Abstract
Rac is a member of the Rho family of small GTPases, which act as molecular switches to control a wide array of cellular functions. In particular, Rac signaling has been implicated in the control of cell-cell adhesions, cell-matrix adhesions, cell migration, cell cycle progression and cellular transformation. As a result of its functional diversity, Rac signaling can influence several aspects of tumorigenesis. Consistent with this, in vivo evidence that Rac signaling contributes to tumorigenesis is continuously emerging. Additionally, our understanding of the mechanisms by which Rac signaling is regulated is rapidly expanding and consequently adds to the complexity of how Rac signaling could be modulated during tumorigenesis. Here we review the numerous biological functions and regulatory mechanisms of Rac signaling and discuss how they could influence the different stages of tumorigenesis.
Key words: Rac, Tiam1, tumorigenesis, adhesion, migration, cell cycle, survival
Regulation of Rac Signaling
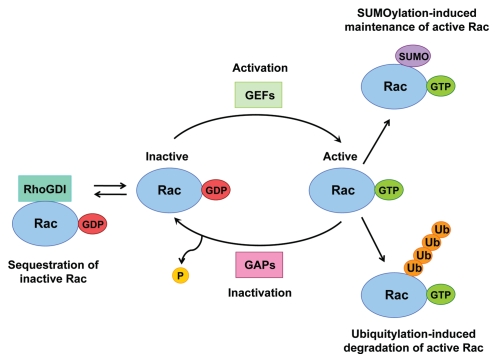
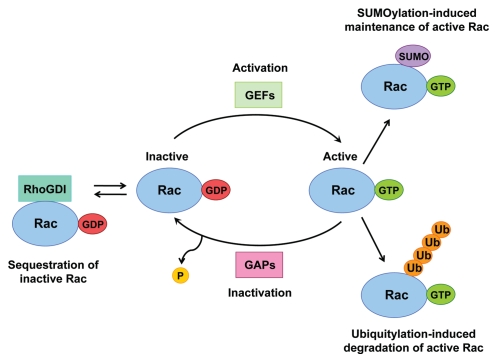
The Rac1 GTPase binds to either GTP or GDP, the exchange of which controls its activation status. Rac is inactive when in a GDP-bound state and is activated upon exchange of its GDP for GTP, which enables downstream signaling to proceed. The conformation of the guanine nucleotide binding domain is altered upon exchange of GDP for GTP, which permits effector binding.1 Multiple mechanisms exist to control Rac activation (Fig. 1). Rac activity can be regulated through its association with various guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), which control the cycling between the GDP- and GTP-bound states.2 The activated GTP-bound state of Rac is promoted by its association with GEFs, whereas its inactive GDP-bound state is promoted by its association with GAPs. Moreover, through association with RhoGDI in the cytosol, Rac can be maintained in its inactive state.2 In addition to working as chaperones for RhoGTPases, RhoGDIs also control their homeostasis through protecting them from degradation.3 Furthermore, post-translational modifications (PTMs) of Rac can also regulate its activity. Modification of the carboxyl-terminal CAAX motif on Rac with the addition of either farnesyl or geranylgeranyl isoprenoid lipids increases its hydrophobicity, facilitating its membrane localization and therefore also its activation.4 Ubiquitin-like (Ubl)-type modifications of Rac, including ubiquitylation5–7 and SUMOylation,8 have now also been described as additional regulatory mechanisms of Rac activity, adding huge complexity to the regulation of Rac signaling.
Figure 1.
Multiple mechanisms exist to regulate Rac activity. The Rac GTPase cycles between inactive GDP-bound and active GTP-bound states. Rac activation is facilitated by the action of GEFs, which promote GDP dissociation from Rac and allow GTP to bind instead. Through the association with GAPs the intrinsic GTPase activity of Rac is accelerated, thereby inactivating Rac. Through association with RhoGDIs Rac can be sequestered in its inactive state. Activated Rac can also be removed through Ubiquitylation-induced degradation, or it can be maintained following its modification by SUMO.
Ubiquitin and ubiquitin-like proteins such as SUMO (small ubiquitin-like modifier) are conjugated to many target proteins, either as monomeric units or as polymeric chains, which can vary in length and linkage type, all of which can provide signals to alter protein function in specific ways, i.e., through changing protein stability, activity or localization.9 Lynch et al. showed that activated Rac is ubiquitylated and subjected to proteasome-mediated degradation during the early stages of Hepatocyte Growth Factor (HGF)-induced epithelial cell scattering.6 Additionally, ubiquitylation of activated Rac1 has been shown to occur in response to the CNF1 toxin, also resulting in its degradation and which was shown to be dependent on its prenyl-acceptor residue Cys189.10 More recently, another study reported that the major target site for Rac1 ubiquitylation is Lys147, which is stimulated by downstream Rac1 signaling through JNK, creating a negative feedback loop to terminate Rac signaling.5 Moreover, it has also been shown that the ubiquitylation of active Rac and its subsequent degradation is regulated by its association with calveolin-1, and that Rac1 ubiquitylation is important for Rac1 dynamics at the cell periphery.7 Together, these studies have shown a positive correlation between Rac activation, its ubiquitylation and its consequent degradation. Consequently, it is believed that ubiquitylation and the resultant degradation of activated Rac acts as a mechanism to terminate Rac signaling downstream of multiple stimuli.
Furthermore, we have recently shown that the SUMO ligase PIAS3 preferentially interacts with and SUMOylates the active form of Rac, and that Rac1 SUMOylation is important for optimal Rac-mediated cell migration and invasion, but not proliferation.8 Therefore, the active form of Rac is preferentially ubiquitylated and SUMOylated; however, in contrast to ubiquitylation, we found that SUMOylation of Rac promotes its activated state and influences the magnitude of Rac activation. We therefore believe SUMOylation to be required to maintain activated Rac during some cellular functions, including cell motility.8 The polybasic region (PBR) within the C terminus of Rac provides the main sites for SUMO conjugation, and, interestingly, this region is also involved in regulating its degradation.7,11,12 However, loss of the lysine residues on Rac that are required for SUMOylation does not affect its ubiquitylation or other functions in which the PBR has previously been implicated, such as nuclear or plasma membrane localization or protein-protein interactions.8
The identification of Ubl-type PTMs of Rac has greatly enhanced our understanding of the complexity but also the plasticity of the regulation of Rac signaling. An interesting question is whether GTP hydrolysis alone is sufficient to inactivate Rac signaling. Other key unanswered questions are whether these Ubl-type PTMs of Rac become deregulated during tumorigenesis and/or whether their manipulation could provide novel therapeutic strategies to inhibit cancer progression.
Rac Signaling in Intercellular Adhesion and Apical-Basal Polarity
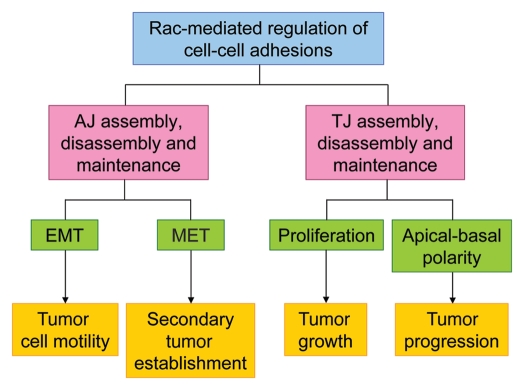
Alterations in intercellular adhesions are known to occur during several stages of tumorigenesis, including tumor initiation, growth, progression and metastasis. Several types of intercellular adhesion exist, including tight junctions (TJ), adherens junctions (AJ), desmosomes and gap junctions (Fig. 2A). TJ are comprised of members of the Claudin, Occludin and junctional adhesion molecule (JAM) families of proteins, which are associated with various cytoplasmic-signaling and scaffolding proteins, such as the zonula occludens (ZO), partitioning defective 3 (Par3) and atypical protein kinase C (aPKC) (Fig. 2B). TJ provide so-called “barrier” and “fence” functions to epithelial tissues. Their barrier function regulates the passage of ions, solutes and cells through the intercellular space. The fence function of TJ prevents diffusion of apical and basolateral membrane components, which is necessary for the establishment and maintenance of apical-basal polarity.13,14 Directly below TJ are AJ complexes. AJ consist of both Cadherin-Catenin and Nectin-Afadin complexes (Fig. 2B), which cooperate to promote efficient assembly of both AJ and TJ.15,16 A large body of evidence has implicated Rac signaling in the regulation of both AJ and TJ, including their assembly, disassembly and maintenance. Here, we review the evidence and discuss how Rac-mediated regulation of intercellular adhesion may contribute to different stages of tumorigenesis (summarized in Fig. 3).
Figure 2.
Schematic representation of epithelial cell-cell adhesions. (A) Typical organization of epithelial cell-cell junctions and apical-basal polarity. The different types of cell-cell junctions are shown. (B) The main constituents of epithelial adherens junctions (AJ) and tight junctions (TJ).
Figure 3.
Schematic representation summarizing how Rac-mediated regulation of cell-cell adhesions may contribute to different stages of tumorigenesis.
Numerous reports have demonstrated that Rac regulates AJ. Ehrlich et al. showed that Rac1-mediated lamellipodia formation expands initial cadherin-based cell-cell contacts in MDCKII cells in a zipper-like fashion, and that Rac1 is lost from more mature sites of intercellular adhesion, suggesting Rac1 is required at the early stages of cell-cell adhesion assembly.17 Supporting these findings, induction of cadherin-based intercellular adhesion using a calcium switch procedure leads to the activation of Rac1, which can be inhibited with E-cadherin function-blocking antibodies.18,19 Also in agreement, the E-cadherin ligand (hE/Fc) recruits Rac1 to nascent sites of cell-cell contact in CHO cells and induces its activation.20 Similarly, the trans-interaction of Nectins also recruits and activates Rac1,21,22 for which the Rac GEF Vav2 has been proposed to mediate.23 Moreover, expression of dominant-negative Rac1 in keratinocytes perturbs calcium-induced E-cadherin accumulation at cell-cell junctions.24 Furthermore, Hordijk et al. showed that expression of either constitutively active Rac1 or the Rac activator Tiam1 restores E-cadherin-based intercellular adhesion and an epithelioid morphology to Ras-transformed MDCK cells (MDCKF3), which is reminiscent of a mesenchymal to epithelial transition (MET).25 Consistent with this, Malliri et al. later reported that E1A-induced epithelioid morphology in primary MEFs and MDCKF3 cells requires Tiam1-induced Rac activity.26 In addition, we have more recently shown that optimal Src-induced AJ disassembly in MDCKII cells requires the phosphorylation and subsequent degradation of Tiam1 at cell-cell junctions in a Calpain-dependent manner.27 Importantly, we showed that this Tiam1 phosphorylation positively correlates with Src phosphorylation in human tumors. These data provide further evidence that Tiam1-induced Rac activity is likely to be important for AJ maintenance. However, we cannot exclude the possibility that Src-induced, Tiam1-mediated activation of Calpain could be necessary for degradation of E-cadherin28,29 or of other junctional proteins,30,29 which could provide the driving force for AJ disassembly rather than the loss of Tiam1 itself. It is therefore possible that signaling through the Tiam1/Rac module plays a role in AJ disassembly as well as AJ maintenance. Consistent with this idea, another report has shown that Tiam1-mediated Rac1 activation drives PAF-induced disassembly of interendothelial junctions.31
Furthermore, several other reports have shown Rac1 activity to negatively regulate AJ. In PANC1 cells, E-cadherin levels are reduced by overexpression of constitutively active Rac1 but are increased by dominant-negative Rac1.32 In addition, overexpression of constitutively active Rac1 in keratinocytes induces the disassembly of E-cadherin-based adhesions in a PAK1-dependent manner33,34 and also induces internalization of E-cadherin into endosomal structures.35 Moreover, expression of constitutively active Rac1 promotes Ras-induced transformation and the loss of AJ in primary epithelial cells.36,37 Rac1 is also required for the disruption of AJ during cell scattering induced by HGF38 and Collagen I.39 In addition, increased Rac1 activity has been observed in primary mouse keratinocytes from squamous mouse epithelia with defective cell-cell junctions.40 Consequently, there is substantial evidence that Rac signaling contributes to the disassembly of AJ.
Rac has also been shown to regulate TJ in both a positive and negative manner. Several reports have demonstrated that altered Rac signaling can perturb TJ assembly. Chen and Macara reported that Par3-mediated inhibition of Tiam1-induced Rac activity is required for efficient TJ assembly in MDCKII cells.41 In contrast to this, Mertens et al. reported that Tiam1-mediated Rac activity is required for efficient TJ assembly in keratinocytes.42 Moreover, Bruewer et al. reported that expression of constitutively active Rac1 increases paracellular permeability in MDCK cells 18 h after seeding, which is reflective of defective TJ assembly.43 They also showed that both constitutively active and dominant-negative Rac1 can disrupt the localization of certain TJ proteins. In addition, Jou et al. reported that both constitutively active and dominant-negative Rac1 disrupt TJ function in MDCK cells, but the localization of some TJ proteins was only affected by constitutively active Rac1.44 In summary, it is apparent that Rac signaling must be tightly controlled for the establishment, maintenance and disassembly of epithelial AJ and TJ.
The loss or gain of AJ are hallmarks of the epithelial to mesenchymal transition (EMT) and the mesenchymal to epithelial transition (MET), respectively. As discussed above, Rac signaling has been implicated in both the assembly and disassembly of AJ and is therefore likely to be involved in both EMT and MET processes. EMT increases cell motility and invasiveness and therefore promotes the acquisition of a metastatic phenotype. Aberrant Rac signaling can perturb AJ integrity, and therefore, changes in Rac signaling are likely to be important in driving EMT and promoting tumor progression and metastasis. On the other hand, Rac activity has been shown to be important for the establishment of AJ, and therefore, Rac activity may be required for metastatic tumor cells to undergo MET at distant sites to facilitate secondary tumor formation.
The role of Rac in the regulation of TJ could also be important for tumorigenesis because functional TJ restrict cell proliferation.45 TJ can restrict cell proliferation through the sequestration of various proteins, including the Y-box transcription factor ZONAB and the ZO proteins that can localize to both cell-cell junctions and to the nucleus, with their nuclear activities promoting cell proliferation.45–49 Cell differentiation and TJ maturation causes such proteins to translocate from the nucleus to TJ, thereby inhibiting their pro-proliferative functions, and conversely, the loss of functional TJ can promote their nuclear localizations, where their activities can enhance proliferation. Defects in the TJ barrier function can also lead to increased cell proliferation through enhancing the accessibility of luminal growth factors to basolateral cell surface receptors.50 In summary, aberrant Rac signaling may disrupt TJ functions and, consequently, increase cell proliferation, thereby promoting tumor initiation and growth.
Additionally, the regulation of cell-cell adhesion is tightly coordinated with the establishment and maintenance of apical-basal polarity.51 The formation of intercellular junctions provides spatial organization along the lateral domain and sets up the polarity axis. In particular, the fence function of TJ is essential for the maintenance of apical-basal polarity through preventing diffusion of proteins between the apical and basolateral regions of the plasma membrane. Disruption of this fence function can cause protein mislocalization along the lateral membrane, resulting in the loss of apical-basal polarity. Interestingly, expression of dominant-negative Rac1 has been shown to invert apical-basal polarity in cysts of MDCKII cells grown in Collagen I matrix as a result of defective Laminin assembly.52 Therefore, alterations in Rac signaling could disrupt apical-basal polarity through perturbation of both cell-cell adhesions and matrix-induced polarization. Loss of apical-basal polarity is frequently observed in tumor cells and is believed to be an early event in tumorigenesis and a driving force for tumor progression.51,53 Therefore, deregulated Rac signaling could drive changes in apical-basal polarity and, consequently, could promote tumorigenesis.
Rac Signaling in Cell Migration
In addition to regulating cell motility and invasiveness indirectly through modulating AJ integrity, Rac signaling has also been directly implicated in the regulation of cell migration through its ability to regulate membrane protrusions and cell-matrix adhesions. The actin cytoskeleton is a major driving force for cell migration due to its ability to promote both cell protrusions and cell contraction. The RhoGTPases are key regulators of the actin cytoskeleton, and therefore, coordination of their activities is essential for controlled cell migration. The activities of RhoA, Rac1 and Cdc42 have been shown to be tightly coordinated to regulate both membrane protrusions and cell-matrix adhesions at or near the leading edge of migrating cells to control forward cell movement.54
Rac1 activity can induce reorganization of the actin cytoskeleton to form lamellipodia and membrane ruffles in fibroblasts.55 Expression of dominant-negative Rac1 in wound edge migrating cells blocks lamellipodia formation and also cell movement.56,57 Moreover, HGF-induced migration of epithelial cells requires Rac activation for both the initial spread of cell colonies through lamellipodia-membrane ruffle formation and also for the subsequent cell scattering.58 Our recent finding that SUMO modification of Rac is important for Rac-mediated cell migration and invasion8 has reiterated the importance of Rac activity for these cellular functions.
In addition to regulation of Rac activation level, the spatial regulation of Rac activity is also important for controlled cell migration. Rac-induced lamellipodia must form specifically at the front of the cell to drive forward cell movement. Cdc42 has been shown to be important for restricting Rac-induced lamellipodia to the front of the cell, since inhibition of Cdc42 activity results in Rac-mediated lamellipodia formation around the whole periphery of the cell due to a loss of front-rear polarity and thereby inhibits directional cell migration.56 Similarly, it has been shown by Bass and co-workers that Syndecan-4 signaling via PKCalpha is also important for the localization of Rac activity and Rac-induced membrane protrusions to the leading edge of migrating fibroblasts.59 Moreover, several studies have made use of FRET-based probes to detect Rac activity in live migrating cells, which have clearly demonstrated spatial regulation of Rac activity during cell migration. In one such study, Kraynov et al. revealed that gradients of Rac activity exist in migrating fibroblasts, with high Rac activity near the leading edge and in membrane ruffles.60
In addition to promoting membrane protrusions, Rac also regulates focal adhesions (FA), which must also be efficiently regulated to drive forward cell movement. Continuous assembly and disassembly of FA is required for cell movement, and therefore, the turnover of FA must be efficiently regulated to control cell migration. One regulatory mechanism of FA turnover is through microtubule (MT) dynamics, since multiple targeting of FA by MT promotes FA disassembly.61,62 We have previously shown that Rac activity induced by its activator STEF is important for MT-induced FA disassembly and, consequently, is required for optimal cell migration.63
Interestingly, Rac activity has also been shown to regulate the mode of cell movement. Cells can move using either a mesenchymal- or an amoeboid-type movement, and these two types of cell movement are interconvertible.64 Mesenchymal-type cell movement is characterized by elongated cell morphology, and it requires extracellular proteolysis to generate tracks for cells to move through. In contrast, cells moving via an amoeboid-type movement display a rounded morphology and are less dependent on proteolytic activity, but do require Rho-ROCK-mediated actomyosin contractility. Sanz-Moreno et al. showed that Rac signaling is important for determining these two different modes of cell movement through its regulation of MLC2 phosphorylation. 65 Rac-mediated inhibition of MLC2 phosphorylation was shown to repress amoeboid movement but promote mesenchymal-type movement. It was also shown that ROCK signaling activates ARHGAP22, which inactivates Rac, thereby permitting MLC2 phosphorylation and stimulating amoeboid movement. In addition, the Rac activator DOCK3, in a complex with NEDD9, promotes Rac activation and its signaling through WAVE2. This signaling is required for both reducing MLC2 phosphorylation and, therefore, suppressing amoeboid movement, but also for the formation of actin-based membrane protrusions to stimulate mesenchymal-type movement. Consistent with these findings, Rac activity has been implicated in the expression of various matrix metalloproteinases (MMPs), secretion of which is important for generating tracks for mesenchymal- type movement.64 For tumor cells to efficiently metastasise both amoeboid and mesenchymal-type cell movement may be required. For example, mesenchymal-type movement may be required for cells to invade through a more rigid environment through its ability to degrade the matrix and generate tracks. On the other hand, amoeboid movement is more rapid, promotes lung colonization and may also protect tumor cells from shear stress during circulation.65
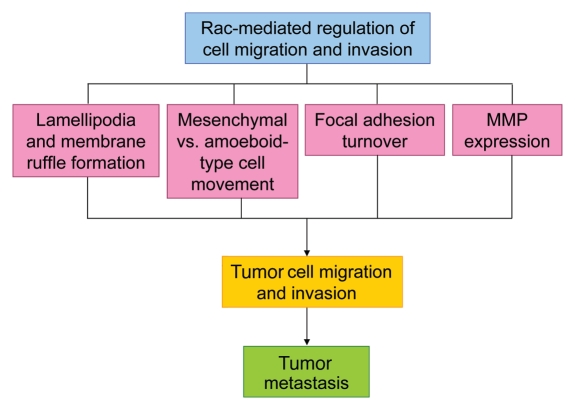
In summary, Rac signaling can modulate cell motility and invasion through a variety of mechanisms (summarized in Fig. 4) and, consequently, it has a complex role in these processes and likely plays an important part in the tumor metastasis process. Efficient regulation of Rac signaling may be required for efficient tumor cell metastasis, or conversely, deregulated Rac signaling may either promote or repress tumor cell metastasis or could alter the location where metastases develop. Recent advances in our understanding of the molecular details of how Rac signaling regulates cell-cell adhesion, migration and invasion has provided many opportunities to develop novel therapeutic strategies to target Rac signaling at different stages of tumor cell metastasis.
Figure 4.
Schematic representation summarizing how Rac-mediated regulation of cell migration and invasion may contribute to tumor metastasis.
Rac Signaling in Cell Survival and the Cell Cycle
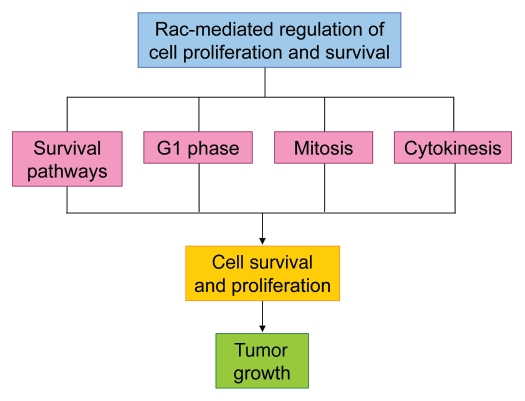
The observation that Rac plays a critical role in oncogenic, Ras-mediated foci formation in fibroblasts66 highlighted a potential function for Rac in regulating cell growth and survival. It has since become apparent that Rac has important roles in the regulation of cell survival and apoptosis in addition to the regulation of gene expression required for progression through the early stages of the cell cycle. To complicate matters, recent studies have also revealed an intriguing role for Rac in mitosis, and have shown it is also involved in the process of cytokinesis. There are, therefore, many separate pathways by which Rac could influence tumor growth and survival (Fig. 5). The following sections will review the known roles of Rac signaling in regulating cell survival and apoptosis, in addition to various stages of the cell cycle.
Figure 5.
Schematic representation of how Rac-mediated regulation of cell proliferation and survival may contribute to tumor growth.
Rac Signaling in Cell Survival
It has been known for many years that cancer cells are capable of evading normal cell death pathways by the altered expression of cell survival genes. There are many reports demonstrating a role for Rac in positively regulating cell survival. For example, Rac has been shown to suppress Ras-induced apoptosis in fibroblasts through signaling to NFκB.67 In another study, deletion of Rac1 in MEFs was shown to promote apoptosis and premature senescence.68 Rac has also been shown to promote survival in mammary acini cells in 3D culture via PAK and NFκB69 and in non-adherent cells via interaction with Akt.70 In addition, an alternative splice variant of Rac, Rac1b, has been shown to increase NFκB-mediated survival in fibroblasts,71 and its expression in colorectal tumor cells also promotes survival.72 Despite these numerous reports that Rac can promote cell survival through activation of Akt, Erk and NFκB, it has also been shown to induce apoptosis by stimulation of p38 and JNK in rat PC-12 pheochromocytoma cells73 and to promote Fas-mediated apoptosis in T cells,74 and therefore, in some circumstances, Rac can apparently also act to promote cell death.
Rac Signaling in G1/S Phase of the Cell Cycle
The ability to progress from a quiescent state (G0) to G1 and S phase in the absence of either mitogenic or adhesion signals can allow tumor cells to proliferate in abnormal conditions. It has been shown that Rac, along with Rho and Cdc42, is required for progression of cells from G1 to S phase of the cell cycle.75 Progression through the G1 phase is controlled by the activity of certain cyclin-dependent kinases, which are activated when they bind to their specific cyclin partners (reviewed in ref. 76). The activity of CDK4 and/or CDK6 in complex with D-type cyclins is essential to commit a cell to proliferate. These active CDK complexes phosphorylate and inhibit the tumor suppressor protein retinoblastoma (Rb), which allows activity of the E2F transcription factor and subsequent transcription of genes required for cell cycle progression to S phase.77 CDK2 then combines with E- and A-type cyclins to promote the replication of DNA.
The D-type cyclin (mostly D1 in fibroblastic and epithelial cells) is required for Ras-induced tumorigenesis in vivo78 and is overexpressed in a variety of tumors.79 It has been shown that dual signaling from growth factor receptors and integrin receptors leads to sustained ERK activity, which is required for the expression of cyclin D1.80 Several studies have demonstrated that activated Rac stimulates cyclin D1 expression, though it appears there may be several possible mechanisms. Although it has been shown that sustained ERK activity is required for cyclin D1 expression, several studies have shown that Rac can induce cyclin D1 expression independently of ERK.81–84 Joyce et al. showed that induction of the cyclin D1 promoter by Rac required NFκB and ATF2 binding sites in fibroblasts.85 In this study NFκB binding to the cyclin D1 promoter was increased by Rac, and this correlated with Rac's ability to promote superoxide production.85 This agreed with another study which showed that inhibition of superoxide production inhibited the mitogenic effect of Rac in fibroblasts.86 More recently, however, it was shown that Rac and NFκB can stimulate cyclin D1 gene expression in parallel yet separate pathways.82 Moreover, in addition to its effect on cyclin D transcription, it has been shown that Rac activation downstream of integrin engagement on the extracellular matrix component fibronectin leads to upregulation of cyclin D1 translation independently of ERK, via SOS and PI3-K.84
Other studies have tried to dissect these various mechanisms by focusing more closely on the timing of cyclin D1 expression. Firstly, Welsh et al. found that Rho is required to inhibit the ERK-independent induction of cyclin D1 by Rac (or Cdc42) in early G0, and this inhibition is necessary for the correct timing of cyclin D1 expression in mid G1-phase in NIH-3T3 cells.87 A more recent study using MCF10A cells confirmed that Rac can induce cyclin D1 in early G1 independently of ERK, but also showed that mid-G1 phase induction of cyclin D1 required parallel signaling from both ERK and Rac.88 Interestingly, the early ERK-independent cyclin D1 expression was not seen in mesenchymal cells (and was not sufficient to induce Rb phosphorylation or S-phase entry), but the mid-G1 induction is conserved in both epithelial and mesenchymal cells,88 highlighting the potential differences in Rac-dependent cell cycle events in different cell types.
Rac Signaling in Mitosis and Cytokinesis
There have also been reports implicating Rac in regulation of the G2/M phase of the cell cycle. Moore et al. showed that Rat2 fibroblasts expressing dominant-negative Rac1 accumulate in the G2/M phase, whereas no such accumulation was seen with cells expressing dominant-negative Rho or dominant-negative Cdc42.89 In another more recent study, it was found that Rac localizes to the nucleus in late G2 phase and expression of a mutant of Rac that was confined to the nucleus increased the mitotic rate.90 It has since become apparent that Rac is involved specifically in the process of cell division, both in the early stages of mitosis and in the regulation of cytokinesis. These processes need to be carefully regulated in order to maintain genomic stability; loss or gain of chromosomes (chromosomal instability or CIN) is known to be a driving force for tumorigenesis.91 At the beginning of mitosis, the two centrosomes (the MT organizing centers of the cell) begin to separate around the intact nucleus, then, upon nuclear envelope breakdown, the MTs invade the nuclear space to form the mitotic bipolar spindle.92 This structure is responsible for the capture of chromosomes to the metaphase plate, and only when all chromosomes are properly aligned (with the kinetochore of each sister chromatid attached to opposite poles) will the spindle assembly checkpoint allow progression into anaphase.93 Whilst the genetic material is separated in anaphase, the separate process of physical division of the cells (cytokinesis) also begins.
A few studies have suggested that RhoGTPases are important for proper alignment of chromosomes in the earlier stages of mitosis,94 and Rac has also been shown to be involved in correct spindle organization in meiosis of mouse oocytes, where cell division is assymetrical. It was found that Rac-GTP was polarized in the cortex overlying the meiotic spindle, and that inhibition of Rac during oocyte maturation caused a permanent block at prometaphase I and elongation of the spindle.95 We have also recently discovered that Rac is involved in the process of chromosome congression in mitosis.96 We found that the Rac activator Tiam1 is required for proper centrosome separation in the early stages of mitosis, and that exerting a force opposing the major MT kinesin Eg5 promotes the efficient alignment of chromosomes in prometaphase.96 Intriguingly, both Rac and Tiam1 localize to the centrosomal regions in the early stages of mitosis. The same chromosome alignment and centrosomal separation defects seen with Tiam1 depletion were also apparent after treatment with a Rac inhibitor. Importantly, unlike wild-type Tiam1, expression of a GEF mutant form of Tiam1 was unable to rescue the phenotypes. We also found evidence that the same mechanism functions in vivo using a mouse model in which Rac can be conditionally deleted from the intestine. We found that cells in the crypts of Rac-null intestines were less susceptible to arrest by Eg5 inhibitor than wild-type mice, which mirrored our results for Tiam1 depletion in cell culture. Therefore Tiam1-induced Rac activity is important for balancing the forces during formation of the mitotic spindle to allow proper chromosome alignment and mitotic progression.
The process of cytokinesis involves the formation and ingression of a cleavage furrow (CF), which is driven by the assembly and contraction of a dynamic, highly organized actomyosin structure known as the actomyosin contractile ring (CR).97 It has long been known that the activation of Rho is required for CR assembly and constriction,98 but the role of Rac in the process of cytokinesis has only recently begun to emerge. Activation of RhoA at the cleavage site is regulated by the evolutionarily conserved two-protein complex known as centralspindlin,99 which comprises a plus-end directed MT motor MKLP1 and the Rho family GAP MgcRacGAP. Studies in Drosophila have shown that the RacGAP component of centralspindlin binds to and activates a RhoGEF that subsequently activates Rho at the cleavage site.100 However, conversely, the RacGAP has also been shown to be important for local inactivation of RhoA to promote the formation and maintenance of the Rho activity zone at the CF.101 The role that Rac plays in this process has not been as easy to interpret. Genetic experiments in Drosophila had suggested that RacGAP50C (the ortholog of MgcRacGAP) was able to inhibit Rac function, leading to the hypothesis that this GAP was required to simultaneously inhibit Rac and activate RhoA at the cleavage site.102 Subsequently, Yoshizaki et al. measured a decrease in Rac activity at the CF in mammalian cells by FRET, and demonstrated that expression of a dominant-negative MgcRacGAP abolished this decrease.103 The authors also showed that expression of constitutively active Rac1 induces a multinucleated phenotype in both HeLa and Rat1A cells, indicating that the decrease in Rac activity is essential for cytokinesis. A recent study in C. elegans has also added evidence to this hypothesis, since depletion of its two Rac proteins or depletion of the Rac effectors WASP, WAVE or Arp2/3 were able to rescue the ingression defects caused by expression of inactive RacGAP (CYK-4 in C. elegans).104 These studies suggest that GAP activity is necessary to decrease Rac activity at the cleavage site to promote CF ingression and allow normal progression of cytokinesis.
In Vivo Evidence Implicating Rac Signaling in Tumorigenesis
Despite the in vitro evidence that activation of Rac promotes several biological processes that are relevant to tumorigenesis, no constitutively active mutant forms of Rac have been found in tumors. However, there are several lines of evidence that Rac signaling is altered in cancer, including a number of reports of increased Rac expression in tumors. Overexpression of Rac1 correlates with progression of gastric carcinoma,105 testicular cancer106 and breast cancer.107 Rac1 is also overexpressed in oral squamous cell carcinoma.108 An alternative splice variant of Rac1, Rac1b, which encodes a highly active isoform, was also increased in colon cancer.109 Rather than changes at the protein level, the activity of another isoform of Rac, Rac3, was found to be increased in highly proliferative breast cancer cell lines.110 Altered Rac activity could also be a result of differential expression of its regulating proteins. Consistent with this, it has been shown that high expression levels of the Rac GEFs Vav, Trio and Tiam1 correlate with poor prognosis in breast cancer.111 Another Rac-specific GEF P-Rex1 was shown to be upregulated in human prostate adenocarcinoma and lymph node metastases.112
The role of Rac in regulating tumorigenesis in vivo has been studied by production of various mouse models. For example, Kissil et al. produced a lung cancer model in which an oncogenic allele of K-Ras (K-RasG12D) could be activated by Cre-mediated recombination in the presence or absence of conditional deletion of Rac1.113 Using this model, they found that mice with Rac1 deletion had reduced tumor formation and prolonged survival compared to control mice.114 Moreover, all tumors which formed in the Rac-knockout mice were actually derived from Rac1 expressing cells that had escaped the gene deletion, suggesting that Rac1 is essential for K-Ras-induced tumorigenesis in this model. Additionally, in a colorectal carcinoma model whereby adenocarcinoma cells were orthotopically injected into mice, overexpression of Rac1 accelerated tumor formation, whereas inhibition of Rac1 completely suppressed tumor formation.115
Indirect evidence that Rac is involved in skin tumorigenesis has come from the findings of Malliri et al., who showed that Tiam1-knockout mice (which displayed approximately a 50% reduction in GTP-bound Rac) are resistant to a Ras-induced skin carcinogenesis protocol (DMBA/TPA).116 More recently, this has been tested directly using a mouse model with a keratinocyte-specific loss of the Rac1 gene.117 Using the same Ras-mediated transformation protocol (DMBA/TPA) as was used with the Tiam1-knockout mice, Rac1 was shown to be essential for the formation of Ras-induced skin tumors. It was also shown that this was via Rac1 promoting, Erk-dependent hyperproliferation through a Rac1-Pak1-Mek-Erk pathway. Rac1 was also required for Pak2-dependent hyperactivation of Akt in this model. Interestingly, no change in proliferation was detected in untreated Rac1-deficient skin, indicating a hyperproliferation-specific function of Rac1 in this system.117
Rac signaling has also been found to be important in chronic myelogenous leukemia (CML), which is characterized by expression of the p210-BCR-ABL fusion protein. Bcr is a GEF, and the p210-BCR-ABL oncoprotein was shown to activate Rac1 in addition to Cdc42 and RhoA.118 In a mouse model of p210-BCR-ABL-induced myeloproliferative disease, gene targeting of Rac significantly delayed or abrogated disease development, apparently through severe disruption of p210-BCR-ABL-induced downstream signaling.119 In the same study, it was shown that treatment with the Rac inhibitor NSC-23766 significantly inhibited p210-BCR-ABL-induced proliferation of primary human CML cells in vitro and in a mouse model in vivo. In addition, when CML cells from human patients were transplanted into immunodeficient (NOD/SCID) mice, NSC-23766 induced an 85% reduction in CML development. These data implicate Rac inhibition as a potential therapy for CML, perhaps in combination with tyrosine kinase inhibition of p210-BCR-ABL.120 Interestingly, NSC-23766 has also been shown to significantly impair proliferation and survival of some human acute myelogenous leukemia (AML) cell lines in vitro and development of AML in vivo,121 indicating that targeting Rac may be beneficial in a subgroup of AML cases.
Conclusions/Perspectives
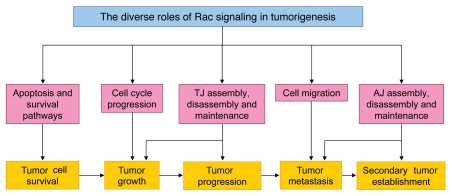
It is clear from the extensive evidence described here that Rac signaling is involved in a wide range of cellular functions, the modulation of which can be crucial for tumor formation and progression (Fig. 6). Despite the complexity already apparent from these studies, it is likely that we are still far from understanding the full extent of the contribution of Rac signaling to tumorigenesis. In addition to the role of Rac in cancer cells themselves, we must consider that Rac-mediated signaling pathways in other cell types can also alter the behavior of tumor cells. For example, there is growing evidence that the stromal microenvironment surrounding cancer cells can affect their growth, invasiveness and metastatic potential. For example, the levels of the Rac activator Tiam1 in fibroblasts has been shown to influence the invasiveness of epithelial cells in experimental models in vitro and also to affect breast cancer invasion in vivo.122 It therefore seems likely that changes in Rac activity in stromal fibroblasts could affect tumor progression. Additionally, through regulating many essential signaling pathways in endothelial cells, Rac has an important role in angiogenesis,123 a process which is well known to be important for tumor progression. In addition, the relatively recent discovery of the involvement of Rac in mitosis and cytokinesis may reveal another link between Rac and tumorigenesis, since the deregulation of both of these processes can lead to chromosomal instability (CIN). As CIN is known to contribute to tumorigenesis, this could be another important mechanism by which Rac signaling contributes to tumor progression.
Figure 6.
Schematic representation summarizing how Rac-mediated regulation of various important cellular processes may contribute to multiple stages of tumorigenesis.
Despite the in vitro evidence that Rac is involved in cell cycle progression, it is interesting that in vivo studies thus far have found that Rac may not be required for proliferation of nontransformed cells, but instead, its function in the cell cycle may be specific to hyperproliferating cells. It has been suggested that if Rac is indeed not required for normal proliferation in vivo, blocking its function may allow tumor-specific growth repression.117 However, the complexity of the involvement of Rac in tumorigenesis suggests that caution is required when considering Rac as a direct therapeutic target. An important task for the future is therefore to further dissect the molecular mechanisms underlying the positive and negative effects of Rac on the various aspects of tumorigenesis. This will involve more research to decipher which regulating molecules and post-translational modifications are important in defining the effector pathways downstream of Rac. Additionally, we need to understand the importance of the various Rac effector pathways in different tumor types. Answering these questions will not only further our understanding of the function of Rac and its associated proteins, but may reveal new potential targets for cancer therapy.
Acknowledgements
Work in the laboratory of A.M. is funded by Cancer Research UK, grant number C147/A6058. S.C.L. was supported by an EMBO Long-Term Fellowship.
Abbreviations
- AJ
adherens junctions
- AML
acute myelogenous leukemia
- aPKC
atypical protein kinase C
- CF
cleavage furrow
- CIN
chromosomal instability
- CML
chronic myelogenous leukemia
- CR
contractile ring
- EMT
epithelial to mesenchymal transition
- GAP
GTPase-activating protein
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- GTP
guanosine triphosphate
- HGF
hepatocyte growth factor
- JAM
junctional adhesion molecule
- MDCKF3
Ras-transformed MDCKII cells
- MET
mesenchymal to epithelial transition
- MT
microtubule
- Par3
partitioning defective 3
- PTM
post-translational modification
- STEF
Sif and Tiam1 like exchange factor
- Tiam1
T-cell lymphoma invasion and metastasis 1
- TJ
tight junctions
- Ubl
ubiquitin-like
- ZO
zonula occludens
References
- 1.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 2.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 3.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, et al. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visvikis O, Lores P, Boyer L, Chardin P, Lemichez E, Gacon G. Activated Rac1, but not the tumorigenic variant Rac1b, is ubiquitinated on Lys 147 through a JNK-regulated process. FEBS J. 2008;275:386–396. doi: 10.1111/j.1742-4658.2007.06209.x. [DOI] [PubMed] [Google Scholar]
- 6.Lynch EA, Stall J, Schmidt G, Chavrier P, D'Souza-Schorey C. Proteasome-mediated degradation of Rac1-GTP during epithelial cell scattering. Mol Biol Cell. 2006;17:2236–2242. doi: 10.1091/mbc.E05-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nethe M, Anthony EC, Fernandez-Borja M, Dee R, Geerts D, Hensbergen PJ, et al. Focal-adhesion targeting links caveolin-1 to a Rac1-degradation pathway. J Cell Sci. 2010;123:1948–1958. doi: 10.1242/jcs.062919. [DOI] [PubMed] [Google Scholar]
- 8.Castillo-Lluva S, Tatham MH, Jones RC, Jaffray EG, Edmondson RD, Hay RT, et al. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat Cell Biol. 2010;12:1078–1085. doi: 10.1038/ncb2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denuc A, Marfany G. SUMO and ubiquitin paths converge. Biochem Soc Trans. 2010;38:34–39. doi: 10.1042/BST0380034. [DOI] [PubMed] [Google Scholar]
- 10.Doye A, Boyer L, Mettouchi A, Lemichez E. Ubiquitin-mediated proteasomal degradation of Rho proteins by the CNF1 toxin. Methods Enzymol. 2006;406:447–456. doi: 10.1016/S0076-6879(06)06033-2. [DOI] [PubMed] [Google Scholar]
- 11.Lanning CC, Daddona JL, Ruiz-Velasco R, Shafer SH, Williams CL. The Rac1 C-terminal polybasic region regulates the nuclear localization and protein degradation of Rac1. J Biol Chem. 2004;279:44197–44210. doi: 10.1074/jbc.M404977200. [DOI] [PubMed] [Google Scholar]
- 12.Pop M, Aktories K, Schmidt G. Isotype-specific degradation of Rac activated by the cytotoxic necrotizing factor 1. J Biol Chem. 2004;279:35840–35848. doi: 10.1074/jbc.M404346200. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Nakanishi H, Kakunaga S, Okabe N, Kawakatsu T, Shimizu K, et al. Role of nectin in formation of E-cadherin-based adherens junctions in keratinocytes: Analysis with the N-cadherin dominant negative mutant. Mol Biol Cell. 2003;14:1597–1609. doi: 10.1091/mbc.E02-10-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuramitsu K, Ikeda W, Inoue N, Tamaru Y, Takai Y. Novel role of nectin: Implication in the co-localization of JAM-A and claudin-1 at the same cell-cell adhesion membrane domain. Genes Cells. 2008;13:797–805. doi: 10.1111/j.1365-2443.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol-3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino T, Shimizu K, Honda T, Kawakatsu T, Fukuyama T, Nakamura T, et al. A novel role of nectins in inhibition of the E-cadherin-induced activation of Rac and formation of cell-cell adherens junctions. Mol Biol Cell. 2004;15:1077–1088. doi: 10.1091/mbc.E03-05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y. Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J Biol Chem. 2002;277:50749–50755. doi: 10.1074/jbc.M209846200. [DOI] [PubMed] [Google Scholar]
- 23.Kawakatsu T, Ogita H, Fukuhara T, Fukuyama T, Minami Y, Shimizu K, et al. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem. 2005;280:4940–4947. doi: 10.1074/jbc.M408710200. [DOI] [PubMed] [Google Scholar]
- 24.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 26.Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem. 2004;279:30092–30098. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- 27.Woodcock SA, Rooney C, Liontos M, Connolly Y, Zoumpourlis V, Whetton AD, et al. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell. 2009;33:639–653. doi: 10.1016/j.molcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA, et al. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem. 2003;278:1372–1379. doi: 10.1074/jbc.M208772200. [DOI] [PubMed] [Google Scholar]
- 29.Chun J, Prince A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe. 2009;5:47–58. doi: 10.1016/j.chom.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rios-Doria J, Kuefer R, Ethier SP, Day ML. Cleavage of beta-catenin by calpain in prostate and mammary tumor cells. Cancer Res. 2004;64:7237–7240. doi: 10.1158/0008-5472.CAN-04-1048. [DOI] [PubMed] [Google Scholar]
- 31.Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, et al. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem. 2009;284:5381–5394. doi: 10.1074/jbc.M808958200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hage B, Meinel K, Baum I, Giehl K, Menke A. Rac1 activation inhibits E-cadherin-mediated adherens junctions via binding to IQGAP1 in pancreatic carcinoma cells. Cell Commun Signal. 2009;7:23. doi: 10.1186/1478-811X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozano E, Frasa MA, Smolarczyk K, Knaus UG, Braga VM. PAK is required for the disruption of E-cadherin adhesion by the small GTPase Rac. J Cell Sci. 2008;121:933–938. doi: 10.1242/jcs.016121. [DOI] [PubMed] [Google Scholar]
- 34.Braga VM, Betson M, Li X, Lamarche-Vane N. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell-cell adhesion in normal human keratinocytes. Mol Biol Cell. 2000;11:3703–3721. doi: 10.1091/mbc.11.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell. 2001;12:847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer RS, Quinlan MP. Identification of a novel mechanism of regulation of the adherens junction by E1A, Rac1 and cortical actin filaments that contributes to tumor progression. Cell Growth Differ. 1998;9:905–918. [PubMed] [Google Scholar]
- 37.Quinlan MP. Rac regulates the stability of the adherens junction and its components, thus affecting epithelial cell differentiation and transformation. Oncogene. 1999;18:6434–6442. doi: 10.1038/sj.onc.1203026. [DOI] [PubMed] [Google Scholar]
- 38.Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide-3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shintani Y, Wheelock MJ, Johnson KR. Phosphoinositide-3-kinase-Rac1-c-Jun NH2-terminal kinase signaling mediates collagen I-induced cell scattering and upregulation of N-cadherin expression in mouse mammary epithelial cells. Mol Biol Cell. 2006;17:2963–2975. doi: 10.1091/mbc.E05-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagi R, Waguri S, Sumikawa Y, Nada S, Oneyama C, Itami S, et al. C-terminal Src kinase controls development and maintenance of mouse squamous epithelia. EMBO J. 2007;26:1234–1244. doi: 10.1038/sj.emboj.7601595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 42.Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL. RhoA, Rac1 and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol. 2004;287:327–335. doi: 10.1152/ajpcell.00087.2004. [DOI] [PubMed] [Google Scholar]
- 44.Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–2398. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, et al. Beta-catenin and ZO-1: Shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- 50.Mullin JM. Potential interplay between luminal growth factors and increased tight junction permeability in epithelial carcinogenesis. J Exp Zool. 1997;279:484–489. doi: 10.1002/(sici)1097-010x(19971201)279:5<484::aid-jez11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Brennan K, Offiah G, McSherry EA, Hopkins AM. Tight junctions: A barrier to the initiation and progression of breast cancer? J Biomed Biotechnol. 2010;2010:460607. doi: 10.1155/2010/460607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 53.Feigin ME, Muthuswamy SK. Polarity proteins regulate mammalian cell-cell junctions and cancer pathogenesis. Curr Opin Cell Biol. 2009;21:694–700. doi: 10.1016/j.ceb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 56.Nobes CD, Hall A. Rho GTPases control polarity, protrusion and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 58.Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 61.Kaverina I, Rottner K, Small JV. Targeting, capture and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rooney C, White G, Nazgiewicz A, Woodcock SA, Anderson KI, Ballestrem C, et al. The Rac activator STEF (Tiam2) regulates cell migration by microtubule-mediated focal adhesion disassembly. EMBO Rep. 2010;11:292–298. doi: 10.1038/embor.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 66.Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 67.Joneson T, Bar-Sagi D. Suppression of Ras-Induced Apoptosis by the Rac GTPase. Mol Cell Biol. 1999;19:5892–5901. doi: 10.1128/mcb.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Debidda M, Williams DA, Zheng Y. Rac1 GTPase regulates cell genomic stability and senescence. J Biol Chem. 2006;281:38519–38528. doi: 10.1074/jbc.M604607200. [DOI] [PubMed] [Google Scholar]
- 69.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. {alpha}6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-{kappa}B-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 70.Chaigne-Delalande B, Anies G, Kramer I, Genot E. Nonadherent cells switch to a Rac-mediated, SHIP regulated, Akt activation mode for survival. Oncogene. 2007;27:1876–1885. doi: 10.1038/sj.onc.1210830. [DOI] [PubMed] [Google Scholar]
- 71.Matos P, Jordan P. Expression of Rac1b stimulates NF[kappa]B-mediated cell survival and G1/S progression. Exp Cell Res. 2005;305:292–299. doi: 10.1016/j.yexcr.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 72.Matos P, Jordan P. Increased Rac1b Expression Sustains Colorectal Tumor Cell Survival. Mol Cancer Res. 2008;6:1178–1184. doi: 10.1158/1541-7786.MCR-08-0008. [DOI] [PubMed] [Google Scholar]
- 73.Xia Z DM, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 74.Ramaswamy M, Dumont C, Cruz AC, Muppidi JR, Gomez TS, Billadeau DD, et al. Cutting Edge: Rac GTPases Sensitize Activated T Cells to Die via Fas. J Immunol. 2007;179:6384–6388. doi: 10.4049/jimmunol.179.10.6384. [DOI] [PubMed] [Google Scholar]
- 75.Olson MFAA, Hall A. An essential role for Rho, Rac and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 76.Pines J. The cell cycle kinases. Semin Cancer Biol. 1994;5:305–313. [PubMed] [Google Scholar]
- 77.Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117:2173–2181. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- 78.Robles AI, Rodriguez-Puebla ML, Glick AB, Trempus C, Hansen L, Sicinski P, et al. Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 1998;12:2469–2474. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JK, Diehl JA. Nuclear cyclin D1: An oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK. alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197–3204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, et al. Rac regulation of transformation, gene expression and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klein EA, Chengfeng Yang, Kazanietz MG, Richard K. Assoian. NFκB-Independent Signaling to the cyclin D1 gene by Rac. Cell Cycle. 2007;6:1115–1121. doi: 10.4161/cc.6.9.4147. [DOI] [PubMed] [Google Scholar]
- 83.Page K, Li J, Hodge JA, Liu PT, Vanden Hoek TL, Becker LB, et al. Characterization of a Rac1 signaling pathway to cyclin D1 expression in airway smooth muscle cells. J Biol Chem. 1999;274:22065–22071. doi: 10.1074/jbc.274.31.22065. [DOI] [PubMed] [Google Scholar]
- 84.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, et al. Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 85.Joyce D, Bouzahzah B, Fu M, Albanese C, Da’Amico M, Steer J, et al. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-ΰB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 86.Joneson T, Bar-Sagi D. A Rac1 effector site controlling mitogenesis through superoxide production. J Biol Chem. 1998;273:17991–17994. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- 87.Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 88.Klein EA, Campbell LE, Kothapalli D, Fournier AK, Assoian RK. Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells. J Biol Chem. 2008;283:30911–30918. doi: 10.1074/jbc.M804537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore KA, Sethi R, Doanes AM, Johnson TM, Pracyk JB, Kirby M, et al. Rac1 is required for cell proliferation and G2/M progression. Biochem J. 1997;326:17–20. doi: 10.1042/bj3260017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michaelson D, Abidi W, Guardavaccaro D, Zhou M, Ahearn I, Pagano M, et al. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J Cell Biol. 2008;181:485–496. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jefford CE, Irminger-Finger I. Mechanisms of chromosome instability in cancers. Crit Rev Oncol Hematol. 2006;59:1–14. doi: 10.1016/j.critrevonc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Rosenblatt J. Spindle assembly: Asters part their separate ways. Nat Cell Biol. 2005;7:219–222. doi: 10.1038/ncb0305-219. [DOI] [PubMed] [Google Scholar]
- 93.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 94.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Curr Opin Cell Biol. 2006;18:199–205. doi: 10.1016/j.ceb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 96.Woodcock SA, Rushton HJ, Castañeda-Saucedo E, Myant K, White GRM, Blyth K, et al. Tiam1-Rac signaling counteracts Eg5 during bipolar spindle Assembly to facilitate chromosome congression. Curr Biol. 2010;20:669–675. doi: 10.1016/j.cub.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barr FA, Gruneberg U. Cytokinesis: Placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organization of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- 99.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 100.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 101.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D'Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol. 2004;166:61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshizaki H, Ohba Y, Parrini MC, Dulyaninova NG, Bresnick AR, Mochizuki N, et al. Cell type-specific regulation of RhoA activity during cytokinesis. J Biol Chem. 2004;279:44756–44762. doi: 10.1074/jbc.M402292200. [DOI] [PubMed] [Google Scholar]
- 104.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, et al. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–1546. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, et al. Expression of seven main Rho family members in gastric carcinoma. Biochem Biophys Res Com. 2004;315:686–691. doi: 10.1016/j.bbrc.2004.01.108. [DOI] [PubMed] [Google Scholar]
- 106.Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, et al. Overexpression of RhoA, Rac1 and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10:4799–4805. doi: 10.1158/1078-0432.CCR-0436-03. [DOI] [PubMed] [Google Scholar]
- 107.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, et al. Rac1 in human breast cancer: overexpression, mutation analysis and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 108.Shyun-Yeu L, Ching-Yu Y, Shun-Chun Y, Wei-Fan C, Kuo-Wei C. Overexpression of Rac-1 small GTPase binding protein in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2004;62:702–707. doi: 10.1016/j.joms.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Jordan P, Brazão R, Boavida MG, Gespach C, Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18:6835–6839. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 110.Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Nat Acad Sci USA. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lane J, Martin T, Mansel R, Jiang W. The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast cancer. Int Semin Surg Oncol. 2008;5:23. doi: 10.1186/1477-7800-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, et al. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene. 2009;28:1853–1863. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 114.Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 115.Espina C, Cespedes MV, Garcia-Cabezas MA, del Pulgar MTG, Boluda A, Oroz LG, et al. A critical role for Rac1 in tumor progression of human colorectal adenocarcinoma cells. Am J Pathol. 2008;172:156–166. doi: 10.2353/ajpath.2008.070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 117.Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S, et al. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene. 29:3362–3373. doi: 10.1038/onc.2010.95. [DOI] [PubMed] [Google Scholar]
- 118.Harnois T, Constantin B, Rioux A, Grenioux E, Kitzis A, Bourmeyster N. Differential interaction and activation of Rho family GTPases by p210bcr-abl and p190bcr-abl. Oncogene. 2003;22:6445–6454. doi: 10.1038/sj.onc.1206626. [DOI] [PubMed] [Google Scholar]
- 119.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABLinduced myeloproliferative disease. Cancer cell. 2007;12:467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 120.Thomas EK, Cancelas JA, Zheng Y, Williams DA. Rac GTPases as key regulators of p210-BCR-ABL-dependent leukemogenesis. Leukemia. 2008;22:898–904. doi: 10.1038/leu.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muller LUW, Schore RJ, Zheng Y, Thomas EK, Kim MO, Cancelas JA, et al. Rac guanosine triphosphatases represent a potential target in AML. Leukemia. 2008;22:1803–1806. doi: 10.1038/leu.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu K, Rajagopal S, Klebba I, Dong S, Ji Y, Liu J, et al. The role of fibroblast Tiam1 in tumor cell invasion and metastasis. Oncogene. 2010;29:6533–6542. doi: 10.1038/onc.2010.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bryan B, D'Amore P. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]