Abstract
Context
The study of symptom clusters is gaining increased attention in the field of oncology in an attempt to improve the quality of life of patients diagnosed with cancer.
Objectives
The aims of the present study were to: (1) determine the prevalence and distribution of pain, fatigue, and symptoms of depression and their covariation as a cluster in people with hepatobiliary carcinoma; (2) characterize how variation in each individual symptom and/or their covariation as a cluster are associated with changes in immunity; and (3) determine if the symptom clusters, and associated biomarkers, are related to survival in people diagnosed with hepatobiliary carcinoma.
Methods
Two hundred and six participants diagnosed with hepatobiliary carcinoma completed a battery of standardized questionnaire measuring cancer-related symptoms. Peripheral blood leukocytes were measured at diagnosis, three- and six-month follow-ups. Survival was measured from the date of diagnosis to death.
Results
Cancer-related symptoms were prevalent and two-step hierarchical cluster analyses yielded three clusters. High levels of pain, fatigue, and depression were found to be associated with elevated eosinophil percentages (F[1,78]=3.1, P=0.05) at three-month and six-month follow-ups using repeated measures ANOVA. Using multivariate latent growth curve modeling, pain was the primary symptom associated with elevated eosinophil percentages between diagnosis and six months (z=2.24, P=0.05). Using Cox regression, vascular invasion and age were negatively associated with survival (Chi-square=21.6, P=0.03). While stratifying for vascular invasion, Kaplan Meier survival analysis was performed and eosinophil levels above the median for the sample were found to be related to increased survival in patients with and without vascular invasion (Breslow Chi-square=4.9, P=0.03). Symptom clusters did not mediate the relationship between eosinophils and survival.
Conclusion
Cancer-related symptoms, particularly pain and depression, were associated with increased percentages of eosinophils. The presence of symptoms may reflect tumor cell death and be indicative of response to treatment, or other processes, in patients with hepatobiliary carcinoma.
Keywords: Symptom cluster, immunity, cancer, pain, depression, fatigue, hepatobiliary cancer
Introduction
The National Cancer Institute (NCI) State-of-the-Science consensus statement reported that the three most prevalent and undertreated cancer-related symptoms are pain, fatigue, and depression (1). In a clinical context, it is well established that these symptoms co-occur, but are treated independently. Although the concept of “symptom clusters” has been employed in other areas of medicine, this conceptual framework has only been recently introduced to the field of oncology (2–6). Dodd and colleagues (2) defined a symptom cluster as “three or more concurrent symptoms that are related to each other.” In the present study, we examined the prevalence and distribution of the three most common cancer-related symptoms in patients with hepatobiliary carcinoma. A growing body of research suggests that these cancer-related symptoms, at a molecular level, may have shared underlying biological mechanisms -- the cytokine immunological model (5–6).
Pain has been previously described as an evolutionarily adaptive constellation of responses that enhance survival of the host (7). Pro-inflammatory cytokines have been found to facilitate inflammatory and neuropathic pain (7). Spinal glial cells (astrocytes and microglia) when activated lead to increased levels of pain but also release pro-inflammatory cytokines within the central nervous system (8–10). Furthermore, the exogenous administration of proinflammatory cytokines facilitates the induction of pain and agents that antagonize proinflammatory responses have been shown to block pain (11).
In regard to fatigue, cytokines act as autocrine or paracrine growth factor for neoplastic tissue that result in fatigue (12). In other disease states, such as multiple sclerosis, fatigue has been found to be associated with elevations in IFN-γ and TNF-α. In patients with acute myelogenous leukemia or myelodysplastic syndrome, fatigue was found to be associated with elevations in interleukin (IL)-6, IL-1 and TNF- α levels in the serum. Bower and colleagues also recently found that fatigue was associated with higher levels of serum IL-1RA, soluble TNF-RII, and neopterin in women diagnosed with breast cancer (13). Anemia, which is commonly associated with fatigue, has been shown to be associated with blunting of erythropoietin response and cytokine changes, including elevations in IL-1, IL-6, and TNF- α, which suppress erythropoiesis (14).
Finally, abnormal secretion of IL-1 and IFN-γ, as well as IL-2 and IL-6, have been observed in people who report depressive symptoms (15–18). According to Raison and Miller, changes in these cytokines may contribute to depressive symptoms in several ways, including: (1) causing alterations in metabolism of monoamines, such as norepinephrine, serotonin, and dopamine; (2) activating the HPA-axis and stimulating corticotrophin-releasing hormone (19–20); (3) leading to the resistance of nervous, endocrine and immune system tissues to circulating glucocorticoid hormones through direct inhibitory effects on the expression or function of glucocorticoid receptors (21); and (4) reducing L-trypotophan and induction of enzyme indolamine 2,3 dioxygensase (IDO), which breaks down tryptophan into kynureinin and inhibit immunity (22–25). Suarez and colleagues found that, even while controlling for age, race, alcohol use, and body mass index, mild to moderate depression was associated with monocyte-associated (CD14) expression of interleukin-1β, tumor necrosis factor-α, IL-8, and monocyte chemotactic protein-1 (MCP-1) after in vitro lipopolysaccharide stimulation of undiluted whole blood (26). Finally, it is also well established that the introduction of exogenous interferon-alpha can lead to depression (27).
Although the cytokine-immunological theory has not been empirically tested, a plethora of evidence has accumulated regarding the association between cancer-related symptom and cytokines from research investigating the underlying biological mechanisms of “sickness behavior” (5–6); administration of exogenous cytokines and subsequent induction of symptoms (27), and the reduction of these symptoms with cytokine antagonists across a variety of disease states (28). A separate but related literature regarding the role of tumor-associated tissue and blood eosinophilia (TATE and TABE, respectively), has lead to important advances in the treatment of cancer (29–30), namely the development of kinase inhibitors (e.g., imatinib mesylate, sorafenib) (31–32). TABE followed by treatment with kinase inhibitors has been shown to be associated with a favorable prognosis in patients with solid tumors (31–37).
Eosinophils and cytokines are closely wedded. The production of eosinophils require Interleukin IL-5, IL-3, and granulocyte-macrophage colony stimulating factor (38–39). Eosinophils also result in the production of some of the pro-inflammatory cytokines including TNF–α and transforming growth factor–beta (TGF-β) (38–39). Conceivably, cancer-related symptom clusters may be not only associated with changes in cytokines but may also be related to changes in eosinophils. Eosinophils, in the setting of neoplasia, have two primary functions (1) a destructive effector function that limits the growth of the tumor (e.g., gastrointestinal cancers), and (2) an immunoregulative activity to suppress immune response and promote proliferation of tumor cells (oral squamous cell carcinoma (29).
Hepatobiliary carcinoma (HBC) is an excellent model to test the associations between cancer-related symptom clusters, immunity, and survival. Suppression of nonadaptive immunity, as well as changes in cytokines, have been found to be associated with disease progression and survival in people diagnosed with hepatocellular carcinoma, which is the most prevalent of the hepatobiliary carcinomas (40–55). The aims of the study were to prospectively: (1) determine the prevalence and distribution of pain, fatigue, and symptoms of depression and their covariation as a cluster in people with hepatobiliary carcinoma; (2) characterize how variation in each individual symptom and/or their covariation as a cluster are associated with changes in immunity; and (3) determine if the symptom clusters, and associated biomarkers, are related to survival in people diagnosed with hepatobiliary carcinoma.
Methods
Design
Patients were prospectively studied from diagnosis to death. For the purposes of this study, data concerning symptoms were collected at baseline (prior to treatment), and at 3- and 6-months follow-up.
Participants
Two hundred and six patients were recruited from the University of Pittsburgh’s Liver Cancer Center between April 2002 and April 2007. Inclusion criteria for participants in this phase of the study were: (1) diagnosis of hepatobiliary carcinoma, (2) fluency in English, (3) age between 18–85 years. Exclusion criteria were: (1) participants who are too medically ill to participate in the study or had a prognosis of less than 3 months, and (2) patients who reported psychiatric symptoms that include psychosis or thought disorder, or report of suicidal or homicidal ideation.
Instruments/Assessment
Sociodemographic Characteristics
A 25-item questionnaire assessing the participant’s gender, age, ethnic group, educational level, marital status, residence, number of children, occupation, income, religious preference, and health care insurance was administered.
The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep)
(56–57) was used to assess changes in symptoms and side effects of treatment. The FACT-Hep is a combination of the FACT-General, a 27-item instrument that measures four dimensions of quality of life (56), and a module with 18 additional items specific for participants with hepatobiliary disease (57). The module includes questions that pertain to symptoms of the disease as well as side effects of the treatment. The FACT is one of the most widely utilized quality-of-life questionnaires in clinical trials for new cancer treatments and the FACT-G and Hepatobiliary module has been demonstrated to be valid and reliable (56–57).
Single items (“I have pain” and “I have fatigue”) and the emotional well-being (EWB) subscale of the FACT-Hep were employed to measure pain, fatigue, and depressive symptoms, respectively. In a subsample of patients (n=40), the single items (i.e., pain, fatigue) and subscale (i.e., EWB subscale) were found to be significantly correlated with multi-item standardized measures of these symptoms, including the Brief Pain Inventory (58), FACT-Fatigue scale (59), and the Center for Epidemiological Studies-Depression scale (60).
Liver Functioning Tests and Immune System Parameters
Each patient who is evaluated and treated at the Liver Cancer Center has weekly blood draws as part of their routine work up and treatment. The panel of laboratory tests include total bilirubin, PT, PTT, albumin, alkaline phosphatate, GGTP, hemoglobin, hematocrit, alpha-fetoprotein, and creatine were performed at each visit. Peripheral blood leukocytes counts and percentage of distinct cell types were assessed and included lymphocyte subsets such as monocytes, eosinophils, neutrophils and basophils. The lymphocyte subsets, in particular, were collected to assess neutropenia. Although these labs were not originally collected for the purposes of this study, due to the resurgence in interest in TATE and TABE and the link between eosinophils and cytokines, the study of these lymphocyte subsets and cancer-related symptoms was undertaken.
Data Analyses
Using SPSS.v16, the data were entered and verified. Variables were examined to assess the distribution, linearity, and where appropriate, reliability and internal consistency of scales. The response scale for these single items, as well as the EWB subscale, is as follows: 0=not at all; 1= a little bit; 2=somewhat; 3=quite a bit; and 4=very much. A higher score reflected a greater frequency of that symptom in the last week for the single item. However, a higher EWB subscale score reflects a higher level of emotional well-being. To determine whether pain, fatigue and depressive symptoms clustered in unique pattern, a two-step hierarchical cluster analysis was performed on symptoms (pain, fatigue, and depression) to discover homogenous subgroups in the data file using Schwarz’s Bayesian Information Criterion (BIC) on log-likelihood distance measures (58–61). Multivariate latent growth curve modeling (MLGM), using structural equation modeling (SEM), was performed to examine the relationships among rate of change of symptoms or symptom clusters and immune system parameters over time (e.g., a correlation between rate of change of pain and eosinophils). Parameters from these models were estimated using maximum likelihood with Yuan-Bentler robust adjustments, which adjust for non-normal data with missing data (62). The models were evaluated using a Yuan-Bentler model chi-square and fit indices (62). A model Chi-square in SEM is known to be biased against a sample size. Therefore, fit indices are often used to evaluate a model fit. Hu and Bentler recommended a model be evaluated using at least two different fit indices from two different classes (63–64). Comparative fit index (CFI) (65) and root-mean-square-error-of-approximation (RMSEA) (66) were used. A model is considered “good” if CFI is greater than or equal to 0.95 and RMSEA is less than or equal to 0.06.
Cox regression analysis was employed to test the relationship between cancer-related symptoms, immune system parameters, and survival. The variables were categorized to maintain adequate power and provide clinically meaningful groups; they are as follows: gender (male or female); age (≤50 or >50); ethnicity (Caucasian and non-Caucasian); presence or absence of hepatitis B and/or C; presence or absence of cirrhosis, size of lesion (<5cm or ≥5cm); number of lesions (≤2 or >2); vascularity of lesion (hypervascular/mixed vascularity or hypovascular); and the presence or absence of vascular invasion. Kaplan Meier survival analyses were employed to test the differences between eosinophil levels in regard to survival while stratifying for vascular invasion. The data analyses were performed with the entire sample as well as with patients diagnosed with only hepatocellular carcinoma.
Results
Of the total sample (n=206), 72% were male and the mean age was 64 years (range 22–90 years). The majority of participants were Caucasian (91%). The patients were diagnosed with hepatocellular carcinoma (84%), gallbladder carcinoma (6%), cholangiocarcinoma (5%), or neuroendocrine carcinoma (3%), and other primary tumors with liver metastases (2%). Table 1 provides details of sociodemographic and disease-specific characteristics of the sample. Table 2 provides laboratory values at diagnosis for all patients by symptom cluster.
Table 1.
Sociodemographic and Disease-Specific Characteristics of Sample
| Variable | Symptom Cluster | Total | ||
|---|---|---|---|---|
| Asymptomatic | Symptomatic | Fatigue | ||
| Gender (%) | ||||
| Male | 71 | 67 | 75 | 72 |
| Female | 29 | 33 | 25 | 28 |
| Age (years)* | ||||
| Mean | 67.5 | 60.4 | 67.5 | 65 |
| Range | 30–90 | 22–84 | 31–88 | 22–90 |
| Ethnicity (%)a | ||||
| Caucasian | 91 | 94 | 90 | 91 |
| African American | 5 | 2 | 7 | 6 |
| Asian/Pacific Islander | 3 | 6 | 2 | 2 |
| Hispanic | 1 | 0 | 1 | 1 |
| Diagnosis (%) | ||||
| Hepatocellular | 88 | 75 | 87 | 84 |
| Cholangio | 3 | 9 | 6 | 5 |
| Neuroendocrine | 1 | 4 | 3 | 3 |
| Gallbladder | 4 | 10 | 4 | 6 |
| Liver metastases | 4 | 2 | 0 | 2 |
| Hepatitis (%)b | ||||
| B | 7.6 | 5.9 | 1.4 | 6 |
| C | 24.1 | 17.6 | 19.2 | 28 |
| B and C | 3.8 | 5.9 | 9.6 | 8 |
| Cirrhosis (%)b | 57 | 49 | 60 | 45 |
| Tumor Size (cm) | ||||
| Median | 5 | 6.2 | 6 | 7 |
| Range | 0.9–21 | 1–22 | 0–18 | 0.5–22 |
| Number of Lesions | ||||
| Median | 2 | 3 | 2 | 3 |
| Range | 1–6 | 1–6 | 1–6 | 1–6 |
| Vascular invasion (%) | 25.3 | 27.5 | 35.6 | 24 |
| Vascularity (%) | ||||
| Hypervascular | 78 | 74 | 79 | 77 |
| Hypovascular | 20 | 15 | 17 | 17 |
| Mixed | 2 | 10 | 4 | 6 |
| Treatment (%) | ||||
| Transarterial Infusion | ||||
| Chemotherapy | 80 | 83 | 69 | 77 |
| 90-Yttrium | 20 | 17 | 30 | 23 |
| Survival (months) | ||||
| Median | 9.9 | 8.8 | 7.5 | 8 |
| Range | 0.4–52 | 0.9–63.5 | 0.8–91.2 | 0.4–91.2 |
P<0.001.
P<0.01.
Table 2.
Baseline Laboratory Values
| Laboratory Test | Normal | Symptom Cluster | Total | ||
|---|---|---|---|---|---|
| Rangesa | Asymptomatic | Symptomatic | Fatigue | ||
| n=80 | n=52 | n=74 | n=206 | ||
| Bilirubin | 0.1–1.2 | ||||
| Median | 0.8 | 0.65 | 0.9 | 1.2 | |
| Range | 0.2–3.9 | 0.1–3.6 | 0.2–3.5 | 1–4.1 | |
| AFP (mg/ml) | 0–8.9 | ||||
| Median | 104.5 | 44 | 140.5 | 78 | |
| Range | 2–113344 | 2–109600 | 1–98197 | 1–113,340 | |
| Hemoglobin (g/dl) | 14–18 | ||||
| Median | 12.9 | 11.7 | 12.7 | 12.5 | |
| Range | 8.7–15.8 | 8.1–14.4 | 9.4–15 | 8.1–15.8 | |
| Albumin (mg/dl) | 3.3–5.2 | ||||
| Median | 3.5 | 3.5 | 3.4 | 3.4 | |
| Range | 2.3–4.4 | 2.4–4.3 | 21–4.5 | 2.1–4.5 | |
| Akaline Phos (U/L) | 40–125 | ||||
| Median | 188 | 219 | 199 | 215 | |
| Range | 107–1022 | 96–679 | 99–352 | 96–1022 | |
| GGTP (IU/L) | 0–51 | ||||
| Median | 188 | 201 | 176.5 | 190 | |
| Range | 23–780 | 42–747 | 32–750 | 23–780 | |
| PT (seconds) | 9.8–13.8 | ||||
| Median | 12.9 | 12.9 | 12.6 | 12.9 | |
| Range | 10.4–18.6 | 10.4–15.8 | 0.7–15.6 | 10.4–18.6 | |
| WBC (mm3) | 4.8–10.8 | ||||
| Median | 5.7 | 6 | 6.2 | 5.8 | |
| Range | 1.6–19.4 | 2–12.8 | 2.1–14.7 | 1.6–19.4 | |
| Lymphocyte (K/µL) | 0.8–3.5 | ||||
| Median | 18 | 1.1 | 16.5 | 1 | |
| Range | 4–3. | 0.44–.99 | 4–43 | 0.16–2.6 | |
| Monocytes (K/µL) | 0.2–0.8 | ||||
| Median | 8.1 | 8 | 8 | 8 | |
| Range | 0.8–26 | 1.1–15 | 0.9–21 | 0.8–26 | |
| Basophils (K/µL) | 0.01–0.20 | ||||
| Median | 0 | 1 | 0 | 0.15 | |
| Range | .8–5 | 0.3–3 | 0.5–2 | 0.3–5 | |
| Eosinophils (K/µL) | 0.04–0.5 | ||||
| Median | 2 | 2 | 2 | 2.3 | |
| Range | 0–9 | 0–12 | 0–12 | 0–12 | |
Please note values of labs vary across laboratories, race, age, and gender.
Prevalence and Distribution of Symptoms Their Covariation as a Cluster in People with Hepatobiliary Carcinoma
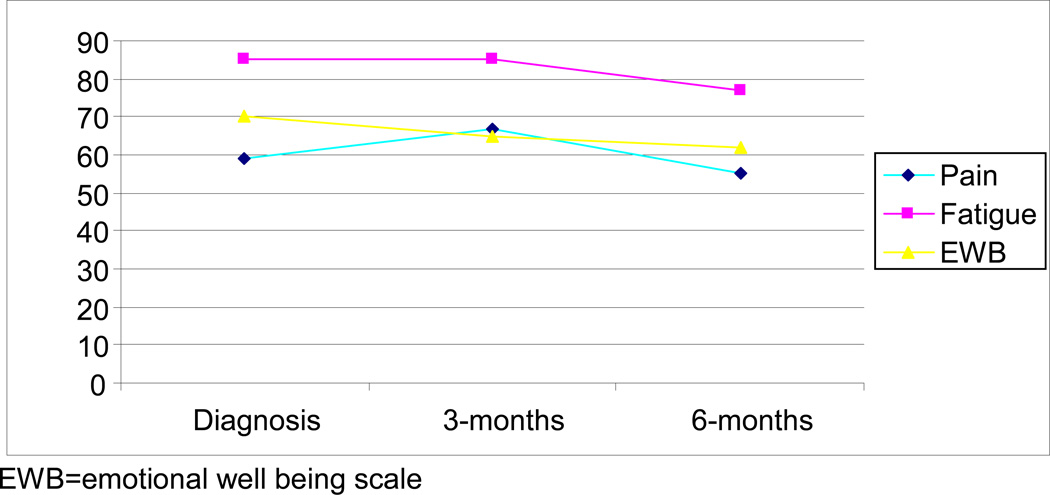
Fifty-nine percent of the patients reported pain at diagnosis and 67% reported pain at the three-month follow-up; at the six-month follow-up, 62% reported pain. At diagnosis, approximately 85% of patients reported fatigue. At the three- and six-month follow-ups, 85% and 77% of patients, respectively, reported fatigue. At diagnosis, three- and six-month follow-ups, 70%, 65%, and 62% of patients, respectively, reported feelings of anxiety and depression.
The cluster analyses yielded three clusters of patients who reported symptoms (Table 3). The first cluster of patients (called asymptomatic) reported low levels of pain, low levels of fatigue, and high levels of EWB (40%). The second cluster of patients (called symptomatic) reported high levels of pain, high levels of fatigue, and low levels of EWB (25%). The third cluster (called fatigue), about 35% of the participants, reported low levels of pain, high levels of fatigue, and moderate EWB. Post-hoc analyses with only patients diagnosed with hepatocellular carcinoma were performed and the same three-cluster solution was yielded.
Table 3.
Mean (SD) of Eosinophils for Patients with and without Comorbid Conditions Known to Be Associated with Eosinophilsa
| Time Point | No Comorbid Condition | Comorbid Condition |
|---|---|---|
| Diagnosis (n=126) | 2.3 (3.2) | 2.1 (1.8) |
| 3-months (n=85) | 2.3 (2.4) | 2.0 (2.2) |
| 6-months (n=43) | 2.9 (3.2) | 2.4 (2.7) |
SD = standard deviation.
For example, allergies or asthma.
Using repeated measures multivariate analysis of variance (MANOVA) with dependent variables including lymphocytes, basophils, polymorphonuclear leukocytes (poly), and eosinophils, a significant between-group difference was found between symptom clusters and eosinophils (F[1,78]=3.1, P=0.05). The symptomatic cluster had significantly higher eosinophils at three months (3.1 vs. 2.9 and 1.6) and six months (3.6 vs. 3.0 and 1.5) when compared to the asymptomatic and the fatigued symptom cluster, respectively (Figure 1).
Figure 1.
Percent of patients reporting pain, fatigue, and sadness from diagnosis to 6-months
Post-hoc analyses were performed to determine if comorbid diseases (e.g., allergies, asthma, and autoimmune diseases) were associated with percentage of eosinophils. Using ANOVA at each time point, no significant association was found between these medical conditions and eosinophils at diagnosis (F[1,125]=0.03, P=0.86), three-months (F[1,84]=0.09, P=0.76), and six months (F[1,42]=0.09, P=0.77). When employing repeated measures ANOVA, no significant differences were found between patients with medical conditions known to be associated with eosinophils and those without such conditions (F[2,27]=0.18, P=0.84) (Table 3).
Variation in Symptoms and Its Association with Changes in Immunity
To investigate changes over time in symptom clusters and immune system parameters, Multivariate Latent Growth Curve Models (MLGM) were employed. Three MLGMs were performed on the FACT-EWB subscale, FACT-pain, and FACT-Fatigue. Yuan and Bentler adjustment for non-normal data is analogous to Satorra and Bentler (67). The initial values of EWB and FACT-Pain were negatively correlated (r=−0.909, z=−6.103). The rate of change of EWB and FACT-pain was negatively correlated (r=−0.704; Yuan-Bentler χ2 (11) = 22.893, CFI=1.00, RMSEA=0.071, SRMR=0.031). The variance of rate of change of FACT-Fatigue was not significant (i.e., within individuals, there was no change in fatigue;σ̂ = .001, z=0.021). Similarly, there was no significant variance of rate of change of FACT-Fatigue. The correlation between the rate of change of FACT-Fatigue and other variables (FACT-Pain and EWB) was, therefore, not tested. The initial values of FACT-Fatigue and FACT-Pain was positively correlated (r=0.764, z=5.129; Yuan-Bentler χ2 (13) = 49.371, CFI=1.000, RMSEA=0.113, SRMR=0.042). The RMSEA is high but it tends to provide a biased measure with a simple model. SRMR was added to assess fit and a good fit is indicated by the fact that SRMS is below 0.08. The initial FACT-Fatigue and EWB were negatively correlated (r=−0.725, z=−3.503; Yuan-Bentler χ2 (13) = 33.096, CFI=1.000, RMSEA=0.084, SRMR=0.037). The rate of change in the symptom clusters and immune system parameters were not significant but the correlation between rate of change in symptom and immune system parameters were large, with a strong trend toward significance for the rate of change for FACT-Pain and eosinophils, z=1.914, P = 0.056 (Table 4). Post-hoc analyses that included only hepatocellular carcinoma patients yielded the same results, with eosinophils and pain being significantly associated over time and a trend toward significance in depressive symptoms and eosinophils.
Table 4.
Rate of Change in Individual Symptoms and Immune System Parameters
| Model | Chi-square (df) |
CFI | RMSEA | SRMR | r (initial values) | r (rate of change) | |
|---|---|---|---|---|---|---|---|
| Depression | Lymphocytes | 6.552 (11) | 1.000 | 0.000 | 0.020 | −0.204 (z=−0.824) | 0.347 (z=0.846) |
| Eosinophils | 6.441 (11) | 1.000 | 0.000 | 0.023 | −0.230 (z=−1.717) | 0.229 (z=0.907) | |
| Basophils | 7.155 (11) | 1.000 | 0.000 | 0.025 | −0.004 (z=−0.033) | 0.088 (z=0.482) | |
| Poly | 10.442 (11) | 1.000 | 0.000 | 0.030 | 0.281 (z=1.586) | 0.008 (z=0.035) | |
| Pain | Lymphocytes | 13.232 (11) | 1.000 | 0.031 | 0.026 | 0.107 (z=0.684) | −0.047 (z=−0.142) |
| Eosinophils | 19.192 (11) | 1.000 | 0.058 | 0.034 | 0.191 (z=2.239)a | −0.211 (z=−1.914) | |
| Basophils | 15.349 (11) | 1.000 | 0.042 | 0.026 | −0.042 (z=−0.386) | 0.127 (z=0.761) | |
| Poly | 19.493 (11) | 1.000 | 0.059 | 0.029 | −0.035 (z=−0.439) | −0.190 (z=−0.924) | |
| Fatigue | Lymphocytes | 8.726 (11) | 1.000 | 0.000 | 0.018 | 0.043 (z=0.260) | −0.148 (z=−0.385) |
| Eosinophils | 12.080 (11) | 1.000 | 0.021 | 0.030 | −0.034 (z=−0.330) | 0.005 (z=0.044) | |
| Basophils | 9.404 (11) | 1.000 | 0.000 | 0.023 | −0.125 (z=−1.046) | 0.279 (z=1.520) | |
| Poly | 11.542 (11) | 1.000 | 0.015 | 0.028 | −0.063 (z=−0.615) | −0.071 (z=−0.234) | |
Poly=polymorphonuclear leukocytes; CFI=Comparative fit index; RMSEA=Root-mean-square-error-of-approximation; SRMR= Standardized Root Mean Residual
P<0.05.
Associations Between Symptom Clusters and Associated Biomarkers, and Survival
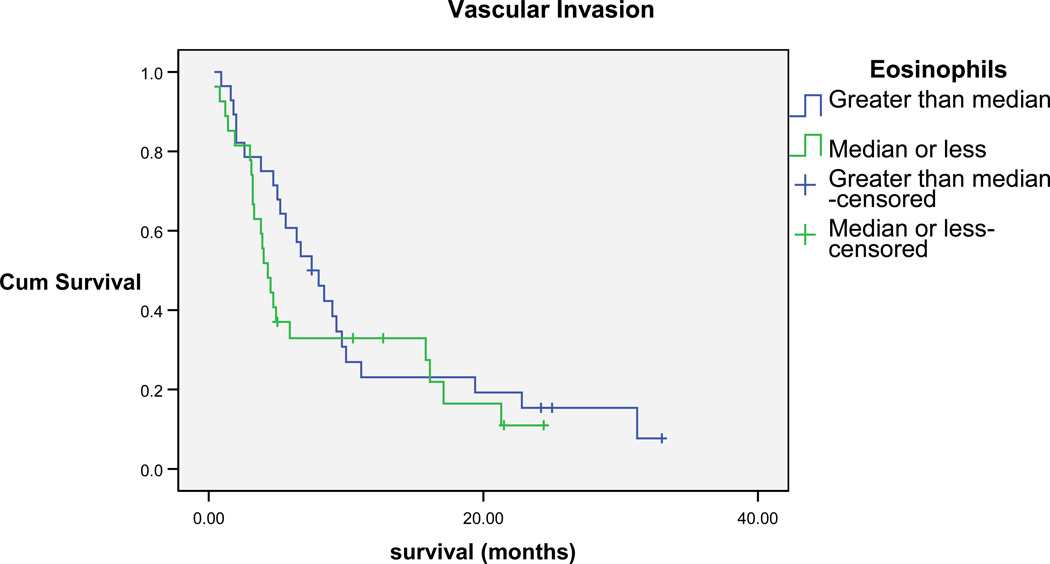
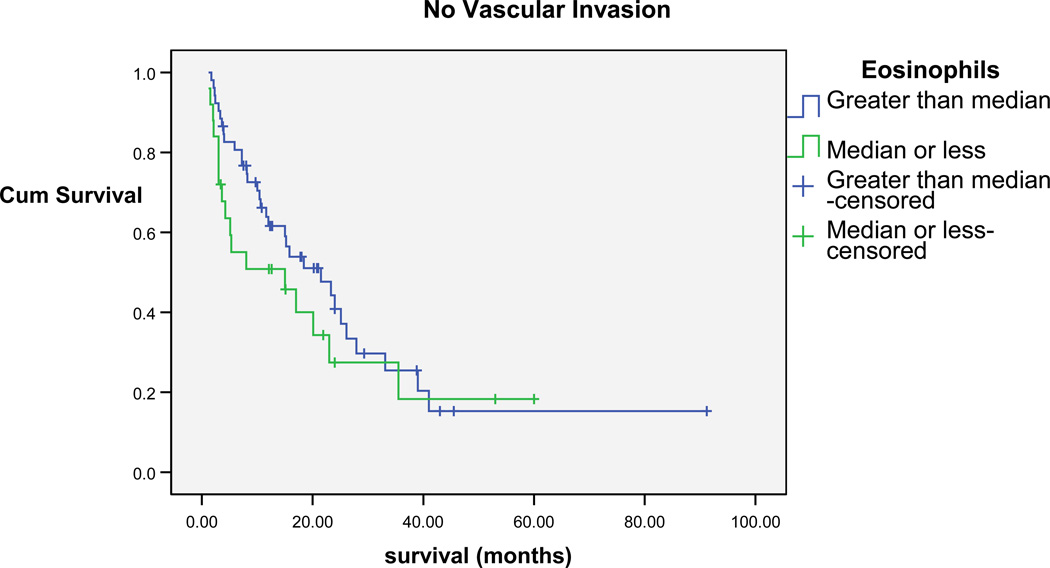
Kaplan Meier analyses were performed to investigate the role of eosinophils on survival. Kaplan Meier survival analyses were then performed after stratifying for vascular invasion, which is consistently found to be associated with poor prognosis. Peripheral blood eosinophils (above and below the median=2) were found to be significantly associated with survival (Breslow Chi-square=4.9, P=0.03). For patients who had vascular invasion and a median eosinophil count less than the median for the sample, a shorter survival (4.3 months median survival; 95% confidence interval [CI]=3.3, 5.3) was found when compared to patients who had eosinophils greater than the median for the sample (7.5 median months; 95% CI=5, 10). The same pattern of results were found with patients without vascular invasion, in that patients who had eosinophil percentages less than the median for the sample, shorter survival (15 median months; 95% CI=0.1, 30.8) was found when compared to patients who had eosinophil percentages higher than the median for the sample (21.5 median months; 95% CI=11.4, 31.6) (Figures 2 and 3).
Figure 2.
Cumulative Survival of patients with vascular invasion Eosinophils greater than and less than median of sample
Figure 3.
Cumulative Survival of patients without vascular invasion Eosinophils greater than and less than median of sample
Cox regression was employed to adjust for and examine the contribution of sociodemographic, disease-specific, symptom clusters, and eosinophils in regard to overall survival of people diagnosed with hepatobiliary carcinoma. The model remained significant (Chi-square=21.1, P=0.03) with vascular invasion (P=0.003), age (P=0.05) and eosinophils (P=0.03) significantly contributing to survival (P=0.01) (Table 5).
Table 5.
Cox Regression Analysis of Predictors of Survival
| Variable | B (SE) | Wald | P- | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|
| level | Lower | Upper | ||||
| Diagnosis | 1.641 | 0.65 | ||||
| 0.675 (0.562) | 1.441 | 0.23 | 1.963 | 0.653 | 5.905 | |
| 0.559 (0.858) | 0.424 | 0.52 | 1.748 | 0.325 | 9.388 | |
| 1.084 (1.194) | 0.825 | 0.36 | 2.958 | 0.285 | 30.685 | |
| Gender | 0.582 (0.322) | 3.259 | 0.07 | 1.789 | 0.951 | 3.364 |
| Age | −0.833 (0.432) | 3.725 | 0.05 | 0.435 | 0.187 | 1.013 |
| Ethnicity | −0.085 (0.463) | 0.034 | 0.85 | 0.918 | 0.371 | 2.274 |
| Cirrhosis | −0.145 (0.341) | 0.180 | 0.67 | 0.865 | 0.444 | 1.688 |
| Tumor Size | 0.295 (0.313) | 0.890 | 0.35 | 1.343 | 0.728 | 2.479 |
| Vascular Invasion | 0.818 (0.273) | 8.989 | 0.003 | 2.266 | 1.327 | 3.868 |
| Vascularity | 3.690 | 0.16 | ||||
| −0.878 (0.601) | 2.135 | 0.14 | 0.416 | 0.128 | 1.349 | |
| −0.409 (0.665) | 0.379 | 0.54 | 0.664 | 0.180 | 2.444 | |
| Eosinophils | 0.547 (0.249) | 4.822 | 0.03 | 1.727 | 1.060 | 2.813 |
| Sx Cluster | 0.529 | 0.77 | ||||
| 0.190 (0.293) | 0.419 | 0.52 | 1.209 | 0.681 | 2.146 | |
| 0.208 (0.342) | 0.372 | 0.54 | 1.232 | 0.630 | 2.408 | |
Discussion
Consistent with the NCI consensus statement, pain, fatigue and depression independently, as well as a symptom cluster, were found to be prevalent in people diagnosed with hepatobiliary carcinoma, with approximately 25% of patients experiencing high levels of pain, depression, and fatigue, and another 35% of patients reporting persistent fatigue. Overall, the three symptoms were reported in 62–85% of patients from diagnosis to six-month follow-up.
Although research has been conducted regarding the association of cancer-related symptoms and cytokines, no study has investigated the association between cancer-related symptoms and eosinophils, which may have a significant role in the context of cancer. The high level of cancer-related symptoms and association with eosinophils could not be accounted for by comorbid diseases that are established as being associated with eosinophilia (e.g., allergy, asthma).
Prospectively, pain was associated with higher percentages of eosinophils both at three-and six-month follow-ups. Eosinophils are multifunctional leukocytes that are involved in numerous inflammatory processes across disease types. Eosinophils, independent of other subtypes of leukocytes, can be recruited from the circulation into inflammatory areas of the body, and modulate immune responses through various mechanisms, including antigen presentation and release of cytokines IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, RANTES and eotaxin-1. Activation and recruitment of eosinophils may regulate vascular permeability and smooth muscle constriction. In addition, eosinophils may serve as a major effector cell, inducing tissue damage by releasing toxic granule proteins.
Our finding are consistent with reports of hypereosinophila outside the context of cancer. Eosinophilia has been associated with upper quadrant pain and fatigue (68), and is hypothesized to be associated with necrotic cell death, particularly during periods of nutrient, hypoxic, and oxidant stress (29). Although a trend toward significance was found in regard to depressive symptoms and eosinophilia over time, fatigue was not found to be associated with eosinophil percentage secondary to the lack of inter-individual variability in the report of fatigue over time.
The link between pain and eosinophils is believed to result from a cascade of events, possibly including increased tissue temperature caused by the release of histamines, which stimulate pain-sensing neurons. This may be followed by an increase in capillary permeability, resulting in the migration of leukocytes and macrophages from the circulatory system to the damaged tissue (69). As a result of the increased leukocytes and macrophages migrating into the tissue, edema and white blood cell body remnants, which increase the cellular pressure, may result in the sensation of pain (69).
In the setting of cancer, pain is often thought to be associated with tumor burden, which may also be indicative of decreased survival. However, post-hoc analyses were performed and pain at diagnosis, as well as at three- and six-month follow-ups, found that pain was not related to tumor size or survival. Prior studies have found that elevations in eosinophils were associated with cirrhosis (70). However, posthoc analyses revealed a lack of association between cirrhosis and eosinophil levels in the present study.
Tumor-associated blood eosinophilia in the present study, as well as previous studies of solid organ tumors, is consistent with a favorable prognosis for people diagnosed with hepatobiliary carcinoma. Although the pathogenesis of eosinophilia is not well understood, necrosis of the tumor has been hypothesized as a possible etiology of eosinophilia (29). Although TATE and TABE often occur independently, TABE is more often observed in advanced disease or metastatic disease (31–37).
Eosinophilia has been observed in a number of disease processes, most notably allergy and parasitic infection (29–30); therefore, the elevations in eosinophils could be related to comorbid diseases rather than the tumor cell death. Although random assignment, which would control for the influence of comorbid medical conditions that may be correlated with eosinophilia, was not the design of this study, post-hoc analyses found no association between eosinophil levels and comorbid disease processes.
Although this is a rather new area of investigation, we did not expect that other leukocyte subsets would necessarily be associated with symptoms or survival. Even though basophils are associated with inflammatory reactions, these granulocytes are specifically associated with allergic reactions or exoparasitic infections and therefore did not expect elevations or associations in this subset. While we did expect neutrophils, and in particular, neutropenia, to be associated with fatigue, secondary to the lack of variability over time and between clusters, an association could not be detected.
A limitation of the present study included the use of single item measures of pain, fatigue, and depression was used for the purposes of this study. However, in a subsample of patients, these single items were found to be highly correlated with multidimensional instruments such as the Center for Epidemiological Studies-Depression scale, Brief Pain Inventory, and the Functional Assessment of Cancer Therapy-Fatigue scale.
Although we did assess other symptoms and side effects of treatment (e.g., nausea and vomiting, itching, fevers), the frequency of these symptoms was low (<10%) and thus we did not include these symptoms in the analyses. Future research should include multidimensional instruments designed to measure each symptom to better understand the “multiplicative” or catalytic effects of symptoms on one another and the use of multilevel factor analysis (3).
The present study provides preliminary support regarding the co-occurrence of cancer-related symptoms and the association between these symptoms, biomarkers, and disease progression. However, cellular infiltration of eosinophils (e.g., TATE) should also be studied in patients with hepatobiliary carcinoma to provide further evidence of the role of tumor-associated tissue eosinophilia in disease progression. Furthermore, the cytokine-immunological model of cancer-related symptoms warrants testing based on the results of this study. Understanding the link between cancer-related symptoms, immunity, and disease progression may contribute to the development of pharmacological interventions to facilitate the management of cancer-related symptoms and potentially slow disease progression in solid tumor cancers.
Acknowledgments
This study was funded by grants from the American Cancer Society, the Pittsburgh Mind Body Center (National Institutes of Health grants nos. HL065111, HL065112, HL076852, and HL076858), and the National Cancer Institute (5K07CA118576).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NIH Consensus Development Program. Symptom management in cancer: pain, depression, and fatigue. NIH Consensus State-of-Science Statements. 2002;(4):1–29. [PubMed] [Google Scholar]
- 2.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 3.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: Conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monogr. 2004;32:17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 6.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 7.Wiesseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. NeuroSignals. 2005;14(4):166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 8.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesisa. Neurosci Let. 2004;361(1–3):184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 9.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192(2):444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Logan HL, Lutgendorf S, Kirchner HL, Rivera EM, Lubaroff D. Pain and immunological response to root canal treatment and subsequent health outcomes. Psychosom Med. 2001;63(3):453–462. doi: 10.1097/00006842-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Hori T, Oka T, Hosoi M, Aou S. Pain modulatory actions of cytokines and prostaglandin E2 in the brain. Ann N Y Acad Sci. 1998;840:269–281. doi: 10.1111/j.1749-6632.1998.tb09567.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92(6 Suppl):1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Dimeo F, Schmittel A, Fietz T, et al. Physical performance, depression, immune status and fatigue in patients with hematological malignancies after treatment. Ann Oncol. 2004;15(8):1237–1242. doi: 10.1093/annonc/mdh314. [DOI] [PubMed] [Google Scholar]
- 15.Anisman AV, Ravidndran J, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- 16.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 17.Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary Findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 18.Sluzewska A. Indicators of immune activation in depressed patients. Adv Exp Med Biol. 1999;461:59–74. doi: 10.1007/978-0-585-37970-8_4. [DOI] [PubMed] [Google Scholar]
- 19.Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 20.Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 21.Rivier C. Influence of immune signals on the hypothalamic-pituitary axis of the rodent. Front Neuroendocrinol. 1995;16:151–182. doi: 10.1006/frne.1995.1005. [DOI] [PubMed] [Google Scholar]
- 22.Pariante CM, Miller AH. Glucocorticoid receptions in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 23.Capuron JF, Gumnick DL, Musselman DH, et al. Neurobehavioral effects of interferon-alpha in cancer patients phenomenology and paroxetine responsiveness of symptoms dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 24.Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 25.Liebaue C, Baltzer AW, Schmidt S, et al. Interleukin 12 and interleukin 18 induced indoleamine 2,3-dioxygenase (IDO) activity in human osteosarcoma cell lines independently from interferon gamma. Anticancer Res. 2002;22:931–936. [PubMed] [Google Scholar]
- 26.Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65(3):362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- 27.Bonaccorso S, Puzella A, Marino V, Pasquini M. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105(1–2):45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 28.Swallow CJ, Partridge EA, Macmillan JC, et al. α2HS-glycoprotein, an antagonist of transforming growth factor β in vivo, inhibits intestinal tumor progression. Cancer Res. 2004;64:6402–6409. doi: 10.1158/0008-5472.CAN-04-1117. [DOI] [PubMed] [Google Scholar]
- 29.Lotfi R, Lee J, Lotze M. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz R. The hypereosinophilic syndrome and the biology of cancer. N Engl J Med. 2003;348:1199–1200. doi: 10.1056/NEJMp030019. [DOI] [PubMed] [Google Scholar]
- 31.Woodgett J. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Griffin J, Leung J, Bruner R, Caligiuri M, Briesewitz R. Discovery of a fusion kinase in EOL-1 cells and idiopathic hypereosinophilic syndrome. Proc Natl Acad Sci U S A. 2003;100:7830–7835. doi: 10.1073/pnas.0932698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies S, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastrnak A, Jansa P. Local eosinophilia in stroma of tumors related to prognosis. Neoplasma. 1984;31:323–326. [PubMed] [Google Scholar]
- 35.Rivoltini L, Colombo MP, Parmiani G, et al. In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int J Cancer. 1993;54:8–15. doi: 10.1002/ijc.2910540103. [DOI] [PubMed] [Google Scholar]
- 36.Silberstein D, Schoof D, Rodrick M, et al. Activation of eosinophils in cancer patients treated with IL-2 and IL-2-generated lymphokine-activated killer cells. J Immunol. 1989;142:2162–2167. [PubMed] [Google Scholar]
- 37.Arinaga S, Karimine C, Takamuku K, et al. Correlation of eosinophilia with clinical response in patients with advanced carcinoma treated with low-dose recombinant interleukin-2 and mitomycin C. Cancer Immunol Immunother. 1992;35:246–250. doi: 10.1007/BF01789330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981;34:1343–1348. doi: 10.1136/jcp.34.12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanderson C. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 40.Shirai M, Watanabe S, Nishioka M. Depressed lymphokine-activated killer activity and analyses of the precursor cells in peripheral blood of patient with hepatocellular carcinoma. Hepato-Gastroenterology. 1990;37(5):465–468. [PubMed] [Google Scholar]
- 41.Shirai M, Watanabe S, Nishioka M. Intramural injection of OK432 and lymphokine-activated killer activity in peripheral blood of patients with hepatocellular carcinoma. Eur J Cancer. 1990;26(9):965–969. doi: 10.1016/0277-5379(90)90621-y. [DOI] [PubMed] [Google Scholar]
- 42.Shirai M, Watanabe S, Nishioka M. Defective immunological functions associated with abnormal lymphokine-activated killer activity in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1990;5(5):542–548. doi: 10.1111/j.1440-1746.1990.tb01438.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Chen H, Wu M, et al. Postoperative immunotherapy for patients with hepatocarcinoma using tumor-infiltrating lymphocytes. Chin Med J. 1997;110(2):114–117. [PubMed] [Google Scholar]
- 44.Haruta I, Yamauchi K, Aruga A, et al. Analytical study of the clinical response to two distinct adoptive immunotherapies for advanced hepatocellular carcinoma: comparison between LAK cell and CTL therapy. J Immunother Emphasis Tumor Immunol. 1996;19(3):218–223. doi: 10.1097/00002371-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Taketomi A, Shimada M, Shirabe K, et al. Natural killer cell activity in patients with hepatocellular carcinoma. Cancer. 1998;83(1):58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 46.Actis GC, Ponzetto A, D'Urso N, et al. Chronic active hepatitis. Interferonactivated natural killer like cells against a hepatoma cell line transfected with the hepatitis B virus nucleic acid. Liver. 1991;11(2):106–113. doi: 10.1111/j.1600-0676.1991.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 47.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56+ cell and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32(5):962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakajima T, Mizushima N, Kanai K. Relationship between natural killer activity and development of hepatocellular carcinoma in patient with cirrhosis of the liver. Jpn J Clin Oncol. 1988;17(4):327–332. [PubMed] [Google Scholar]
- 49.Okanoue T, Itoh Y, Minami M, et al. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. J Hepatol. 1999;30(4):653–659. doi: 10.1016/s0168-8278(99)80196-2. [DOI] [PubMed] [Google Scholar]
- 50.Fang Y, Wang L, Jin J, Zha X. Focal adhesion kinase affect the sensitivity of human hepatocellular carcinoma cell line SMMC-7721 to tumor necrosis factor-alpha/cycloheximide-induced apoptosis by regulating protein kinase B levels. Eur J Biochem. 2001;268(16):4513–4519. doi: 10.1046/j.1432-1327.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 51.Tsujimoto T, Kuriyama S, Yamazaki M, et al. Augmented hepatocellular carcinoma progression and depressed Kupffer cell activity in rat cirrhotic livers. Int J Oncol. 2001;18(1):41–47. doi: 10.3892/ijo.18.1.41. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Yang E, Cheng LY. Effects of IFN-gamma, TNF-alpha and EGF on expression of HLA class I antigen and the proliferation of human hepatocellular carcinoma HepG2 cells. Anticancer Res. 1997;17(1A):181–188. [PubMed] [Google Scholar]
- 53.Feng X, Tang X, Zheng Z. Preliminary studies on the effects of tumor necrosis factor gene transfer on the growth of human hepatocellular carcinoma cells in nude mice. Chin J Oncol. 1995;17(3):167–169. [PubMed] [Google Scholar]
- 54.Osawa Y, Nagaki M, Banno Y, et al. Possible involvement of reactive oxygen species in D-galactosamine-induced sensitization against tumor necrosis factor-alpha induced hepatocyte apoptosis. J Cell Physiol. 2001;187(3):374–385. doi: 10.1002/jcp.1088. [DOI] [PubMed] [Google Scholar]
- 55.Atarashi Y, Yasumura S, Nambu S, et al. A novel human tumor necrosis factor alpha mutein, F4614, inhibits in vitro and in vivo growth of murine and human hepatoma: implications for immunotherapy of hepatocellular carcinoma. Hepatology. 1998;28(1):57–67. doi: 10.1002/hep.510280110. [DOI] [PubMed] [Google Scholar]
- 56.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 57.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20(9):2229–2239. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 58.Cleeland CS, Nakamura Y, Mendoza TR, et al. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 59.Radloff LS. The Center for Epidemiological Studies depression scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 60.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34 suppl 2:13–19. [PubMed] [Google Scholar]
- 61.Zhang T, Ramakrishnon R, Livny M. BIRCH: an efficient data clustering method for very large databases. Proceedings of the ACM SIGMOD Conference on Management of Data; Montreal, Canada: ACM; 1996. [Google Scholar]
- 62.Chiu T, Fang D, Chen J, Wang Y, Jeris C. A robust and scalable clustering algorithm for mixed type attributes in large database environment. Proceedings of the Seventh ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; San Francisco, CA: ACM; [Google Scholar]
- 63.Jamshidian M, Bentler PM. ML estimation of mean and covariance structures with missing data using complete data routines. J Educ Behav Stat. 1999;24(1):21–41. [Google Scholar]
- 64.Satorra A, Bentler PM. Corrections to test statistics and standard errors in covariance structure analysis. In: von Eye A, Clogg CC, editors. Latent variables analysis: Applications for developmental research. Thousand Oaks, CA: Sage Publications, Inc; 1994. pp. 399–419. [Google Scholar]
- 65.Yuan KH, Bentler PM. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol Methodol. 2000;30(30):165–200. [Google Scholar]
- 66.Hu L-T, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3(4):424–453. [Google Scholar]
- 67.Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 68.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 69.Steiger JH, Lind JC. Statistically based tests for the number of common factors. Paper presented at the Psychometric Society; Iowa City, IA: 1980. [Google Scholar]
- 70.Ahmad M, Rees R, Ali S. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noirot C, Leynadier F, Luce H, Abuaf N, Bernard PF. Hypereosinophilia in cancer and cirrhosis: utility of total eosinophil count. [French] Sem Hop. 1982;58:133–137. [PubMed] [Google Scholar]
- 72.Lucey D, Clerici M, Shearer G. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and infammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]