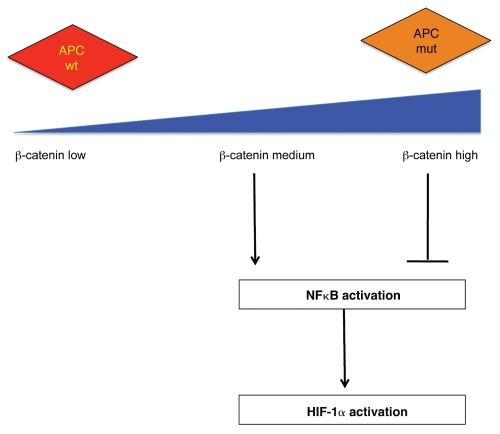
A reciprocal repression of HIF-1α by APC was also observed. APC depletion results in increased HIF-1α levels and activity (Fig. 2). This increase is mediated by NFκB and requires regulation of β-catenin by APC. In this case, the level of active β-catenin seems to be crucial for the ability of APC to regulate HIF. This is likely related to the fact that, while too much β-catenin activity results in NFκB inhibition, low levels of β-catenin can promote NFκB activity to induce HIF-1α (Fig. 2). This observation is in agreement with other studies that have suggested a dose-dependent effect of β-catenin in producing a malignant phenotype in cancer cells.25
Figure 2.
APC represses HIF-1α. APC control of HIF-1α is indirect and requires wild-type APC, low levels of β-catenin and NFκB activity. Mutations in APC result in high levels of β-catenin, which inhibits NFκB, and hence suppresses changes in HIF-1α.
Our results suggest that cells lacking APC are adapted to hypoxia and hence have a survival advantage under hypoxic conditions. This could be an important factor in the progression of colorectal tumors even at the early stages.
Acknowledgements
We would like to thank all the members of the Näthke and Rocha laboratories. This study was funded by the Wellcome Trust and a Tenovus Small grant to S.R. and by a program grant from CR-UK to I.N.
Abbreviations
- APC
adenomatous polyposis coli
- HIF
hypoxia inducible factor
- FAP
familial adenomatous polyposis
- HRE
hypoxia responsive element
- PHD
prolyl-hydroxylase
- NFκB
nuclear factor κB
- GEF
guanine-nucleotide exchange factor
References
- 1.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 2.Ichii S, Takeda S, Horii A, Nakatsuru S, Miyoshi Y, Emi M, et al. Detailed analysis of genetic alterations in colorectal tumors from patients with and without familial adenomatous polyposis (FAP) Oncogene. 1993;8:2399–2405. [PubMed] [Google Scholar]
- 3.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 4.O'Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000;36:151–169. doi: 10.1016/s0959-8049(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 5.Rajaganeshan R, Prasad R, Guillou PJ, Poston G, Scott N, Jayne DG. The role of hypoxia in recurrence following resection of Dukes' B colorectal cancer. Int J Colorectal Dis. 2008;23:1049–1055. doi: 10.1007/s00384-008-0497-x. [DOI] [PubMed] [Google Scholar]
- 6.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 7.McCartney BM, Nathke IS. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr Opin Cell Biol. 2008;20:186–193. doi: 10.1016/j.ceb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JA. HIFing the brakes: Therapeutic opportunities for treatment of human malignancies. Sci STKE. 2006;2006:25. doi: 10.1126/stke.3372006pe25. [DOI] [PubMed] [Google Scholar]
- 9.Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: A complex network. Biochim Biophys Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- 11.Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414:19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- 13.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NFkappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S. Evolutionary conserved regulation of HIF-1beta by NFkappaB. PLoS Genet. 2011;7:1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorlach A, Bonello S. The cross-talk between NFkappaB and HIF-1: further evidence for a significant liaison. Biochem J. 2008;412:17–19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 16.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NFkappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenneth NS, Mudie S, van Uden P, Rocha S. SWI/SNF regulates the cellular response to hypoxia. J Biol Chem. 2009;284:4123–4131. doi: 10.1074/jbc.M808491200. [DOI] [PubMed] [Google Scholar]
- 18.Perkins ND, Gilmore TD. Good cop, bad cop: The different faces of NFkappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 19.Newton IP, Kenneth NS, Appleton PL, Nathke I, Rocha S. Adenomatous polyposis coli and hypoxia-inducible factor-1{alpha} have an antagonistic connection. Mol Biol Cell. 2010;21:3630–3638. doi: 10.1091/mbc.E10-04-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha S. Gene regulation under low oxygen: Holding your breath for transcription. Trends Biochem Sci. 2007;32:389–397. doi: 10.1016/j.tibs.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, et al. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–1197. doi: 10.1126/science.289.5482.1194. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Sato R, Akiyama T. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol. 2003;5:211–215. doi: 10.1038/ncb937. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Nathke IS. Tumor-associated NH2-terminal fragments are the most stable part of the adenomatous polyposis coli protein and can be regulated by interactions with COOH-terminal domains. Cancer Res. 2005;65:5195–5204. doi: 10.1158/0008-5472.CAN-04-4609. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Kroboth K, Newton IP, Nathke IS. Novel self-association of the APC molecule affects APC clusters and cell migration. J Cell Sci. 2008;121:1916–1925. doi: 10.1242/jcs.029470. [DOI] [PubMed] [Google Scholar]
- 25.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]