Abstract
Objective
To summarize the effects of the adipokine adiponectin on the reproductive endocrine system, from the hypothalamic-pituitary axis to the gonads and target tissues of the reproductive system.
Design
A Medline computer search was performed to identify relevant articles.
Setting
Research institution.
Intervention(s)
None.
Result(s)
Adiponectin is a hormone secreted by adipose tissue that acts to reduce insulin resistance and atherogenic damage, but it also exerts actions in other tissues. Adiponectin mediates its actions in the periphery mainly via two receptors, AdipoR1 and AdipoR2. Adiponectin receptors are present in many reproductive tissues, including the central nervous system, ovaries, oviduct, endometrium, and testes. Adiponectin influences gonadotropin release, normal pregnancy, and assisted reproduction outcomes.
Conclusion(s)
Adiponectin, a beneficial adipokine, represents a major link between obesity and reproduction. Higher levels of adiponectin are associated with improved menstrual function and better outcomes in assisted reproductive cycles.
Keywords: Adiponectin, hypothalamus, pituitary, gonads, reproduction, polycystic ovary syndrome, PCOS, pregnancy, embryo development, assisted reproduction
Obesity has reached epidemic proportions in developing and developed countries. According to the practice committee of the American Society for Reproductive Medicine, obesity is the most common chronic disease in the U.S. (1). Despite worldwide awareness and international campaigns, the incidence of obesity is increasing, and obesity has been described as the new worldwide epidemic (2). In 2009, the American Obesity Association reported that obesity affects nearly one-third of the adult American population, ~60 million individuals. Today, 64.5% of adult Americans (~127 million) are categorized as being overweight or obese. Each year, obesity causes at least 300,000 excess deaths in the U.S., and health care costs of American adults with obesity amount to ~$100 billion.
An understanding of adipose tissue and its functions is therefore of special interest to identify mechanisms that account for the metabolic consequences of obesity. Among the main endocrine products of the adipose tissue are the proteins leptin, adiponectin, and resistin. Leptin, first described over a decade ago (3), has been thoroughly studied regarding reproduction and at several levels of the reproductive axis, from the pituitary to end organs (4–6). In contrast, few reports have summarized the effects of adiponectin on the reproductive organs, either at a molecular level or for clinical relevance. The present review focuses on the role of adiponectin as a hormone in order to clarify its role in reproduction.
ADIPOSE TISSUE
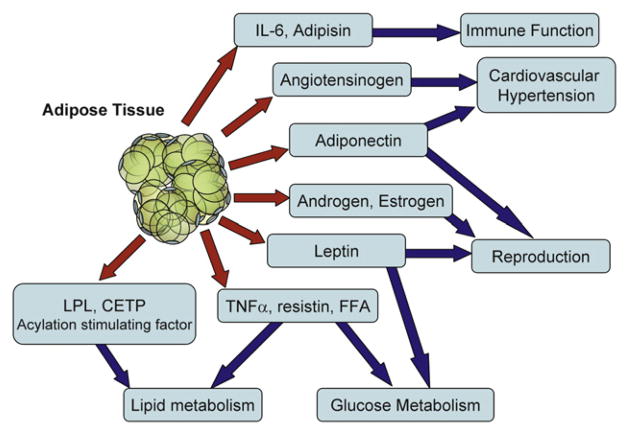
To meet metabolic demands and needs of energy expenditure, adipose tissue secretes several proteins and bioactive peptides—adipokines—(Table 1) that act either locally (paracrine or autocrine action) or systemically (endocrine action) (7). Adipose tissue is considered to be a true endocrine gland (8). Many of these molecules directly or indirectly influence metabolism, cardiovascular function, immunity or reproduction (Fig. 1). An excellent example of the importance of adipose tissue in reproduction is its ability to convert androgens to estrogens, owing to the presence of P450 aromatase in adipose tissue.
TABLE 1.
Products of the adipose tissue.
| Hormones | Leptin, resistin, adiponectin, estrogens |
| Cytokines | Interleukin-6, tumor necrosis factor alpha |
| Extracellular proteins | Fibronectin, laminin, collagen |
| Enzymes | 17β-Hydroxy steroid dehydrogenase (HSD), 11β-HSD 1, p450 aromatase, lipoprotein lipase |
| Renin-aldosterone system proteins | Renin, angiotensinogen I, angiotensinogen II |
| Acute-phase proteins | Haptoglobin |
| Complement factors | Adipsyn, complement C3 |
FIGURE 1.
Adipose tissue functions as an endocrine gland. Adipose tissue secretes proteins and lipids and metabolizes hormones that regulate not only energy homeostasis, but also influence reproduction, immune function, and cardiovascular disease and hypertension. CETP = cholesteryl ester transfer protein; FFA = free fatty acids; IL = interleukin; LPL = lipoprotein lipase; TNF = tumor necrosis factor.
Adipose tissue is primarily composed of lipid-laden adipocytes surrounded by loose connective tissue. In humans, most fat is white adipose tissue, in contrast to animals, which have brown adipose tissue (9). The abundant adipocytes serve as triglyceride storage (9) and are surrounded by a network of collagen fibers, vascular elements, fibroblasts, and immune system cells. The metabolic role of adipose tissue is as an energy storage compartment. In cases of prolonged fasting, lipolysis of adipose tissue promotes fatty acid delivery to be used by muscle, liver, and kidneys. Conversely, a glucose load stimulates lipogenesis through insulin (9), and thus adipose tissue maintains energy homeostasis. Many of the metabolic consequences of obesity are caused by altered secretion of adipokines.
ADIPONECTIN
Structure and Expression
Adiponectin is a member of the adipose-secreted proteins called adipocytokines (Fig. 2). Adiponectin was first described in 1995 as adipocyte complement-related protein of 30 kDa (Acrp30) (10). Adiponectin was discovered independently by several laboratories, thus its various names: Acrp30, adipose most abundant gene transcript 1 (apM1), adipose-specific gene adipoQ (AdipoQ), and gelatin-binding protein of 28 kDa (GBP28). Adiponectin is a 244–amino acid protein (molecular weight 30,000 Da) derived exclusively from adipocytes in white adipose tissue (6). Adiponectin is the protein product of the APM1 gene transcript, which is located at chromosome 3q27, close to the locus responsible for type II diabetes and adiposity (11, 12).
FIGURE 2.
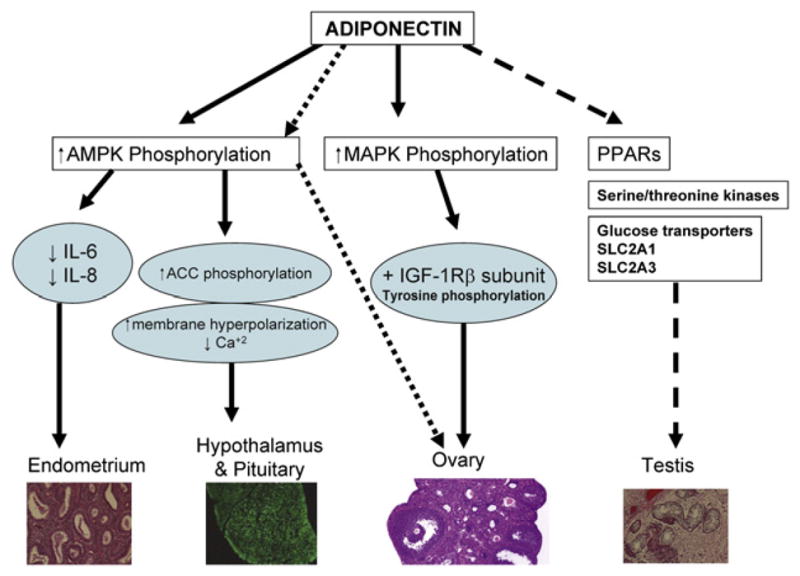
Adiponectin acts through the indicated pathways to influence organs involved in male and female reproduction. ACC = acetyl CoA carboxylase; AMPK = adenosine monophosphate–activated protein kinase; IGF = insulin-like growth factor; IL = interleukin; MAPK = mitogen-activated protein kinase; PPAR = peroxisome proliferator–activated receptor. SLC2A1 (GLUT1) = soluble carrier family 2 (facilitated glucose transporter) member 1; SLC2A3 (GLUT3) = soluble carrier family 2 (facilitated glucose transporter) member 3.
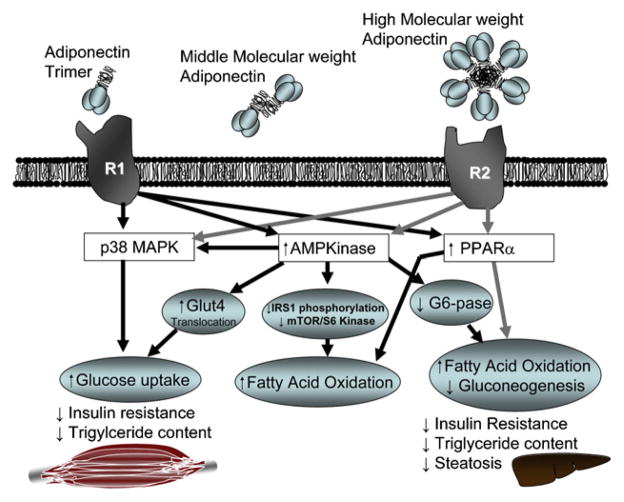
Of note, adiponectin is one of the most abundantly secreted adipokines and corresponds to 0.05% of the serum proteins (13). In fact, serum levels are 3–30 μg/mL in humans and 3–6 μg/mL in rodents (14). Adiponectin is related to the complement 1q family and contains a carboxyl-terminal globular domain and an amino-terminal collagenous domain. Adiponectin is secreted by adipose tissue (Fig. 3) in the form of a trimer as a low-molecular-weight (LMW), a combination of two trimers as a middle-molecular-weight (MMW), or as six trimers as a high-molecular-weight (HMW) form (15) and circulates either as a trimer or an oligomer (16–18). Adiponectin is post-translationally modified by hydroxylation and glycosylation, and circulating levels in mammals have been shown to be differentially glycosylated, with approximate molecular weights of >250,000, 180,000, and 90,000 Da, which correspond to HMW, MMW, and LMW forms of adiponectin (18). Similarly to leptin, adiponectin levels are lower in men (19, 20), obese individuals, individuals with diabetes, and those with coronary artery disease (14, 21, 22). Adiponectin mediates its action in the periphery principally through two receptors, AdipoR1 and AdipoR2 (23), although a third receptor, t-cadherin, has also been identified (24).
FIGURE 3.
Molecular structure of adiponectin and its receptors AdipoR1 and AdipoR2 and the indicated signaling pathways in muscle, adipose tissue, and liver. Adiponectin occurs in three molecular forms with globular and coiled domains: trimeric (low molecular weight), hexameric (middle molecular weight), or multimeric (high molecular weight. Two trimers associate to form a disulfide-linked hexamer (MMW). The HMW complex consists of 12–18 monomers and is most active in suppressing hepatic glucose production. The trimeric form is most potent for induction of AMPK activation and fatty acid β-oxidation in skeletal muscles. G6-pase = glucose-6-phosphatase; Glut = glucose transporter; IRS = insulin receptor substrate; mTOR= mammalian target of rapamycin; other abbreviations as in Figure 2.
AdipoR1 and AdipoR2 receptors are 7-transmembrane receptors that belong to a recently discovered family of 11 progestin AdipoQ receptors (PAQRs), but they are structurally and functionally distinct from G-protein–coupled receptors (25). AdipoR1 and AdipoR2 are also known as PAQR1 and PAQR2, respectively. Both AdipoR1 and AdiopR2 bind adiponectin with an EC50 in the range of 0.7–2.4 pmol/L (26). Related PAQR family members PARQ5 (also known as membrane progesterone receptor gamma [mPRγ]), PAQR 7 (mPRα), and PAQR8 (mPRβ) exhibit strong binding to progesterone (EC50 1–3 nmol/L in yeast), show a similar pattern of activation to known membrane-associated progestin effects, and transduce progestin-dependent signals (26). For example, PAQR5, PAQR7, and PAQR8 are activated by 17α-hydroxyprogesterone, an agonist that is known to activate membrane progesterone, but not the nuclear progesterone receptor. Thus, PAQR receptors closely related to AdipoR1 and AdipoR2 (25, 26) may have an important role in reproduction, although incontrovertible evidence for the in vivo role of PAQR5, PAQR7, and PAQR8 in progesterone action would require targeted deletion in mice or a similar approach.
Adiponectin functions as an agonist of PAQR3, a previously incompletely characterized member of the newly discovered PAQRs. Therefore, PAQR3 could potentially be a newly discovered target receptor of adiponectin in the PAQR family. AdipoR1 receptor is expressed mainly in skeletal muscle, activates the adenosine monophosphate-activated protein kinase (AMPK) pathway, and shows a high affinity to globular adiponectin and low affinity for full-length adiponectin (Fig. 3). In contrast, AdipoR2 receptor is enriched in liver and acts through peroxisome proliferator–activated receptor alpha (PPARα) pathway. Both AdipoR1 and AdipoR2 show affinity for both molecular forms of adiponectin. Although studies have failed to demonstrate a blood-brain transport of adiponectin, both AdipoR1 and AdipoR2 are widely distributed in the brain (see below).
Actions of adiponectin are mediated through activation of AMPK, leading to inhibition of acetyl coenzyme A carboxylase and an increased fatty acid beta-oxidation (27). Activation of AMPK acts to regulate energy homeostasis of the cell via fatty acid oxidation and glucose uptake stimulation. Adiponectin is markedly reduced in obesity and rises with prolonged fasting and severe weight reduction.
Adiponectin functions as an insulin-sensitizing agent by reducing hepatic glucose production and enhancing insulin action in the liver (28, 29). Studies have shown that adiponectin lowers levels of glucose, free fatty acids, and triglycerides in vivo (30–32) and increases fatty acid oxidation in the liver via a reduction in CD36 expression, thus reducing fatty acid influx and liver triglycerides (33). Adiponectin levels negatively correlate with levels of fasting insulin, glucose, and triglyceride (21), and the administration of insulin sensitizers significantly increase serum adiponectin levels in insulin-resistant humans (34). Adiponectin, particularly HMW adiponectin, is increased by thiazolidinediones (TZDs) and mediates the insulin-sensitizing effect of this class of antidiabetic drug (35). Adiponectin levels are low in insulin-resistant states and increase in insulin-sensitive states, such as with weight loss or treatment with thiazolidinediones (21). Further evidence for a key role for adiponectin in glucose homeostasis is the hepatic insulin resistance found in rodents and humans lacking adiponectin. It is important to mention that in vivo studies in humans are associative and that more direct evidence for the importance of adiponectin is provided by experiments in animals. In contrast, adiponectin treatment enhances insulin sensitivity, primarily by suppressing glucose production. High adiponectin levels may protect the cardiovascular system and reduce the incidence of myocardial infarction (36, 37) while accelerating endothelial renewal (38).
In addition to its effects on insulin sensitivity, adiponectin increases lipoprotein lipase and lowers lipid levels (39, 40), increases nitric oxide production in endothelial cells and induces angiogenesis (41), and mediates antiinflammatory (42) and anti-atherogenic actions (43). It was recently reported that adiponectin levels were low in patients with breast cancer (44), endometrial cancer (45), gastrointestinal cancer (46, 47), and prostate cancer. (48). Therefore, adiponectin acts as a hormone to fine-tune energy homeostasis involving food intake and the catabolism of carbohydrate and lipid (49) and may offer an interesting alternative treatment for type II diabetes, obesity, and metabolic disturbances (2).
Adiponectin in the Hypothalamus and Pituitary
Both AdipoR1 and AdipoR2 are expressed in human pituitary (Table 2), suggesting local modulation of the central reproductive endocrine axis by adiponectin (50). Although earlier studies (51) reported that adiponectin did not cross the blood-brain barrier, subsequent studies (50) showed that AdipoR1 was expressed in the hypothalamus and nucleus basalis of Meynert. In 2008, Wen et al. (52) reported that both adiponectin receptors were expressed in arcuate and lateral hypothalamic nuclei. Moreover, the expression and regulation of multiple adipose-related hormones (including adiponectin) in the central nervous system and pituitary gland was described (53).
TABLE 2.
Adiponectin and its receptors’ molecular actions in the various organs of the reproductive system.
| AdipoR1 expression | AdipoR2 expression | Receptor mRNA (PCR) | Mode of action | Endocrine effects | Immunoreactivity | |
|---|---|---|---|---|---|---|
| Hypothalamus neurons | + | + | AMPK JAK2–STAT354 | ↓GnRH-driven LH release52 |
+ | |
| Nucleus basalis of Meynert | +50 | +50 | ||||
| Pituitary | +50 | +50 | AMPK54 | ↓LH54 ↓GH54 |
+ | |
| Ovaries | +59 | +59 | +59 | ↓HR ↓CYP11A1 ↓CYP17A159 |
+59 | |
| Theca/granulosa cells | +66 | +66 | +60 | AMPK PPARγ66 | ↓P ↓ A68 |
|
| Oviduct | + in rats85 | |||||
| Glandular endometrium | +87 | +87 | AMPK87 | +87 | ||
| Stromal fibroblasts | +87 | +87 | AMPK87 | |||
| Testis | +92 | +92 | + (rats, chicken Leydig cells)92,93 | ↓T | + in chicken93 | |
| Trophoblast-placenta | +102 | +102 | ||||
| Cyncytiotrophoblast-cytotrophoblast | + | + | PPARγ | +103 |
Note: Superscript numbers correspond to references in text. CYP = cytochrome P; JAK = Janus kinases; STAT = signal transducers and activators of transcription; AMPK = adenosine monophosphate–activated protein kinase; PPAR = peroxisome proliferator-activated receptor; LHR = LH receptor.
Adiponectin has also been shown to regulate hormone secretion and gene expression in two critical endocrine cell types of the pituitary involved in reproduction: somatotrophs and gonadotrophs. Adiponectin inhibited LH and GH release as well as both ghrelin-induced GH release and GnRH-stimulated LH secretion in short-term (4 h) GT1–7 cells derived from GnRH neurons. Adiponectin inhibited GnRH secretion via activation of AMPK (52). These results were supported by Lu et al. (54), who reported that adiponectin acutely reduced basal and GnRH-stimulated LH secretion through increased phosphorylation of AMPK but had no impact on FSH levels. At the level of the hypothalamus, adiponectin also influenced oxytocin-secreting neuron excitability, perhaps explaining increased oxytocin secretion in the obese population (55). Furthermore, adiponectin increased GHRH-R and GH secretagogue–R mRNA content, which are the two main stimulatory receptors in somatotrophs (56). Taken together, these results suggest a role for adiponectin as a link between adiposity and reproduction. Specifically, low levels of adiponectin may contribute to chronically elevated LH levels.
Adiponectin and the Gonadal Organs
Ovaries
The effects of adipokines on the process of ovulation and ovarian steroidogenesis have not been extensively studied (57). Although leptin levels have been correlated with progesterone levels throughout the menstrual cycle, Lord et al. (58) were the first to demonstrate that pig ovaries and ovarian follicles expressed AdipoR1 and AdipoR2. Subsequent studies confirmed expression of these receptors in the human ovary (59). The isoforms of adiponectin (trimer, hexamer, and HMW) were present in both porcine and human follicular fluid at concentrations equivalent to serum concentrations (60, 61).
Page et al. (62), as well as Lanfranco et al. (63), showed that increased T levels in humans were inversely correlated with circulating adiponectin levels, and a similar inverse relationship has been observed in mice (64). Ledoux et al. (60) found that AdipoR1 and AdipoR2 mRNA was present in theca and granulosa cells, and that adiponectin receptors (AdipoR1, AdipoR2) were detected in human granulosa cells and mediated adiponectin action for increased production of P and E2 by insulin-like growth factor (IGF) I (65). Dupont et al. (66) highlighted the expression of PPARs and AMPK (the main adiponectin mediators) in the ovary.
In 2008, Gutman et al. (67) first demonstrated the in vivo induction of adiponectin by gonadotropins in the human ovary after treatment with recombinant LH (Table 2). The addition of recombinant LH during the late follicular phase may enhance follicular insulin sensitivity, resulting in reduced androgen levels through a cascade mediated by increased production of adiponectin. Lagaly et al. (68) showed that adiponectin inhibited P- and LH-dependent A production and insulin in theca cells in vitro. This was accompanied by reduction of LHr, Cyp11a1, and Cyp17a1 transcripts in theca cells; thus adiponectin reduced theca cell steroidogenesis. However, adiponectin did not affect insulin-induced proliferation of theca cells from large follicles or granulosa cell function. In addition, LH was found to increase AdipoR2 mRNA in theca cells, but not granulosa cells (68). Thus, adiponectin can directly induce gene expression in theca cells, which has potential relevance to the pathophysiology of polycystic ovary syndrome (PCOS; see below). The synergistic role of adiponectin with insulin or IGF-I is consistent with the insulin-sensitizing role of adiponectin.
Anovulation (PCOS)
Polycystic ovary syndrome is characterized by the presence of clinical or biochemical hyperandrogenism, chronic anovulation, and polycystic ovaries (69) and is frequently associated with insulin resistance. Obesity and insulin resistance are accompanied by a decrease in SHBG and an increase in the free androgen index (70). Considering the insulin-sensitizing actions (71–74) of adiponectin, its lower levels in obesity (75), and the fact that adiponectin levels were reduced by T (76), it is reasonable to postulate that adiponectin levels would be low in women with PCOS. Several studies have addressed this point (Table 3). Some studies (77, 78) observed no difference in adiponectin levels between PCOS women and normal weight-matched control subjects, whereas other studies showed lower adiponectin levels in PCOS women (79, 80). Meta-analyses of these studies, in combination with the homeostasis model assessment of insulin resistance (HOMA-IR), supported the conclusion that adiponectin levels were lower in women with PCOS (81). According to one meta-analysis (81), adiponectin levels were lower in PCOS women compared with healthy control subjects of a similar body mass index (BMI). Furthermore, adiponectin levels were lower in obese PCOS women compared with non-PCOS obese women. Adiponectin levels were related to insulin sensitivity: The more insulin-resistant patients had lower adiponectin levels (81). As mentioned above, it is possible that lower levels of adiponectin may contribute to the increased levels of LH observed in some women with PCOS.
TABLE 3.
Adiponectin in disease and clinical disorders.
| Organ | Hormone interactions | Related diseases |
|---|---|---|
| Hypothalamus and pituitary | LH (via GnRH) inhibition of release52 | Obesity: increased oxytocin55 |
| Control of oxytocin-secreting neurons55 | ||
| GH (via ghrelin) inhibition of release56 | ||
| Ovary | Induction of adiponectin by gonadotropins (recombinant LH)67 | PCOS: ↓ adiponectin in anovulatory PCOS women83; no negative correlation between adiponectin and androgens81; inverse correlation with CHOL, Trigl, Glu, and DBP82 |
| ↓Androgen levels as a result of increased production of adiponectin68 | ||
| Inhibition of P and A production68 | ||
| Endometrium | Increased adiponectin expression in the midluteal phase (implantation)87 | Endometriosis: Y adiponectin87 |
| Adhesion scores: inverse correlation with adiponectin87 | ||
| Leiomyomas: ↓ adiponectin89 | ||
| Endometrial cancer: ↓ adiponectin87 | ||
| Placenta pregnancy | Placental contribution to maternal adiponectin105 ↓ estrogens = ↓ adiponectin113 | Preeclampsia: ↓ first-trimester adiponectin105 |
| GDM: ↓ adiponectin (pregnancy and postpartum)114 | ||
| Fetus | Fetal source of adiponectin116 | Fetuses large for placental weight: ↓ cord adiponectin118 |
| Offspring BMI: ↓ cord adiponectin levels relate to more pronounced weight gain in first6 mo and increased BMI in 3 y119 | ||
| IUGR pregnancies: ↓ adiponectin121 |
Note: Superscript numbers correspond to references in text. BMI = body mass index; CHOL = total cholesterol; DBP = diastolic blood pressure; GDM = gestational diabetes mellitus; Glu = glucose; IUGR = intrauterine growth restriction; PCOS = polycystic ovary syndrome; Trigl = triglycerides.
In women without PCOS, adiponectin has been negatively correlated to T (76). In women with PCOS, adiponectin levels were also negatively related to T levels (82) and to free androgen index (83), cholesterol, triglycerides, glucose levels, and diastolic blood pressure (82). Because adiponectin levels are related to insulin resistance, low adiponectin levels can not be considered to be characteristic of PCOS. Instead, the alterations relate to the pathophysiology of insulin resistance, and for this reason measurement of adiponectin in a patient with PCOS is not indicated. Insulin and glucose levels—which are often measured in PCOS—in combination with BMI correlate as expected with adiponectin levels (81).
Oviduct
Though far less studied, adiponectin may play a role in oviductal function. The oviduct has been found to produce soluble factors that influence reproduction and possibly fetal development. The oviductal fluid consists of glycoproteins, protease inhibitors, regulatory molecules, cytokines, growth factors, cytokines, enzyme-binding proteins, and immunoglobulins (84). Arcancho et al. (85) were the first to show that the oviduct of cycling rats produces and secretes leptin and adiponectin (Table 2). Immunoreactivity for both adipokines was found in the apical region of the secretory epithelial cells, but only in the isthmus and ampulla, and immunostaining was stronger in the isthmus and changed throughout the estrous cycle in the ampulla, increasing from proestrus to estrus. A specific role for the protein in the oviduct remains to be shown.
Endometrium
Recent findings suggest a possible role of adiponectin in endometrial function. Both AdipoR1 and AdipoR2 are highly expressed in the pig endometrium (86). In 2006, both receptors were shown to be present in the endometrial and glandular human epithelium and in stromal fibroblasts (87), and transcript levels were higher during the midluteal phase of the cycle. Adiponectin acts through adenosine monophosphate–activated protein kinase phosphorylation in epithelial and stromal endometrial cells. The fact that the expression of both genes was increased in the midluteal phase when the implantation occurs suggests that the homeostatic and antiinflammatory effects of adiponectin in the endometrium might affect implantation. Interestingly, adiponectin levels were lower in women with endometriosis compared with healthy women. In women with endometriosis, adiponectin levels were inversely correlated to endometriosis and adhesion scores (88). Only one study evaluated adiponectin and uterine leiomyomas (89), and the investigators reported that women with leiomyomas had significantly lower adiponectin levels.
Testes
Whereas most studies of obesity and infertility focus on the female partner, evidence suggests a significant effect of obesity upon male reproductive function (Table 3). Despite a recent study showing no significant change in sperm parameters relative to BMI (90), oligozoospermia and asthenozoospermia have been found to increase with an increased BMI, worsening from overweight to obese men (91). Based on the role of adiponectin in the ovary, investigators studied the possible expression of adiponectin in the rat testis. Interstitial Leydig cells were found to express adiponectin mRNA, whose levels were marginally regulated by pituitary gonadotropins (92). In addition, expression of transcripts encoding AdipoR1/R2 was detected and ex vivo and recombinant adiponectin inhibited T secretion, whereas it failed to change levels of stem cell factor and antimullerian hormone (92). Furthermore, Ocon-Grove et al. (93) reported expression of AdipoR1/R2 mRNA in chicken testis, adding that sexual maturation was likely associated with an up-regulation in testicular AdipoR1/R2 and consequent influence on steroidogenesis, spermatogenesis, Sertoli cell function, and spermatozoa motility.
Adiponectin and Assisted Reproduction
Obesity of the female partner is associated with a suboptimal outcome during an assisted reproduction procedure. Although most studies consider obesity as a negative predictive factor, others report differences only when marked obesity (BMI >35) is encountered. Nevertheless, the majority of studies support the conclusion that obesity predicts reduced success at ART (94). To date, few investigators have tested the correlation of adiponectin levels with ART outcomes.
Based on the fact that adiponectin is reduced by increasing concentrations of estrogens and increased by hCG, Liu et al. (95) studied whether adiponectin levels were altered in women undergoing assisted reproduction. In that study, the investigators followed 52 women during an IVF cycle and found a decrease in adiponectin levels from day 0 (baseline) to the day of hCG injection. This decrease was partly explained by high E2 levels, though the E2 levels on day of hCG injection were not correlated with adiponectin levels. On the contrary, post-hCG adiponectin levels showed a subsequent increase, which in turn correlated with increased levels of P after transfer, possibly explained by lipid accumulation and subsequent P production due to adiponectin (95). Consistent with these observations, adiponectin levels were found to be higher in 9 out of 32 women participating in IVF-ICSI who became pregnant, compared with lower circulating levels in unsuccessful cycles. Levels of adiponectin were higher among the group with successful pregnancy on the day of oocyte retrieval as well as the preceding 3 days. Interestingly, adiponectin levels were higher in the group with successful outcome, despite no difference at the beginning of ovarian stimulation (96). Moreover, in the same study, adiponectin was present in the follicular fluid, but levels were not correlated with fertilization rates or the gonadotropin dosage. The authors speculated that because follicular fluid adiponectin levels differed at various stages of blastomere development, levels of adiponectin might hold predictive value (96). A second case-control study of 56 women found that adiponectin levels on the day before gonadotropin administration correlated with the number of oocytes retrieved and that adiponectin levels were higher in women who conceived (97).
Independently of the number of oocytes obtained or estrogen levels, adiponectin levels in the follicular fluid were found to be higher in women that received recombinant LH, possibly relating to lower androgen and increased insulin sensitivity (67). In addition to the correlations observed in vivo, adiponectin receptors were found to be present in human granulosa cells, promoting an increase in P and E2 locally in granulosa cells (65), and AdipoR1 and AdipoR2 were found to be regulated by hCG treatment in rats (98). The mechanism responsible for the improved pregnancy outcome might be enhanced development of oocytes (observed in porcine embryos, possibly through an inhibitory mitogen-activated protein kinase [MAPK] pathway), with a positive effect on the meiotic maturation and a superior rate of embryo development to the blastocyst stage (99).
Adiponectin in Pregnancy
The fact that adiponectin is implicated in the pathogenesis of insulin-resistant states, and that an increase in adiponectin levels promotes insulin sensitivity, raises the question of whether adiponectin plays a metabolic role in pregnancy. The beginning of pregnancy is characterized by tissue accretion, whereas late pregnancy is notable for insulin resistance and facilitated lipolysis (100). In early pregnancy, insulin secretion increases, although insulin sensitivity is unchanged, decreased, or even increased (100, 101). In late gestation, because of the progressive increase in postprandial glucose, insulin requirements must increase (97).
Adiponectin and the Placenta
The human placenta was found to express virtually all known cytokines (101). Cytokines are produced by three different placental cell types: the Hofbauer cells, the trophoblast cells, and the vascular endothelium cells (101). Adiponectin receptors are present in the human and rat placenta (102). Both human and rodent placentas express adiponectin (103). Of note, placental cytokine release is positively correlated to adiponectin levels (104). AdipoR2, in particular, is expressed in human syncytiotrophoblast and cytotrophoblast (103). The trophoblast and the placenta are also local sources of adiponectin secretion (102). Placental adiponectin secretion has been demonstrated in vitro, implicating placenta in a complex system of source and target function. Placental adiponectin acts through a pathway common for adiponectin, by altering the phosphorylation status of p38 and MAPK (102).
Studies have also suggested a role for adiponectin in the function of the placenta (Table 3). Obesity is recognized as a risk factor for preeclampsia, and adiponectin levels were lower during the first trimester in women with preeclampsia (105), whereas adiponectin and HMW adiponectin levels were increased in preeclampsia, perhaps a physiologic response to restore insulin sensitivity (106). Placental tissues from women with severe preeclampsia show reduced expression of adiponectin (107). Whether adiponectin has a direct role in this condition as related to obesity (108) or gestational diabetes (109) remains to be confirmed, but recent data suggest a role for adiponectin and AdipoR1 in placental angiogenesis and placental apoptosis (110).
Recent findings also show that adiponectin secretion and adiponectin transcript levels in white adipose tissues decline as gestation progresses, even in lean women, which suggests that the adiponectin decrease is due to pregnancy-associated factors (111, 112). High E2 levels in vivo tend to lower adiponectin levels, possibly adding to the decrease observed during pregnancy (113). Although women tend to have higher levels of adiponectin in general, gestational diabetes mellitus (GDM) is associated with reduced serum adiponectin levels both during pregnancy and after delivery. Adiponectin is negatively correlated with HOMA-IR, and a decrease in maternal adiponectin after delivery indicates a significant placental contribution to adiponectin production (114). Moreover, adiponectin levels correlate with whole-body insulin sensitivity (115). This is understandable because of the insulin-sensitizing effects of adiponectin in muscle and liver; adiponectin reduces hepatic glucose production and enhances insulin action in the liver and peripheral utilization of glucose. Given these data regarding expression of AdipoR1/R2 in the placenta, adiponectin may affect the constantly changing metabolic state throughout pregnancy. Studies between women with gestational diabetes mellitus and matched normal control subjects showed an alteration of proinflammatory cytokines in women with GDM; adiponectin levels were reduced in women with GDM, whereas interleukin-beta levels were increased. Moreover, birth weight was negatively correlated with second-trimester adiponectin levels (114).
Adiponectin and the Fetus
Expanding the link between adiponectin and the placenta, and given the data that relate adiponectin to fat distribution and insulin resistance, raises the question of adiponectin levels in the fetus. Cord adiponectin levels were found to be significantly higher and not related to maternal adiponectin levels, thus demonstrating a fetal source of adiponectin (116). While adiponectin concentrations were found to be relevant only between maternal serum and breast milk (117), low levels of adiponectin in cord serum tended to occur in fetuses disproportionally large for their placental weight and vice versa (118). Long-term follow-up of children with low cord adiponectin levels during fetal life predicted a more pronounced weight gain at in the first 6 months of life, as well as an increased BMI and central adiposity at age 3 (119). Both HMW and LMW adiponectin were detected in fetal plasma and were stained in in vascular endothelial cells of fetal organs, skeletal muscle, kidney, and brain. Moreover, the HMW adiponectin and total adiponectin levels are found to be higher in umbilical plasma than in adult plasma and were associated with lower insulin concentration and possibly lower insulin resistance in umbilical plasma, reflecting higher insulin sensitivity of the fetus compared with the adult (120). Furthermore, although the relationship between intrauterine growth retardation (IUGR) and perinatal and long-term complications is established, attempts to correlate low adiponectin levels with IUGR are inconclusive, because the majority of children resulting from IUGR are not found to have low adiponectin levels (121, 122). Nevertheless, mothers who carried IUGR pregnancies were found to have reduced adiponectin levels, possibly explaining the insulin insensitivity of their offspring in term (123).
CONCLUSION
Obesity is an epidemic, causing serious health issues, including anovulation and infertility. Because obesity is associated with low adiponectin levels, and given that many reproductive endocrine tissues express adiponectin receptors, adiponectin represents an important hormonal link between adipose tissue and the reproductive system. The relationship between obesity and normal endocrine reproductive function is fertile ground for future research.
Acknowledgments
Supported in part by the Program in Reproductive and Adult Endocrinology, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland.
The authors recognize the support and assistance of Dr. Alan H. DeCherney and Dr. George Chrousos. Dr. Michalakis is a visiting fellow from Greece.
Footnotes
K.G.M. has nothing to disclose. J.H.S. has nothing to disclose.
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the U.S. government.
References
- 1.Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90:S21–9. doi: 10.1016/j.fertnstert.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Metwally M, Ledger W, Li TC. Reproductive endocrinology and clinical aspects of obesity in women. Ann N Y Acad Sci. 2008;1127:140–6. doi: 10.1196/annals.1434.000. [DOI] [PubMed] [Google Scholar]
- 3.Barinaga M. “Obese” protein slims mice. Science. 1995;269:475–6. doi: 10.1126/science.7624769. [DOI] [PubMed] [Google Scholar]
- 4.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–44. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130:583–97. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 6.Budak E, Sanchez MF, Bellver J, Cervero A, Simon C, Pellicer A. Interactions of the hormones leptin, ghrelin, adiponectin, resistin and PYY3-36 with the reproductive system. Fertil Steril. 2006;86:1563–81. doi: 10.1016/j.fertnstert.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 7.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to metabolic syndrome. Endocrine Reviews. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 8.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS, Flier Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–31. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 10.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 11.Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James R, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci U S A. 2000;97:14478–83. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, et al. Genomewide search for type 2 diabetes–susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2–diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000;67:1470–80. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tonita M. Isolation and characterization of GBP28, a novel gelatine-binding protein purified from human plasma. J Biochem (Tokyo) 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 14.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 15.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 16.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Lam KSL, Chan L, Chan LW, Lam JBB, Lam MC, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 19.Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, et al. Adiponectin and the development of type 2 diabetes. The Atherosclerosis Risk in Communities Study. Diabetes. 2004;53:2473–8. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 20.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 21.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–41. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 22.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilisation and fatty acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 24.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. t-Cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–80. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 26.Smith JL, Kupchak BR, Garitaonandia I, Hoang LK, Maina AS, Regalla LM, et al. Heterologous expression of human mPRα, mPRβ, and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–73. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963–74. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 29.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhbited by the adipose-derived protein Acrp 30. J Clin Invest. 2001;108:1875–81. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fruebis J, Tsao T-S, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDA adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomas E, Tsao T-S, Saha AK, Murrey HE, Zhang Cc C, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by Acrp30 globular domain:acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 34.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 35.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–90. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 36.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–6. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimental DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK and COX2 dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPAR γ agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 40.Laplante M, Sell H, MacNaul KL, Richard D, Berger JP, Deshaies Y, et al. PPAR-gamma activation mediates adipose depot-specific effects on gene expression and lipoprotein lipase activity: mechanisms for modulation of postprandial lipemia and differential adipose accretion. Diabetes. 2003;52:291–9. doi: 10.2337/diabetes.52.2.291. [DOI] [PubMed] [Google Scholar]
- 41.De Vries R, Wolffenbuttel BH, Sluiter WJ, van Tol A, Dullaart RP. Post-heparin plasma lipoprotein lipase, but not hepatic lipase activity, is related to plasma adiponectin in type 2 diabetic patients and healthy subjects. Clin Lab. 2003;51:403–9. [PubMed] [Google Scholar]
- 42.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 43.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the function of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 44.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 45.Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–7. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 46.Wei E, Giovannucci E, Fuchs C, Willett WC, Mantzoros CS. Plasma adiponectin levels and the risk of colorectal cancer in men. J Natl Cancer Inst. 2005;97:1688–94. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–72. [PubMed] [Google Scholar]
- 48.Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–13. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 49.Liu YH, Tsai EM, Wu LC, Chen SY, Chang YH, Jong SB. Higher basal adiponectin levels are associated with better ovarian response to gonadotropin stimulation during in vitro fertilization. Gynecol Obstet Invest. 2005;60:167–70. doi: 10.1159/000086633. [DOI] [PubMed] [Google Scholar]
- 50.Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2009;89:38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- 51.Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2004;55:141–7. [PubMed] [Google Scholar]
- 52.Wen JP, Lv WS, Yang J, Nie AF, Cheng XB, Yang Y, et al. Globular adiponectin inhibits GnRH secretion from GT1–7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun. 2008;371:756–61. doi: 10.1016/j.bbrc.2008.04.146. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86:191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- 54.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–71. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoyda TD, Fry M, Ahima RS, Ferguson AV. Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol. 2007;585:805–16. doi: 10.1113/jphysiol.2007.144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–10. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2000;130:583–97. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 58.Lord E, Ledoux S, Murphy BD, Beaudry D, Palin MF. Expression of adiponectin and its receptors in swine. J Anim Sci. 2005;83:565–78. doi: 10.2527/2005.833565x. [DOI] [PubMed] [Google Scholar]
- 59.Campos DB, Palin MF, Bordignon V, Murphy BD. The beneficial adipokines in reproduction and fertility. Int J Obes. 2008;32:223–31. doi: 10.1038/sj.ijo.0803719. [DOI] [PubMed] [Google Scholar]
- 60.Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006;147:5178–86. doi: 10.1210/en.2006-0679. [DOI] [PubMed] [Google Scholar]
- 61.Bersinger NA, Birkhauser MH, Wunder DM. Adiponectin as a marker of success in intracytoplasmic sperm injection/embryo transfer cycles. Gynecol Endocrinol. 2006;22:479–83. doi: 10.1080/09537100600931316. [DOI] [PubMed] [Google Scholar]
- 62.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 63.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–7. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 64.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–41. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 65.Chabrolle C, Tosca L, Rame C, Lecomte P, Royere D, Dupont J. Adiponectin increase insulin-like growth factor I–induced progesterone and estradiol secretion in human granulose cells. Fertil Steril. 2008;92:1988–96. doi: 10.1016/j.fertnstert.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Dupont J, Chabrolle C, Rame C, Tosca L, Coyral-Castel S. Role of the peroxisome proliferator–activated receptors, adenosine monophosphate–activated kinase, and adiponectin in the ovary. PPAR Res. 2008;2008:176275. doi: 10.1155/2008/176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Recombinant luteinizing hormone induces increased production of ovarian follicular adiponectin in vivo: implications for enhanced insulin sensitivity. Fertil Steril. 2008;91:1837–41. doi: 10.1016/j.fertnstert.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulose cell function. Mol Cell Endocrinol. 2008;284:38–45. doi: 10.1016/j.mce.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Carmina E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva Ginecol. 2004;56:1–6. [PubMed] [Google Scholar]
- 70.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 71.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 72.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 73.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 74.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 75.Stefan N, Stumvoll M. Adiponectin—its role in metabolism and beyond. Horm Metab Res. 2002;34:469–74. doi: 10.1055/s-2002-34785. [DOI] [PubMed] [Google Scholar]
- 76.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 77.Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1790–6. doi: 10.1093/humrep/deg353. [DOI] [PubMed] [Google Scholar]
- 78.Ducluzeau PH, Cousin P, Malvoisin E, Bornet H, Vidal H, Laville M, et al. Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in nonobese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:3626–31. doi: 10.1210/jc.2003-030219. [DOI] [PubMed] [Google Scholar]
- 79.Katsikis I, Mouslech T, Kourtis A, Panidis D, Georgopoulos NA. Oligo-ovulation or anovulation and hyperandrogenemia contribute to the decreased serum adiponectin levels in normal-weight women with PCOS with obesity and insulin resistance. Fertil Steril. 2009;91:e3. doi: 10.1016/j.fertnstert.2008.12.075. [DOI] [PubMed] [Google Scholar]
- 80.Yilmaz M, Bukan N, Demirci H, Ozturk C, Kan E, Ayvaz G, et al. Serum resistin and adiponectin levels in women with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:246–52. doi: 10.1080/09513590802653833. [DOI] [PubMed] [Google Scholar]
- 81.Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15:297–307. doi: 10.1093/humupd/dmp006. [DOI] [PubMed] [Google Scholar]
- 82.Bik W, Baranowska-Bik A, Wolinska-Witort E, Chmielowska M, Martynska L, Baranowska B. The relationship between metabolic status and levels of adiponectin and ghrelin in lean women with polycystic ovary syndrome. Gynecol Endocrinol. 2007;23:325–31. doi: 10.1080/09513590701260169. [DOI] [PubMed] [Google Scholar]
- 83.Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril. 2009;91:1332–5. doi: 10.1016/j.fertnstert.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Miller BT, Leondires MP. The fallopian tube in health and disease. In: Seifer DB, Samuels P, Kniss DA, editors. The physiologic basis of gynecology and obstetrics. Philadelphia, Lippincott: Williams & Wilkins; 2001. [Google Scholar]
- 85.Arcancho M, Gomez-Ambrosi J, Tena-Sempere M, Fruhbeck G, Burrell MA. Expression of leptin and adiponectin in the rat oviduct. J Histochem Cytochem. 2007;55:1027–37. doi: 10.1369/jhc.6A7128.2007. [DOI] [PubMed] [Google Scholar]
- 86.Lord E, Ledoux S, Murphy BD, Beaudry D, Palin MF. Expression of adiponectins and its receptors in swine. J Anim Sci. 2005;83:565–78. doi: 10.2527/2005.833565x. [DOI] [PubMed] [Google Scholar]
- 87.Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, et al. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology. 2006;147:3203–10. doi: 10.1210/en.2005-1510. [DOI] [PubMed] [Google Scholar]
- 88.Takemura Y, Osuga Y, Harada M, Hirata T, Koga K, Morimoto C, et al. Serum adiponectin concentrations are decreased in women with endometriosis. Hum Reprod. 2005;20:3510–3. doi: 10.1093/humrep/dei233. [DOI] [PubMed] [Google Scholar]
- 89.Chen HS, Chan TF, Chung YF, Su JH, Yuan SS. Aberrant serum adiponectin levels in women with uterine leiomyomas. Gynecol Obstet Invest. 2004;58:160–3. doi: 10.1159/000079553. [DOI] [PubMed] [Google Scholar]
- 90.Nicopoulou SC, Alexiou M, Michalakis K, Ilias I, Venaki E, Koukkou E, et al. Body mass index vis-à-vis total sperm count in attendees of a single andrology clinic. Fertil Steril. 2009;90:346–91. doi: 10.1016/j.fertnstert.2008.12.093. [DOI] [PubMed] [Google Scholar]
- 91.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–5. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 92.Caminos JE, Nogueiras R, Gaytan F, Pineda R, Gonzalez CR, Barreiro ML, et al. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 2008;149:3390–402. doi: 10.1210/en.2007-1582. [DOI] [PubMed] [Google Scholar]
- 93.Ocon-Grove OM, Krzysik-Walker SM, Maddineni SR, Hendricks GL, 3rd, Ramachandran R. Adiponectin and its receptors are expressed in the chicken testis: influence of sexual maturation on testicular ADIPOR1 and ADIPOR2 mRNA abundance. Reproduction. 2008;136:627–38. doi: 10.1530/REP-07-0446. [DOI] [PubMed] [Google Scholar]
- 94.Bellver J, Ayllón Y, Ferrando M, Melo M, Goyri E, Pellicer A, et al. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2009;93:447–54. doi: 10.1016/j.fertnstert.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 95.Liu YH, Tsai EM, Chen YL, Chen HS, Chen YC, Wu LC, et al. Serum adiponectin levels increase after human chorionic gonadotropin treatment during in vitro fertilization. Gynecol Obstet Invest. 2006;62:61–5. doi: 10.1159/000092260. [DOI] [PubMed] [Google Scholar]
- 96.Bersinger NA, Birkhauser MH, Wunder DM. Adiponectin as a marker of success in inracytoplasmic sperm injection/embryo transfer cycles. Gynecol Endocrinol. 2006;22:479–83. doi: 10.1080/09537100600931316. [DOI] [PubMed] [Google Scholar]
- 97.Liu YH, Tsai EM, Wu LC, Chen SY, Chang YH, Jong SB, et al. Higher basal adiponectin levels are associated with better ovarian response to gonadotropin stimulation during in vitro fertilization. Gynecol Obstet Invest. 2005;60:167–70. doi: 10.1159/000086633. [DOI] [PubMed] [Google Scholar]
- 98.Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotropin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction. 2007;133:719–31. doi: 10.1530/REP-06-0244. [DOI] [PubMed] [Google Scholar]
- 99.Chappaz E, Albornoz MS, Campos D, Che L, Palin MF, Murphy BD, et al. Adiponectin enhances in vitro development of swine embryos. Domest Anim Endocrinol. 2008;35:198–207. doi: 10.1016/j.domaniend.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Ramos MP, Crespo-Solans MD, del Campo S, Cacho J, Herera F. Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am J Physiol Endocrinol Metab. 2003;285:E318–28. doi: 10.1152/ajpendo.00456.2002. [DOI] [PubMed] [Google Scholar]
- 101.Zavalza-Gomez AB, Anaya-Prado R, Rincon-Sanches AR, Mora-Martinez JM. Adipokines and insulin resistance during pregnancy. Diab Res Clin Pract. 2008;80:8–15. doi: 10.1016/j.diabres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 102.Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 103.Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, García-Caballero T, et al. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005;90:4276–86. doi: 10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- 104.Lappas M, Yee k, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186:457–65. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 105.D’Anna R, Baviera G, Corrado F, Giordano D, De Vivo A, Nicocia G, et al. Adiponectin and insulin resistance in early- and late-onset pre-eclampsia. BJOG. 2006;113:1264–9. doi: 10.1111/j.1471-0528.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 106.Fasshauer M, Waldeyer T, Seeger J, Schrey S, Ebert T, Kratzsch J, et al. Eur J Endocrinol. 2008;158:197–201. doi: 10.1530/EJE-07-0454. [DOI] [PubMed] [Google Scholar]
- 107.Cheng MH, Wang PH. Placentation abnormalities in the pathophysiology of preeclampsia. Expert Rev Mol Diagn. 2009;9:37–49. doi: 10.1586/14737159.9.1.37. [DOI] [PubMed] [Google Scholar]
- 108.Suwaki N, Masuyama H, Nakatsukasa H, Masumoto A, Sumida Y, Takamoto N, et al. Hypoadiponectinemia and circulating angiogenic factors in overweight patients complicated with pre-eclampsia. Am J Obstet Gynecol. 2006;195:1687–92. doi: 10.1016/j.ajog.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Thyfault JP, Hedberg EM, Anchan RM, Thorne OP, Isler CM, Newton ER, et al. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig. 2005;12:41–5. doi: 10.1016/j.jsgi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 110.Jarvenpaa J, Vuoristo JT, Santaniemi M, Ukkola O, Savolainen ER, Jaaskelainen M, et al. Adiponectin induced placental cell apoptosis could be mediated via the AdipoR1-receptor in pre-eclampsia with IUGR. J Perinat Med. 2009;37:257–62. doi: 10.1515/JPM.2009.046. [DOI] [PubMed] [Google Scholar]
- 111.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–85. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 112.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–7. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 113.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–76. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 114.Vitoratos N, Deliveliotou A, Vlachos NF, Mastorakos G, Ppadias K, Botsis D. Serum adiponectin during pregnancy and postpartum in women with gestational diabetes and normal controls. Gynecol Endocrinol. 2008;24:614–9. doi: 10.1080/09513590802342866. [DOI] [PubMed] [Google Scholar]
- 115.Hara K, Yamauchi T, Kadowaki T. Adiponectin: an adipokine linking adipocytes and type 2 diabetes in humans. Curr Diab Rep. 2005;5:136–40. doi: 10.1007/s11892-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 116.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, et al. Determining the source of fetal adiponectin. J Reprod Med. 2007;52:774–8. [PubMed] [Google Scholar]
- 117.Weyermann M, Beermann C, Brenner H, Rothenbacher D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin Chem. 2006;52:2095–102. doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- 118.Kadowaki K, Waguri M, Nakanishi I, Miyashita Y, Nakayama M, Suehara N, et al. Adiponectin concentration in umbilical cord serum is positively associated with the weight ratio of fetus to placenta. J Clin Endocrinol Metab. 2006;91:5090–4. doi: 10.1210/jc.2005-2846. [DOI] [PubMed] [Google Scholar]
- 119.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–9. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pinar H, Basu S, Hotmire K, Laffineuse L, Presley L, Carpenter M, et al. High molecular mass multimer complexes and vascular expression contribute to high adiponectin in the fetus. J Clin Endocrinol Metab. 2008;93:2885–90. doi: 10.1210/jc.2008-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lopez-Bermejo A. Insulin resistance after prenatal growth restriction: is it mediated by adiponectin deficiency? Clin Endocrinol. 2006;64:479–80. doi: 10.1111/j.1365-2265.2006.02496.x. [DOI] [PubMed] [Google Scholar]
- 122.Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol. 2009;160:337–47. doi: 10.1530/EJE-08-0621. [DOI] [PubMed] [Google Scholar]
- 123.Kyriakakou M, Malamitsi-Puchner A, Militsi H, Boutsikou T, Margeli A, Hassiakos D, et al. Leptin and adiponectin concentrations in intrauterine growth restricted and appropriate for gestational age fetuses, neonates and their mothers. Eur J Endocrinol. 2008;158:343–8. doi: 10.1530/EJE-07-0692. [DOI] [PubMed] [Google Scholar]