Abstract
Tuberculosis (TB) caused by Mycobacterium tuberculosis continues to be a leading cause of mortality among bacterial diseases, and the bacillus Calmette-Guerin (BCG) is the only licensed vaccine for human use against this disease. TB prevention and control would benefit from an improved method of BCG vaccination that simplifies logistics and eliminates dangers posed by hypodermic needles without compromising immunogenicity. Here, we report the design and engineering of a BCG-coated microneedle vaccine patch for a simple and improved intradermal delivery of the vaccine. The microneedle vaccine patch induced a robust cell-mediated immune response in both the lungs and spleen of guinea pigs. The response was comparable to the traditional hypodermic needle based intradermal BCG vaccination and was characterized by a strong antigen specific lymphocyte proliferation and IFN-γ levels with high frequencies of CD4+IFN-γ+, CD4+TNF-α+ and CD4+IFN-γ+TNF-α+ T cells. The BCG-coated microneedle vaccine patch was highly immunogenic in guinea pigs and supports further exploration of this new technology as a simpler, safer, and compliant vaccination that could facilitate increased coverage, especially in developing countries that lack adequate healthcare infrastructure.
Keywords: Tuberculosis, BCG, Microneedle patch, Hypodermic needle, Guinea pigs, Immune response
1. Introduction
The bacillus Calmette-Guerin (BCG) vaccine has been extensively used to prevent tuberculosis (TB) since its introduction nearly nine decades ago and still remains the only commercially available vaccine for human use against this scourge. BCG is a live attenuated vaccine that is derived from Mycobacterium bovis and has been recognized by the World Health Organization (WHO) for its effectiveness in preventing serious childhood complications of TB and averting many human deaths [1]. The BCG vaccine is administered as a part of childhood vaccination programs in more than 64 high-burden countries and is available in more than 167 countries.
Although the vaccine is one of the most widely used vaccines in human history, its effectiveness in preventing adult pulmonary disease is questionable. Many meta-analyses of clinical studies have estimated that the efficacy of the BCG vaccine in preventing adult pulmonary TB is highly variable, ranging from 0-80% depending on the geographical location [2, 3]. Multiple factors have been attributed for this variable efficacy, including the waning of immunity within 10-20 years of its administration as a neonatal vaccine [4].
Extensive research efforts are currently ongoing to develop novel vaccines that will boost childhood BCG vaccination later in adolescent or adult life [5, 6]. Alternatively, viral vectored or subunit vaccine prime followed by selective BCG boost has been proposed for infants of HIV-infected mothers [7]. New recombinant BCG strains with improved immunogenicity are under evaluation in clinical trials as a priming vaccine [8, 9]. These efforts demonstrate that BCG still remains at the center of new TB vaccination strategies, and the effective and technically accurate administration of BCG is required for the induction of optimal immunity and the success of the current TB vaccination strategies.
Currently, the BCG vaccine is administered to newborns or neonates by intradermal injection as per the recommendations of the WHO using a 1 ml tuberculin syringe attached to a 26 or 25-gauge, ½-inch hypodermic needle. This method is widely accepted and available for precise dose control. However, BCG vaccination is often limited by the difficulty of making a reliable and accurate intradermal injection and it requires skilled healthcare professionals [10, 11]. Improper injection technique, such as an accidental intramuscular injection, can deposit BCG vaccine to a poorly immunogenic space beyond the dermal layers [12]. Like other needle-based immunizations, intradermal BCG vaccination using hypodermic needle and syringe can be a deterrent to acceptance of immunization in children and parents due to needle phobia and the pain associated with the injection [13].
Accidental needle sticks in the neonates during mass vaccination is a serious problem. An estimated 5 accidental needle-stick injuries occur per 100 injections worldwide, posing a considerable risk to both vaccinee and healthcare provider [13, 14]. The reuse of needle and syringes, which is common in developing countries for reasons of cost, can result in transmission of blood-borne pathogens and disease. Safe disposal of syringes and needles also creates a substantial logistic and economic burden. Not surprisingly, development of simple and effective approaches for vaccination without using hypodermic needles has now been identified as an important goal in global healthcare [15] .
Needle-free transdermal patches have been reported, but the skin's outer layer (stratum corneum) must be disrupted for the delivery of live attenuated bacterial vaccines or large vaccine molecules [16]. Micron-scale needles assembled on a transdermal patch have been proposed as a hybrid between hypodermic needles and transdermal patches to overcome the individual limitations of both injections and patches [17, 18]. Not only are microneedles painless [19], but they also cannot be intentionally reused without specialized instrumentation to reload the microneedles with vaccine. In addition, the small size of microneedle patches should reduce the risk of accidental needle-stick injury and lessen the amount of biohazardous waste. Polymer microneedles that completely dissolve in the skin are also under development, which completely eliminate the biohazardous sharps waste [20].
To advance this concept, we and others have fabricated microneedles by adapting tools of the microelectronics industry to create micron-scale needles which are assembled into patches that pierce the outer barrier layer of the skin, stratum corneum and administer compounds with accuracy into the epidermal and dermal layers [20-26]. These layers are an important peripheral immune organ rich in potent immune-inducing cells, including Langerhans cells and dermal dendritic cells that have been shown to play an important role in antigen processing and presentation following intradermal immunization [27, 28].
In our earlier studies, we have demonstrated that an inactivated influenza vaccine can be efficiently delivered via skin using vaccine-coated solid microneedles and impart superior protection compared to intramuscular immunization in the mouse model [20, 24, 29]. Microneedle patches have been reported as minimally invasive and painless, are well tolerated in humans and can be administered with ease and without the help of skilled healthcare providers [19, 30].
In this study, we sought to develop solid microneedle patches as a simple, inexpensive and reliable delivery technology for BCG vaccination that could be used especially for mass neonatal vaccination in the developing countries that lack proper healthcare infrastructure. We evaluated the effectiveness of this approach in guinea pigs after a single application of BCG-coated microneedle vaccine patch. Our study demonstrates that the BCG vaccine can be efficiently delivered to the epidermis and dermis of the skin using a microneedle patch and induce a strong cell-mediated immune response comparable to that induced by traditional intradermal vaccination using hypodermic needle.
2. Materials and methods
2.1. BCG vaccine
The BCG vaccine (Danish strain 1331) manufactured by Statens Serum Institute (Copenhagen, Denmark) was provided as a lyophilized TB preclinical vaccine reference standard by the Center for Biologics Evaluation and Research, Food and Drug Administration (FDA) (Bethesda, MD, USA).
2.2. Microneedle vaccine patch preparation
Stainless steel microneedles were fabricated using laser cutting and electro-polishing technology as described previously [31]. To apply the BCG vaccine coating, microneedles were dipped repeatedly into coating solution and then air dried at 25 °C for 2 h. The vaccine coating solution was composed of 1% (w/v) carboxymethylcellulose sodium salt (low viscosity, USP grade, Carbo-Mer, San Diego, CA), 2% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ), 15% (w/v) D-(+)-trehalose dihydrate (Sigma Aldrich, St. Louis, MO) and 3.75 mg ml-1 BCG vaccine. All components of the coating solution were materials previously approved by the US FDA for use in other pharmaceutical formulations.
2.3. Quantification of BCG on microneedles
To determine the amount and viability of BCG coated on the microneedles, vaccine-coated microneedles were first incubated in sterile PBS for 12 h at 4 °C. To determine the mass of vaccine on the microneedles, reconstituted vaccine from 6 arrays of microneedles (total 30 individual microneedles) was assayed by BCA protein assay (Pierce Biotechnology, Rockford, IL, USA) to determine total protein content, which was converted to the absolute mass of vaccine using a calibration curve based on known concentrations of BCG.
The live colony forming units (CFUs) of the reconstituted vaccine from 18 arrays of microneedles (total 90 individual microneedles) from different batches was determined using intracellular ATP assay [32, 33]. The ATP assay was performed using ATP Bioluminescence Assay Kit HS II (Roche Applied Science, Mannheim, Germany) in accordance with the manufacturer's instructions. The microneedle-coated BCG vaccine's viability was further confirmed by culture of BCG bacilli from microneedle washings on Middelbrook 7H10 agar plates for 4 weeks at 37 °C and by counting the CFUs. The coated microneedles were stored in polyethylene plastic containers with desiccant packet of Tri-sorb (Süd-Chemie Performance Packaging, Colton, CA, USA) under refrigerated condition (4 °C) or at room temperature (25 °C) to check the shelf life.
2.4. Dissolution of BCG coating from microneedles and delivery into skin
Microneedles were imaged by bright-field stereo microscopy (Olympus SZX12, Center Valley, PA, USA) with a CCD camera (Leica DC 300, Leica Microsystems, Bannockburn, IL, USA). In some cases, the BCG vaccine was labeled by mixing 200 μl of BCG bacilli (3.75 mg ml-1) with 10 μl of octadecyl rhodamine B chloride (R18, Invitrogen, Carlsbad, CA, USA) and then incubating at 25 °C for 1 h. To remove unbound R18 molecules, BCG vaccine was washed with PBS several times. Guinea pig cadaver skin was pierced with labeled BCG-coated microneedles, frozen in OCT compound (Tissue- Tek, 4583, Sakura Finetek, Torrance, CA, USA), and cut into 10-μm thick sections using a cryostat. Histological examination of skin was performed on frozen sections.
The efficiency of delivery into skin was determined by comparing (i) the residual amount of fluorescently labeled BCG on the microneedle after skin insertion, (ii) the amount of BCG deposited on the skin surface and (iii) the amount initially coated. The amount of BCG deposited on the skin surface was evaluated by applying adhesive tape (Scotch tape, 3M, St. Paul, MN, USA) to the skin surface, immersing the tape in PBS, and determining the BCG amount using calibrated fluorescence spectroscopy (QM-1, Photon Technology International, South Brunswick, NJ, USA). Using a mass balance, the amount of BCG delivered into the skin was determined by subtracting the amount remaining on the microneedles and on the skin surface after insertion from the amount originally on non-inserted microneedles.
2.5. Guinea pigs vaccination
Female Hartley guinea pigs (three weeks old and weighing 200-250 g) were obtained from Charles River Laboratories (Wilmington, MA, USA) and housed at the Georgia Institute of Technology animal facility. All the animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committees (IACUC) of the Georgia Institute of Technology and the Centers for Disease Control and Prevention (Atlanta, GA, USA) using an approved animal protocol. Hair on the back of the guinea pigs was removed by a depilatory lotion (Nair, Church & Dwight, Princeton, NJ, USA) under isoflurane inhalation anesthesia.
The BCG-coated microneedle patch (5×104 CFUs of BCG) was administered using 10 microneedle arrays containing 5 microneedles each, stored under refrigeration for 1 day after coating. The patch was gently pressed into the skin and left in place for 10 min under isoflurane anesthesia for one group of guinea pigs. Another group of guinea pigs received intradermal BCG (5×104 CFUs) vaccine reconstituted in diluted Sauton's medium at two separate places on the back in the volume of 50 μl each using a 26 gauge needle and 1 ml tuberculin syringe. Sham immunized guinea pigs received a microneedle patch (10 microneedle arrays containing 5 microneedles each) coated only with the inert excipients (i.e., no BCG vaccine) inserted into skin on the back. Naïve (untreated) and sham immunized guinea pigs served as negative controls in the study.
2.6. Blood collection, tissue harvest and isolation of immune cells
Following vaccination, guinea pigs were bled by cardiac puncture under anesthesia and euthanized at different time points. Lungs were perfused via the right ventricle with PBS containing heparin (10 U ml-1) to remove intravascular leukocytes and subsequently with an enzyme mixture containing 1 mg ml-1 collagenase type IV (Sigma-Aldrich) and 25 U ml-1 DNase (Roche, Penzberg, Germany) in RPMI 1640 supplemented with 100 IU ml-1 penicillin, 50 μg ml-1 streptomycin, 1 mM L-glutamine, 25 mM HEPES, 1 mM sodium pyruvate, 5 × 10–5 M ß-mercaptoethanol, vitamins and nonessential amino acids (Gibco-Invitrogen, Grand Island, NY, USA) and 10% endotoxin-free heat-inactivated fetal calf serum (FCS; Atlas Biologicals, Fort Collins, CO, USA). Lungs were sliced into small pieces in a sterile dish and the fragments were incubated in the enzyme mixture at 37 °C for 1 h. The digested lung fragments were passed through a 70-μm pore size cell strainer (BD Falcon, Bedford, MA, USA) to obtain a single-cell suspension. The single cell suspensions of spleen were obtained by gently grinding the organ through a 70-μm cell strainer into supplemented RPMI. Erythrocytes from the cell suspensions were lysed with RBC lysis buffer (eBioscience, San Diego, CA, USA) for 4-5 min at room temperature. The lung and spleen cells were washed, recovered by centrifugation, and resuspended in supplemented RPMI for counting using an automated cell counter (Countess, Invitrogen) employing the trypan blue dye exclusion method.
2.7. In vitro lymphocyte proliferation assay
Lung and spleen cells from the vaccinated and control guinea pigs were labeled with carboxyfluoroscein succinimidyl ester (CFSE; 3.0 μM, Invitrogen, Carlsbad, CA, USA), and 2×106 cells/well were seeded in 24-well tissue culture plates in 600 μl of supplemented RPMI-1640 in the presence or absence of BCG whole-cell lysate (WCL) (10 μg ml-1). Phytohemagglutinin (PHA) (5 μg ml-1) was used as a positive control for cell viability and proliferation. The cells were incubated for 72 h at 37 °C in 5% CO2. Dilution of CFSE after 72 h was analyzed by flow cytometry.
2.8. IFN-γrelease assay
Lung and spleen cells from the vaccinated and control guinea pigs were seeded in 24-well tissue culture plates in supplemented RPMI-1640 (2×106 cells/well) in the presence or absence of BCG WCL (10μg ml-1). PHA (5 μg ml-1) was used as a positive control for IFN-γ production and cell viability. The cells were incubated for 96 h at 37 °C in 5% CO2. The culture supernatants were harvested, and IFN-γ levels were quantified using anti-guinea pig IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (Uscn Life Science Inc., Wuhan, China) as per manufacturer's instructions.
2.9. Flow cytometry
For intracellular cytokine staining of T lymphocytes, 2×106 cells from the lungs and spleen of vaccinated and control guinea pigs were stimulated with BCG WCL (10 μg ml-1) for a total of 16 h with the final 5 h of incubation in presence of 5μM brefeldin A and monensin according to the manufacturers instruction (BD Biosciences, San Jose, CA, USA) in supplemented RPMI-1640. After incubation, the cells were washed with FACS buffer (PBS containing 2% FBS) and permeabilized with phosflow Perm buffer II (BD Biosciences). The permeabilized cells were stained with mouse monoclonal anti-guinea pig TNF-α antibody (clone 29T14; Invitrogen) and incubated at room temperature for 30 min. After removing the unbound antibodies with FACS buffer, rat anti-mouse IgG1-PE (Clone X56; BD Biosciences) was added to the cells and incubated at room temperature for 30 min. This was followed by washing with FACS buffer and staining with a cocktail of pretitrated antibodies consisting of anti-guinea pig CD4-FITC (clone CT7; Serotech, Inc., Raleigh, NC, USA) or anti-guinea pig CD8-FITC (clone CT6; Serotech) [34, 35] and anti-IFN-γ-Alexa Fluor-647 (clone CC302; reactive against IFN-γ of multiple animal species [36]; Serotech). Cells were washed with FACS buffer, fixed with 0.5% paraformaldehyde and acquired using BD FACS Calibur system (BD Biosciences). FlowJo software (Tree Star, Inc., Ashland, OR, USA) was used to analyze the results.
2.10. Antibody response
M. bovis BCG WCL-specific IgG antibody levels in the serum of vaccinated and control animals were quantified by ELISA using anti-guinea pig IgG-HRP (1:1000; Sigma-Aldrich) following standard protocol [37]. The reaction was stopped after 15 min by adding 50 μl of 3 M H2SO4. Data are presented as the absorbance measured at 492 nm (A492) or as end-point titers. Titers are expressed as the reciprocal of highest serum dilution having a mean absorbance at 492 nm greater than the mean value + 3 standard deviations of naïve guinea pig serum samples.
2.11. Statistical analysis
The data obtained were analyzed using a Student's t-test and analysis of variance (ANOVA) by Prism Graph Pad software (GraphPad Sofware, La Jolla, CA, USA). A value of p-value < 0.05 was considered significant.
3. Results
3.1. Fabrication of microneedles and development of vaccine coating formulation
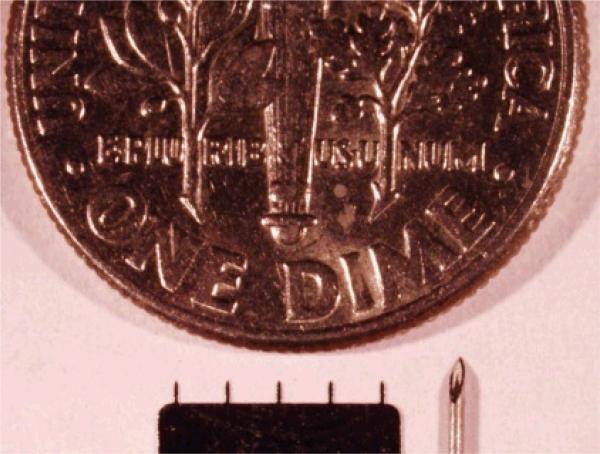
Microneedles fabricated from laser-cut stainless steel sheets were designed to be long enough to penetrate across the stratum corneum barrier and into the skin by gentle manual insertion, but short enough to avoid pain [19]. Each microneedle used for BCG coating was 700 μm long, 170 μm × 55 μm in cross section at the base and tapered toward the tip with a 5 μm radius of curvature. Fig 1 illustrates the size comparison between an array of 5 microneedles, the tip of a 26 gauge hypodermic needle and a U.S. dime coin.
Fig 1.
Size comparison between a microneedle array, the tip of a 26 gauge hypodermic needle, and a U.S. dime coin.
In practice, the BCG vaccine is injected in the liquid form to children. In contrast, microneedle-based vaccination involves coating BCG onto microneedles using a drying process, and represents vaccine delivery in a solid state. In our previously published studies, we showed that inactivated influenza vaccine or virus-like particles can be effectively coated on to microneedles by drying and be used for improved vaccination in the mouse model [24, 38-40]. However, BCG is a live vaccine and the viability of the bacilli in the coating formulation is critical for the induction of effective immune response. Therefore, we used our previously standardized formulation [24, 38, 40] containing carboxymethyl cellulose sodium salt as a viscosity enhancer to enable thicker coating on the microneedles, surfactant Lutrol F-68 NF to facilitate complete coating of the microneedle shaft by reducing surface tension, and a vaccine-stabilizing agent trehalose as a starting point to optimize the coating formulation for BCG vaccine and to preserve its activity and maximize the shelf-life.
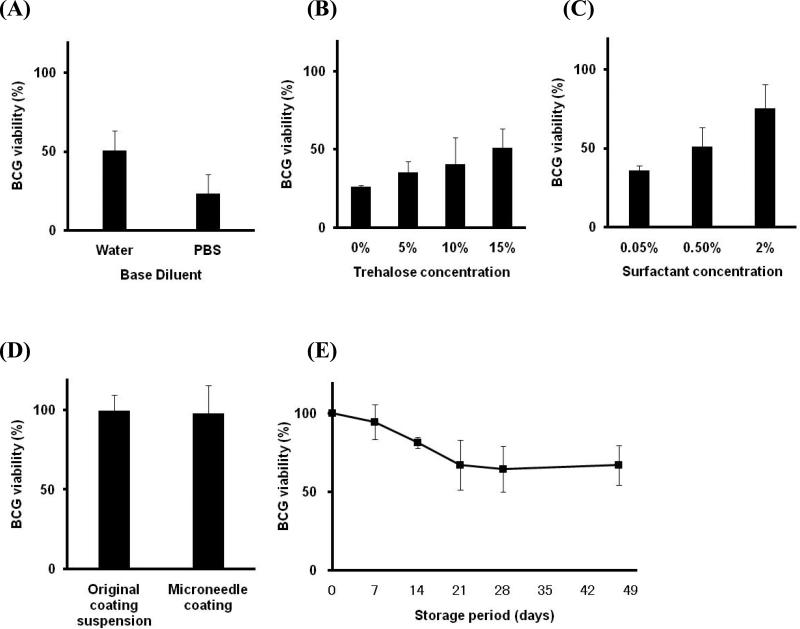
To optimize the formulation, we found that coating with a DI water-based formulation was better than PBS-based formulation for preserving the BCG viability after coating and drying on the stainless steel microneedle material surface and storage at 25 °C for 7 days (Fig. 2A, Student's t-test, p < 0.05). The BCG viability also increased with increasing concentration of trehalose (Fig. 2B, ANOVA, p < 0.05) and the surfactant (Fig. 2C, ANOVA, p < 0.01) used in the coating solution. This optimization process yielded a vaccine coating that retained 75 ± 15% viability after formulation, drying and storage for one week at room temperature and contained 0.375% (w/v) BCG, 1% (w/v) carboxymethylcellulose sodium salt, 2% (w/v) Lutrol F-68 NF and 15% (w/v) trehalose in water (Fig 2C, right most bar).
Fig 2.
BCG-coated microneedle formulation. BCG viability after coating onto microneedles is shown as a function of formulation components after 7 days storage at 25 °C: (A) DI water versus PBS in a base formulation of 1% (w/v) CMC, 0.5% (w/v) Lutrol F-68 NF, 15% (w/v) trehalose and 3.75 mg ml-1 BCG vaccine, (B) trehalose concentration in a base formulation of 1% (w/v) CMC, 0.5% (w/v) Lutrol F-68 NF and 3.75 mg ml-1 BCG vaccine in DI water and (C) surfactant (Lutrol F-68 NF) concentration in a base formulation of 1% (w/v) CMC, 15% (w/v) trehalose and 3.75 mg ml-1 BCG vaccine in DI water. The kinetics of BCG viability loss was also measured. (D) BCG viability measured in coating solution (1% (w/v) CMC, 2% (w/v) Lutrol F-68 NF, 15% (w/v) trehalose and 3.75 mg ml-1 BCG vaccine in DI water) and storage for 0.5 h after drying on microneedles. (E) BCG viability as a function of storage time at 4 °C for microneedles coated using a formulation of 1% (w/v) CMC, 2% (w/v) Lutrol F-68 NF, 15% (w/v) trehalose and 3.75 mg ml-1 BCG vaccine in DI water. BCG viability was determined by an ATP-based assay (see Methods section). All coatings were carried out using 9 dipping cycles. Data represent the average ± standard deviation of n = 3 measurements.
To better understand the kinetics of BCG viability loss and its possible shelf life, we next measured BCG viability immediately after microneedles were coated and dried. The coating formulation was not toxic to the bacilli and the drying process did not adversely affect BCG viability (Fig. 2D, Student's t-test, p = 0.86). Subsequent storage for one week at room temperature decreased the viability (78 ± 6.3% viable, Student's t-test, p < 0.01), whereas storage for one week under refrigeration (4 °C) had no significant effect (Fig. 2E, Student's t-test, p = 0.26). Continued storage under refrigeration resulted in a viability loss to a level of 67 ± 16% after 3 weeks (Fig. 2E, ANOVA, p < 0.01), but there was no additional loss during continued storage from week 3 through week 7 (Fig. 2E, ANOVA, p = 0.94).
3.2. BCG vaccine dissolution from microneedle coatings and delivery into skin
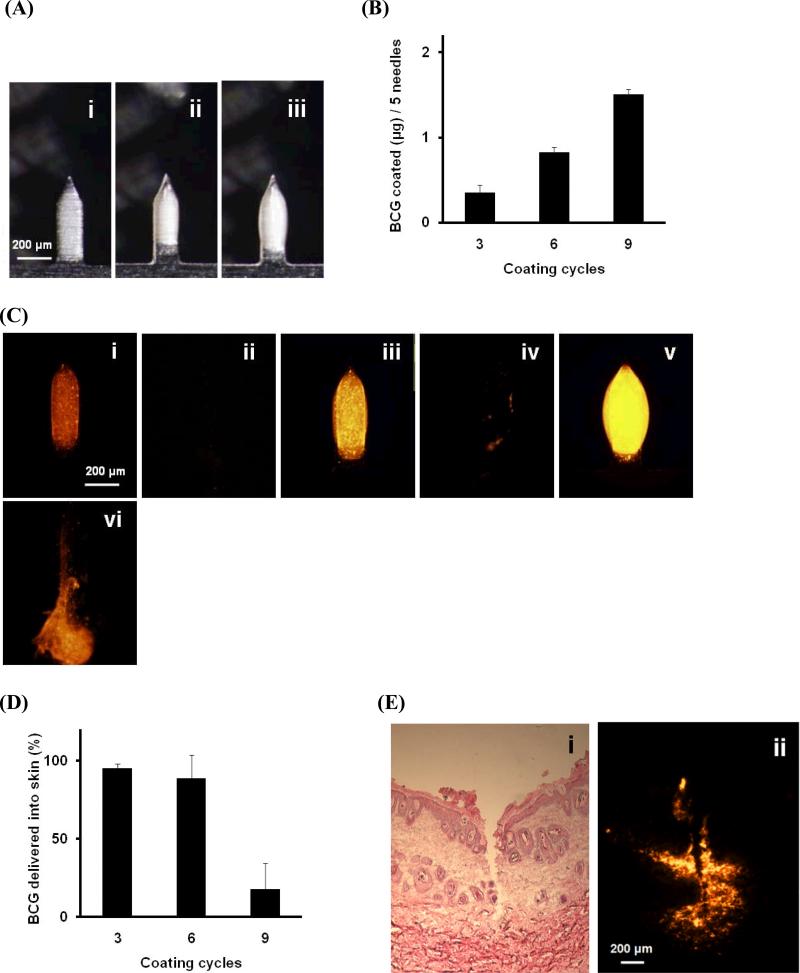
Bright-field micrographs of individual microneedles demonstrated that BCG vaccine can be successfully coated onto the solid metal microneedles, and the dip coating method produced a thick and uniform coating localized to the microneedle shafts (Fig. 3). The amount of BCG vaccine coated on the microneedles depended on the number of coating cycles, such that 3-9 cycles coated 0.4-1.5 μg of BCG per 5-needle array (Fig. 3A - 3B, ANOVA, p < 0.01).
Fig 3.
Coating and dissolution of BCG-coated microneedles. (A) Bright-field micrographs of individual microneedles coated with BCG vaccine as seen in white, bulky parts covering the microneedle surface. Coatings were prepared using (i) three (ii) six and (iii) nine coating cycles. (B) Mass of BCG coated per five-microneedle array as a function of the number of coating cycles using microneedles like those shown in part (A). (C) Fluorescence micrographs of individual microneedles coated with red-fluorescent BCG vaccine shown before (i, iii, v) and 10 min after (ii, iv, vi) insertion into guinea pig cadaver skin using microneedles coating using three (i, ii), six (iii, iv) and nine (v, vi) coating cycles. (D) Percent of BCG coating delivered into skin as a function of the number of coating cycles using microneedles like those shown in part (C). (E) Histologic section of guinea pig cadaver skin fixed after 10-min insertion of microneedles coated with BCG using six coating cycles imaged by (i) bright-field microscopy after H&E staining, which shows skin deformation and needle track across the epidermis and into superficial dermis and (ii) fluorescence microscopy of the frozen section before H&E staining, which shows deposition of red-fluorescent BCG vaccine coating in skin. All coatings were carried out using a formulation of (1% (w/v) CMC, 2% (w/v) Lutrol F-68 NF, 15% (w/v) trehalose and 3.75 mg ml-1 BCG vaccine in DI water). Data represent the average ± standard deviation of n = 3 measurements.
The vaccine delivery rate into guinea pig cadaver skin using microneedles coated with red-fluorescent BCG vaccine was also evaluated, and the fluorescence images of microneedles before and after insertion are shown in Fig. 3C. After insertion into skin for 10 min using microneedles coated with 3 and 6 coating cycles, almost all the BCG was released into the skin (Fig. 3D). However, in the case of microneedles coated 9 times, much of the BCG was pushed back from the needle shaft during insertion and remained at the base of the array (Fig. 3C(vi) and 3D), resulting in only 17% of the coated vaccine delivered into the skin.
Based on these results, we used microneedles prepared with 6 cycles that coated 0.83 μg of BCG per 5-needle array (Fig. 3B) for all subsequent experiments. Each animal was vaccinated using ten 5-needle arrays, which administered approximately 7.3 μg of BCG (i.e., 5×104 CFUs ) into the skin, based on an 88% delivery efficiency (Fig. 3D). Fig. 3E shows a representative histological section in guinea pig skin after the insertion and removal of a microneedle coated with fluorescently tagged BCG. The resulting needle track crosses the epidermis and into the superficial dermis and shows vaccine delivered in both of these two layers. Application of the coated microneedle vaccine patch on the guinea pig skin in vivo under anesthesia was found to be safe without any adverse side effects, inflammation or scaring.
3.3. Lymphocyte responses induced by microneedle vaccination
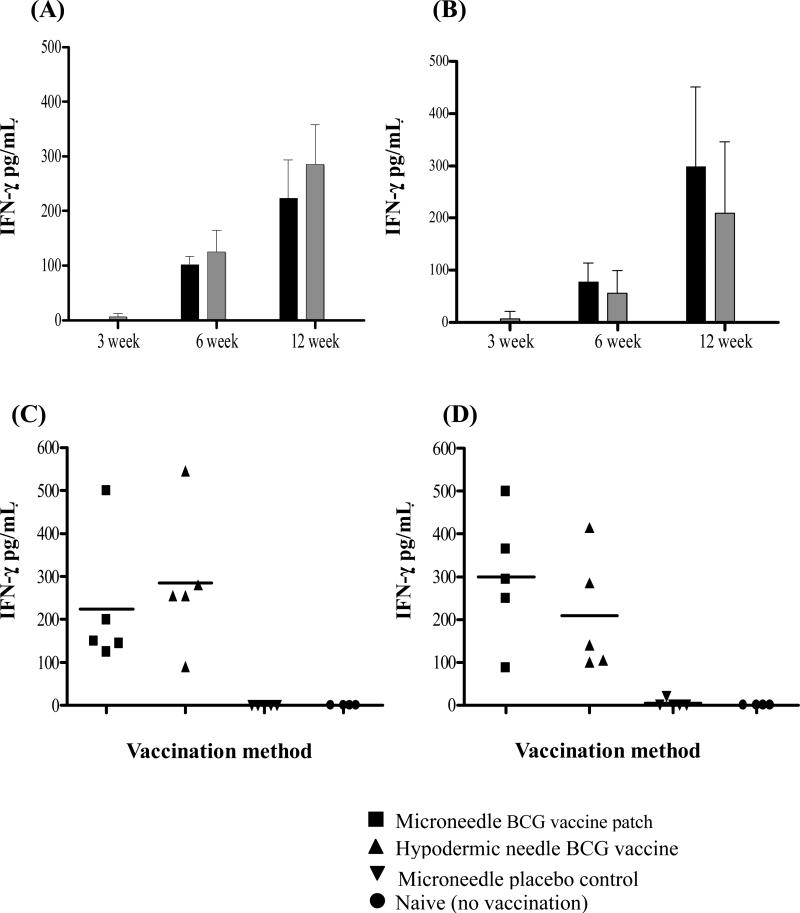
The ability of the BCG-coated microneedle vaccine patch to induce cell-mediated immune response was evaluated at 3, 6, and 12 weeks post vaccination in guinea pigs. The BCG WCL-specific in vitro IFN-γ levels induced by both the lungs and spleen cells of microneedle BCG vaccinated guinea pigs was maximum at 12 weeks (Figs. 4A and 4B, ANOVA, p < 0.05) and the levels were comparable to that induced by intradermal BCG vaccination using a traditional hypodermic needle (Figs. 4A and 4B, Student's t-test, p > 0.05). The IFN-γ response induced by both the types of vaccination was significantly higher than unvaccinated (naïve) and placebo microneedle vaccinated control guinea pigs (Figs. 4C and 4D, ANOVA, p < 0.01).
Fig 4.
Kinetics of IFN-γ responses of spleen (A) and lung (B) cells of BCG-vaccinated guinea pigs at 3, 6 and 12 weeks after vaccination. The black and gray bars depict the responses of two groups of guinea pigs vaccinated using either BCG-coated microneedle vaccine patch or by intradermal BCG administration using conventional hypodermic needle, respectively. Each bar represents the mean ± SD of IFN-γ levels in the culture supernatants of in vitro BCG-whole cell lysate (WCL) stimulated cell cultures of five guinea pigs per group as evaluated by IFN-γ ELISA and expressed as pg ml-1 levels after subtracting responses of respective unstimulated control wells. (C and D) The spleen(C) and lung(D) cell IFN-γ responses of microneedle BCG, hypodermic needle BCG, microneedle placebo control and naïve groups at the 12 week time point. The IFN-γ responses of both the BCG vaccinated groups were statistically significant (ANOVA, p < 0.01) when compared to the placebo microneedle vaccinated or naïve unvaccinated control guinea pigs. Each symbol represents the IFN-γ response of individual guinea pig and the solid horizontal bar represents the mean IFN-γ levels for each group at 12 weeks post vaccination.
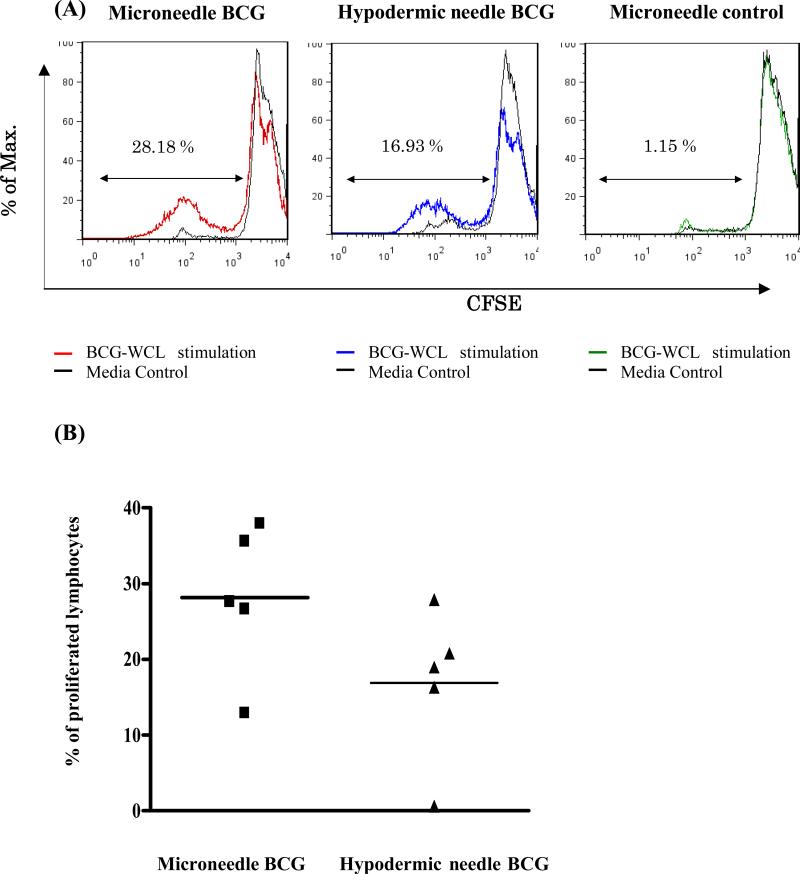
The BCG vaccination using microneedles also induced pronounced WCL-specific splenocyte proliferation comparable to that induced by hypodermic needle-based BCG vaccination of guinea pigs (Fig 5, Student's t-test, p > 0.05) as evaluated by CFSE dilution assay at 12 weeks post vaccination.
Fig 5.
Splenocyte proliferation responses induced by microneedle vaccine patch or hypodermic needle-based BCG vaccination of guinea pigs at 12 weeks post vaccination. (A) A representative FACS histogram showing lymphocyte proliferation after gating on the lymphocyte population based on the forward and side scatter. The histogram depicts WCL-specific in vitro proliferative responses of guinea pigs vaccinated either by BCG-coated microneedle vaccine patch, hypodermic needle injection or placebo microneedles as evaluated by flow cytometry-based CFSE dilution assay in comparison with their respective unstimulated controls (black line). The cells that have undergone the most division are the ones with the lower intensity of the CFSE stain (furthest to the left in the FACScan). (B) The WCL-specific proliferative responses of individual guinea pigs of two BCG vaccinated groups at 12 weeks post vaccination. Each symbol represents percentage of proliferated splenic lymphocytes of individual guinea pig after subtracting the values for unstimulated wells from the respective WCL-stimulated wells and the solid horizontal bar represents the mean lymphocyte proliferation response for each group. The percentage of WCL-specific proliferating splenic lymphocytes of placebo microneedle vaccinated group was 1.15 ± 0.5 % and the response was significantly lower than both the BCG vaccinated groups (ANOVA, p < 0.01).
3.4. Cytokine secreting T cells induced by microneedle vaccination
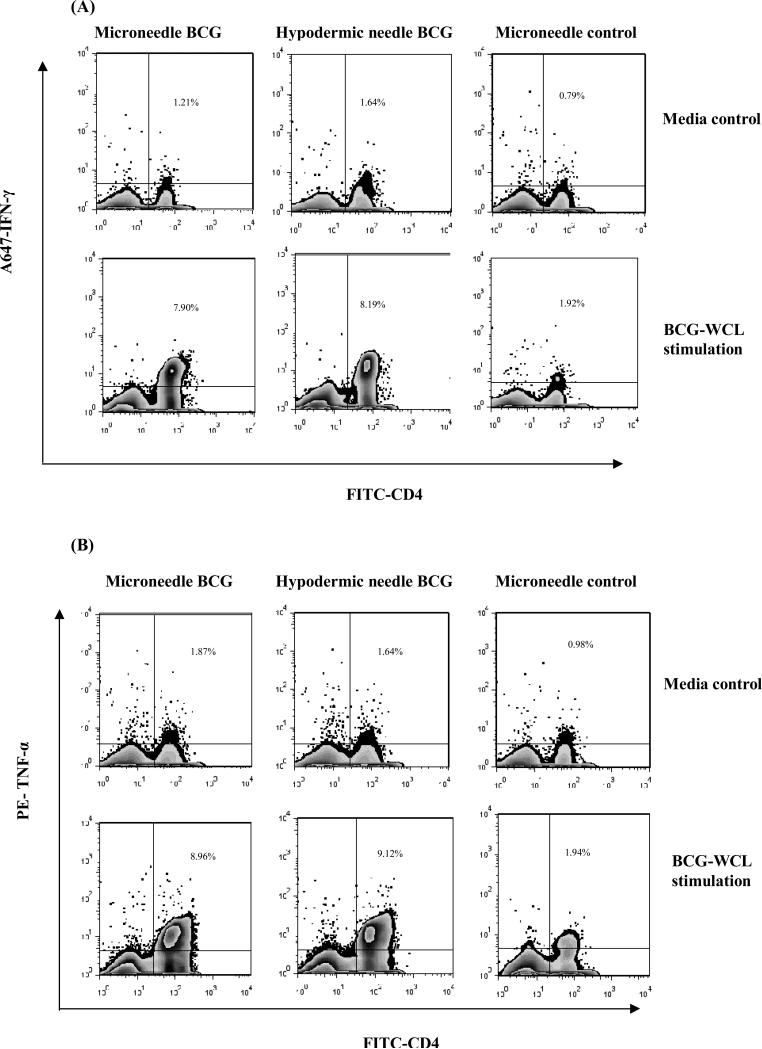
To identify the source of IFN-γ in the lungs and spleen, frequency of BCG WCL-specific IFN-γ secreting CD4+ and CD8+ T cells induced by BCG vaccine administered by microneedles or hypodermic needle was evaluated at 3, 6, and 12 weeks post vaccination by intracellular cytokine staining using flow cytometry. Both types of vaccination predominantly induced CD4+ T cell response, and the frequency of IFN-γ secreting CD4+ T cells in the spleen and lungs increased from 3 to 12 weeks (Figs. S1A and S1B, ANOVA, p < 0.05). However, both the modes of vaccination induced a low frequency of IFN-γ secreting CD8+ T cells in the spleen and lungs (Figs. S1C and S1D).
To further evaluate the quality and polyfunctionality of T cell response, the frequency of both IFN-γ and TNF-α co-producing or only IFN-γ or TNF-α secreting CD4+ T cells was evaluated at 12 weeks. The frequency of WCL-specific IFN-γ (Fig. 6A) or TNF-α (Fig. 6B) secreting splenic CD4+ T lymphocytes was not significantly different whether the BCG vaccine was administered via microneedle patch or hypodermic needle. Both the microneedles and hypodermic needle-administered BCG vaccine induced comparable levels of IFN-γ or/and TNF-α cytokine producing CD4+ T cell responses in the spleen and lungs (Fig. 6C and 6D, Student's t-test, p > 0.05). The percentage of WCL-specific IFN-γ+CD4+, TNF-α+CD4+ and INF-γ+TNF-α+CD4+ T lymphocytes of placebo microneedle vaccinated control group in the spleen and lungs was significantly lower than both the BCG vaccinated groups (ANOVA, p<0.01).
Fig 6.
The frequencies of intracellular IFN-γ or TNF-α cytokine-secreting CD4+ T lymphocytes induced by microneedle vaccine patch or hypodermic needle-based BCG vaccination of guinea pigs at 12 weeks post vaccination. The lymphocytes were gated based on forward and side scatter and the CD4 T cells were subsequently analyzed for the expression of IFN-γ or TNF-α. To obtain the frequency of double cytokine producing CD4 T cells, the cytokine plots were created after gating on CD4 T cells. Representative zebra plots of WCL-specific IFN-γ (A) or TNF-α (B) secreting splenic CD4+ T lymphocyte responses of guinea pigs vaccinated either by BCG-coated microneedle vaccine patch, hypodermic needle or placebo microneedles as evaluated by flow cytometry. The top panel represents the zebra plots of no-antigen stimulated (RPMI-1640 media alone) splenocytes while the bottom panel represents that of the BCG-WCL-stimulated splenocytes. (C and D) The WCL-specific intracellular cytokine secreting CD4+ T lymphocyte responses in the spleen (C) and lungs (D) of individual guinea pigs of the two BCG vaccinated groups at 12 weeks post vaccination. Each symbol represents percentage of WCL-specific either IFN-γ or TNF-α or both IFN-γ and TNF-α co-producing CD4+ T lymphocytes after subtracting the response in the unstimulated control. The solid horizontal bar represents the mean frequency of respective cytokine(s)-secreting cells of each group.
3.5. Antibody response induced by microneedle vaccination
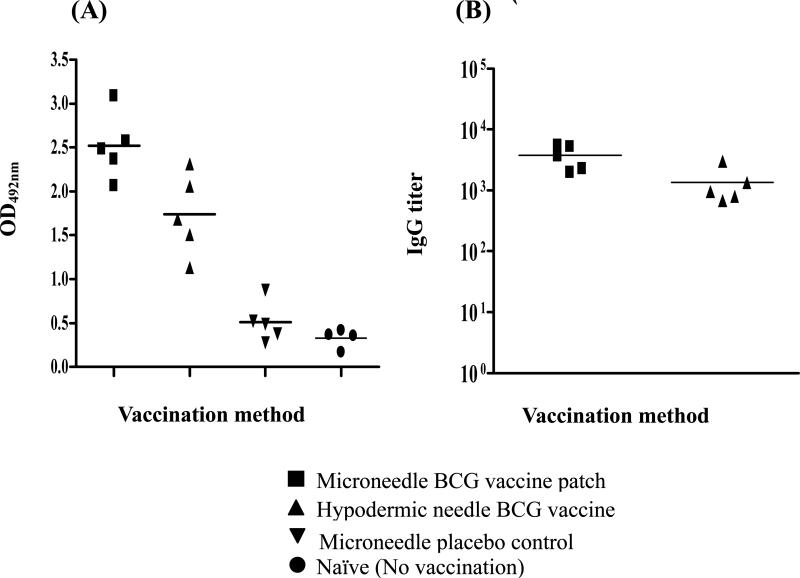
The BCG WCL-specific serum IgG levels induced by BCG vaccination administered by microneedles or hypodermic needle were estimated by ELISA and the kinetics of antibody levels induced at 3, 6 and 12 weeks post vaccination are shown in Fig S2. Both BCG vaccinated groups induced comparable IgG levels at 3 and 6 weeks (Fig. S2, Student's t-test, p > 0.05) when analyzed using 1:100 serum dilutions. At 12 weeks, the antibody response induced by microneedle BCG vaccination was higher than that induced by both hypodermic BCG vaccination (Fig. 7A, Student's t-test, p < 0.05) and the placebo microneedle vaccination (Fig. 7A, Student's t-test, p < 0.001). The 12th week post vaccination end point serum IgG titers were also higher in the microneedle BCG vaccinated group compared to the hypodermic needle vaccinated guinea pigs (Fig. 7B, Student's t-test, p < 0.05).
Fig 7.
Serum antibody (IgG) responses induced by microneedle vaccine patch or hypodermic needle-based BCG vaccination of guinea pigs at 12 weeks post vaccination. (A) The WCL-specific serum IgG levels of individual guinea pigs of the two BCG vaccinated groups at 12 weeks post vaccination as evaluated by ELISA. MaxiSorp plates were coated with 0.5 μg BCG-WCL/well and probed with 1:100 serum dilutions. Each symbol represents the mean absorbance of the test wells at 492nm after subtracting the values for no antigen coated control wells for each individual guinea pig serum and the solid horizontal bar represents the mean antibody response of each group. (B) The end point serum IgG titers of the guinea pigs of the two BCG vaccinated groups at 12 weeks post vaccination. The serum IgG titers of the placebo microneedle vaccinated control guinea pigs were significantly lower compared to microneedle and hypodermic needle-based BCG vaccinated guinea pigs. (ANOVA, p <0.05).
Overall, these results demonstrate that intradermal BCG vaccination using a microneedle vaccine patch was at least as immunogenic as hypodermic needle administration in the guinea pig model and induced comparable levels of cell-mediated immune responses during the 12-week time course of the study.
4. Discussion
Since its first use in humans in 1921, BCG has been administered by a variety of routes, including orally, intradermally by injection, and percutaneously using a multipuncture device. Originally, the BCG vaccine was administered by the oral route, however, this strategy was discontinued due to the loss of viability of the BCG by gastric passage (1-2 log) and frequent cervical adenopathy in children. BCG administration via intra-gastric or intra-rectal routes in animal models demonstrated that an enormous dose is required to achieve a level of protection equivalent to that of BCG vaccination via skin [41, 42]. Because TB is primarily a lung disease, direct pulmonary BCG vaccination has also been evaluated. Aerosolized BCG has been found to be safe in humans [43], however, its efficacy varied from superior protection compared to parenteral inoculation to no apparent advantage over the subcutaneous route in different studies with animal models [44-46].
Administration by intradermal route is the only method currently recommended by the WHO for BCG vaccination. Intradermal administration using hypodermic needle is considered to deliver a precise vaccine dose and induce higher rates of tuberculin skin test conversion and a better immune responses compared to other methods [47]. However, skin test conversion is a poor correlate of BCG-induced protection against TB. Furthermore, the use of hypodermic needles create many technical and safety issues and disposal challenges, and many health organizations are beginning to look for improved alternatives [13].
Therefore, this study aimed to evaluate the use of a simple BCG-coated microneedle vaccine patch to overcome the limitations of intradermal hypodermic needle injection, both in terms of targeting skin antigen presenting cells and avoiding use of hypodermic needles. We first designed and engineered the solid microneedles and developed the BCG coating formulation. The microneedle coating formulation was optimized based on our previously published work with inactivated influenza vaccine [20, 24, 29] to preserve the viability and shelf-life of the BCG vaccine.
The aqueous formulation was found to be significantly better than phosphate-buffered saline-based formulation in preserving BCG viability during coating and drying process. These observations are similar to the findings of Wong et al [48], who demonstrated that by drying bacteria such as BCG in salt-free suspensions, osmotic stresses can be minimized during drying and good activity and shelf-life stability can be achieved.
We also found that BCG viability depended on the concentration of stabilizing agent used in the microneedle coating suspension. In previous studies, we demonstrated that the addition of 15% trehalose to the coating solution significantly improved the retention of hemagglutination (HA) activity of microneedle-coated influenza vaccine up to 64% compared to an untreated vaccine [40]. In this study also we found that 15% trehalose has a better stabilizing effect on microneedle-coated live BCG vaccine. Of note, trehalose was found by others to impart better stabilizing effect compared to other cryoprotectants following freeze drying in a study evaluating encapsulation of BCG in alginate microparticles [49, 50].
The coating formulation and number of coating cycles optimized in this study provided uniform BCG coating on the microneedles and small 50-microneedle patch was sufficient to immunize with optimum dose. Although we applied 50 microneedles to the skin in this study using 10 five-needle arrays, which simplified application to the deformable skin of the guinea pig, the 50 microneedles could also be assembled onto a single patch, as we have done previously in human subjects [22]. The safety exams in guinea pigs showed that the BCG coated vaccine patch had no safety concerns and did not result in any untoward complications.
Of importance, our study demonstrates that BCG vaccine delivery using microneedles can induce robust antigen-specific cellular immune responses after a single application of a vaccine patch coated with a low dose of BCG (5×104 viable bacilli), comparable to those induced by traditional intradermal BCG vaccination using a 26-gauge hypodermic needle. The cell-mediated responses induced by BCG-coated microneedle vaccination are characterized by pronounced IFN-γ production both in terms of in vitro cytokine levels and frequencies of cytokine producing CD4+ T cells in the lungs and spleen and correlated with splenocyte proliferation. Of significance, the IFN-γ response is widely accepted as one of the correlates of protection against intracellular pathogens including M. tuberculosis [51]. In this study, IFN-γ response induced by both microneedle and hypodermic needle based BCG vaccination of guinea pigs increased from 3 to 12 weeks after vaccination. The similar kinetics of BCG-induced IFN-γ response was also observed in the mouse model after subcutaneous vaccination when analyzed by the ELISPOT assay (S. Sable et. al. unpublished observations) and correlated with IFN-γ mRNA kinetics reported after intradermal BCG vaccination of guinea pigs by conventional hypodermic needle [35].
Microneedle BCG vaccination also induced comparable frequencies of TNF-α secreting or both IFN-γ and TNF-α cytokine secreting bi-functional CD4+ T cells. These results are of particular importance because of the probable role of BCG induced polyfunctional CD4+ T-cells in the anti-mycobacterial immunity [52], and the requirement of IFN-γ and TNF-α cytokines produced by these cells in activating and enhancing the antibacterial activity of macrophages, which harbor mycobacteria [53]. However, the frequency of IFN-γ and TNF-α producing CD8+T cells induced by both microneedle and hypodermic needle administered BCG vaccination was found to be low in our study. Although it is accepted that CD8+ T cells play an important role by killing infected macropahges and possibly M. tuberculosis, BCG vaccination is known to induce low CD8+ T-cell responses [8].
The robust IgG antibody production by both the types of BCG vaccination in our study was also not surprising since live BCG vaccination has been shown to induce both cellular and humoral immune responses in humans [54]. As the magnitude and quality of BCG WCL-specific immune responses induced by both microneedle and hypodermic needle-based intradermal BCG vaccination was comparable in guinea pigs, it is tempting to speculate that the BCG-coated microneedle vaccine patch might be equally capable of imparting protection against M. tuberculosis challenge and will be investigated in the future.
This study thus provides proof of principle of the feasibility of a microneedle patch to administer live attenuated BCG vaccine without affecting its immunogenicity in the guinea pig model. With the increase in the number of immunizations that children around the world routinely receive, microneedle patch vaccination might provide a simpler, safer and relatively painless approach for childhood BCG vaccination with increased parental compliance. In addition, mass manufacturing of the microneedle patch itself is expected to cost less than a needle, syringe and vial. This means that the vaccine and its sterile processing will dominate cost, which is common to both conventional injectable formulations and microneedles. The microneedle vaccine patch also has the potential to simplify the logistics of BCG vaccination by reducing the burden on healthcare systems, in developing countries by facilitating storage, stockpiling and distribution for a wider child population coverage where patches can be distributed through various channels and applied without the help of skilled healthcare professionals. Overall, we conclude that microneedle patches can offer an effective means of BCG vaccination in the skin that simplifies vaccination logistics and thereby can increase vaccination coverage, especially in developing countries.
Supplementary Material
Acknowledgments
This work was carried out at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Biosciences at the Georgia Institute of Technology and the Mycobacteriology Laboratory Branch, DTBE, CDC. YH was supported by TAKEDA Pharmaceutical Company Ltd. and SN, by ORISE fellowship. We acknowledge the help of Ellen Kersh at the Division of HIV/AIDS Prevention, CDC with flow cytometry instrumentation, Vladimir Zarnitsyn at Georgia Tech for assistance with microneedle coating and Donna Bondy at Georgia Tech for administrative support. This work was supported by CDC funds to SBS and a grant from the Georgia Research Alliance to MRP. MRP serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
References
- 1.World Health Organization BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004 Jan 23;79(4):27–38. [PubMed] [Google Scholar]
- 2.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994 Mar 2;271(9):698–702. [PubMed] [Google Scholar]
- 3.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995 Nov 18;346(8986):1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005 Aug;3(8):656–62. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 5.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006 Jun;4(6):469–76. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 6.Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008 Jul 12;372(9633):164–75. doi: 10.1016/S0140-6736(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 7.Hatherill M, Mahomed H, Hanekom W. Novel vaccine prime and selective BCG boost: a new tuberculosis vaccine strategy for infants of HIV-infected mothers. Vaccine. 2010 Jun 23;28(29):4550–2. doi: 10.1016/j.vaccine.2010.04.088. [DOI] [PubMed] [Google Scholar]
- 8.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005 Sep;115(9):2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parida SK, Kaufmann SH. Novel tuberculosis vaccines on the horizon. Curr Opin Immunol. 2010 Jun;22(3):374–84. doi: 10.1016/j.coi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn PM, Shenep JL, Mao L, Crawford R, Williams BF, Williams BG. Influence of needle gauge in Mantoux skin testing. Chest. 1994 Nov;106(5):1463–5. doi: 10.1378/chest.106.5.1463. [DOI] [PubMed] [Google Scholar]
- 12.Pasteur MC, Hall DR. The effects of inadvertent intramuscular injection of BCG vaccine. Scand J Infect Dis. 2001;33(6):473–4. doi: 10.1080/00365540152029981. [DOI] [PubMed] [Google Scholar]
- 13.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005 Dec;5(12):905–16. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 14.WHO and UNICEF release first ever “State of the World's Vaccines” report. Indian Pediatr. 1996 Nov;33(11):978–81. [PubMed] [Google Scholar]
- 15.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004 Mar;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 16.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008 Nov;26(11):1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–93. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010 May;17(4):187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008 Sep;24(7):585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010 Jul 18; doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002 Jan;19(1):63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 22.Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, et al. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):18936–41. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Z, Verbaan FJ, Bivas-Benita M, Bungener L, Huckriede A, van den Berg DJ, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009 May 21;136(1):71–8. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009 May 12;106(19):7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010 Aug 16;28(36):5850–6. doi: 10.1016/j.vaccine.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 26.Prow TW, Chen X, Prow NA, Fernando GJ, Tan CS, Raphael AP, et al. Nanopatch-Targeted Skin Vaccination against West Nile Virus and Chikungunya Virus in Mice. Small. 2010 Aug 16;6(16):1776–84. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 27.Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF, et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005 Dec 15;175(12):7905–15. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 28.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–68. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 29.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010 Jan 15;201(2):190–8. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009 Feb;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 31.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007 Feb 12;117(2):227–37. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen SE, Hubrechts P, Klein BM, Haslov KR. Development and validation of an ATP method for rapid estimation of viable units in lyophilised BCG Danish 1331 vaccine. Biologicals. 2008 Sep;36(5):308–14. doi: 10.1016/j.biologicals.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Lundin A. Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Methods Enzymol. 2000;305:346–70. doi: 10.1016/s0076-6879(00)05499-9. [DOI] [PubMed] [Google Scholar]
- 34.Tan BT, Ekelaar F, Luirink J, Rimmelzwaan G, De Jonge AJ, Scheper RJ. Production of monoclonal antibodies defining guinea pig T-cell surface markers and a strain 13 Ia-like antigen: the value of immunohistological screening. Hybridoma. 1985;4(2):115–24. doi: 10.1089/hyb.1985.4.115. Summer. [DOI] [PubMed] [Google Scholar]
- 35.Grover A, Taylor J, Troudt J, Keyser A, Arnett K, Izzo L, et al. Kinetics of the immune response profile in guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect Immun. 2009 Nov;77(11):4837–46. doi: 10.1128/IAI.00704-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen LG, Castelruiz Y, Jacobsen S, Aasted B. Identification of monoclonal antibodies that cross-react with cytokines from different animal species. Vet Immunol Immunopathol. 2002 Sep 25;88(3-4):111–22. doi: 10.1016/s0165-2427(02)00139-3. [DOI] [PubMed] [Google Scholar]
- 37.Sable SB, Verma I, Khuller GK. Multicomponent antituberculous subunit vaccine based on immunodominant antigens of Mycobacterium tuberculosis. Vaccine. 2005 Jul 14;23(32):4175–84. doi: 10.1016/j.vaccine.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 38.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010 Mar 3;142(2):187–95. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010 Aug;84(15):7760–9. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4(9):e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Immunogenicity and protective capacity of Mycobacterium bovis BCG after oral or intragastric administration in mice. Vaccine. 2000 Jan 18;18(13):1186–95. doi: 10.1016/s0264-410x(99)00386-2. [DOI] [PubMed] [Google Scholar]
- 42.Abolhassani M, Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Mycobacterium bovis BCG induces similar immune responses and protection by rectal and parenteral immunization routes. Infect Immun. 2000 Oct;68(10):5657–62. doi: 10.1128/iai.68.10.5657-5662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garner FB, Meyer CA, White DS, Lipton A. Aerosol BCG treatment of carcinoma metastatic to the lung: a phase I study. Cancer. 1975 Apr;35(4):1088–94. doi: 10.1002/1097-0142(197504)35:4<1088::aid-cncr2820350411>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Barclay WR, Busey WM, Dalgard DW, Good RC, Janicki BW, Kasik JE, et al. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973 Mar;107(3):351–8. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 45.Lagranderie M, Ravisse P, Marchal G, Gheorghiu M, Balasubramanian V, Weigeshaus EH, et al. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuber Lung Dis. 1993 Feb;74(1):38–46. doi: 10.1016/0962-8479(93)90067-8. [DOI] [PubMed] [Google Scholar]
- 46.Palendira U, Bean AG, Feng CG, Britton WJ. Lymphocyte recruitment and protective efficacy against pulmonary mycobacterial infection are independent of the route of prior Mycobacterium bovis BCG immunization. Infect Immun. 2002 Mar;70(3):1410–6. doi: 10.1128/IAI.70.3.1410-1416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bricks LF. [Percutaneous or intradermal BCG vaccine?]. J Pediatr (Rio J) 2004 Mar-Apr;80(2):93–8. [PubMed] [Google Scholar]
- 48.Wong YL, Sampson S, Germishuizen WA, Goonesekera S, Caponetti G, Sadoff J, et al. Drying a tuberculosis vaccine without freezing. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2591–5. doi: 10.1073/pnas.0611430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esquisabel A, Hernandez RM, Igartua M, Gascon AR, Calvo B, Pedraz JL. Production of BCG alginate-PLL microcapsules by emulsification/internal gelation. J Microencapsul. 1997 Sep-Oct;14(5):627–38. doi: 10.3109/02652049709006815. [DOI] [PubMed] [Google Scholar]
- 50.Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995 Oct;61(10):3592–7. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agger EM, Andersen P. Tuberculosis subunit vaccine development: on the role of interferon-gamma. Vaccine. 2001 Mar 21;19(17-19):2298–302. doi: 10.1016/s0264-410x(00)00519-3. [DOI] [PubMed] [Google Scholar]
- 52.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 53.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beyazova U, Rota S, Cevheroglu C, Karsligil T. Humoral immune response in infants after BCG vaccination. Tuber Lung Dis. 1995 Jun;76(3):248–53. doi: 10.1016/s0962-8479(05)80013-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.