Abstract
Parkinson disease (PD) is the most common movement disorder affecting people. It is characterized by the accumulation of the protein α-synuclein in Lewy body inclusions in vulnerable neurons. α-Synuclein overexpression caused by gene multiplications is sufficient to cause this disease, suggesting that α-synuclein accumulation is toxic. Here we review our recent study showing that α-synuclein inhibits autophagy. We discuss our mechanistic understanding of this phenomenon and also speculate how a deficiency in autophagy may contribute to a range of pleiotropic features of PD biology.
Key words: Parkinson disease, alpha-synuclein, autophagy, Rab1a, Atg9
Parkinson disease is the second most common neurodegenerative disease in humans. It is pathologically characterized by the loss of dopaminergic neurons and the cytoplasmic accumulation of proteinaceous material within aggregates called Lewy bodies. One of the major constituents of Lewy bodies is a protein called α-synuclein. This protein is likely to be a toxic mediator of pathology in PD, since wild-type α-synuclein gene duplications, which increase its expression levels, cause rare cases of autosomal dominant PD. A number of other genes causing monogenic forms of PD have been identified. Studies of these conditions, as well as the much more common “sporadic” form of PD, have led to the postulation of some possible common themes in this disease, including cell death, protein aggregation, mitochondrial dysfunction and impaired capacity of the cell's degradative machinery, where much of the focus has been on the ubiquitin-proteasome system and chaperone-mediated autophagy.
While a number of intracellular pathways have been implicated as contributors to PD pathogenesis, no single pathway is able to explain the range of pathologies seen in neuronal tissue of PD patients. In a previous study, our lab showed that overexpression of α-synuclein increases mutant huntingtin aggregation. Subsequently, we discovered that mutant huntingtin was a macroautophagy (henceforth called autophagy) substrate and that its levels increase when autophagy is compromised. Since the levels of mutant huntingtin fragments correlate directly with the proportion of cells developing aggregates, autophagy inhibition also increases this phenomenon. Because inhibition of autophagy may also have an impact upon a number of PD pathologies (including mitochondrial dysfunction and cell death), we tested if α-synuclein affected autophagy.
We found that α-synuclein overexpression inhibits autophagy at a very early stage of autophagosome formation. These data were further confirmed in vivo in mice overexpressing α-synuclein. Interestingly, RNAi depletion of α-synuclein increases autophagy. This suggests a regulatory role for α-synuclein in autophagosome synthesis, and also implies that the effects of this protein are not restricted to levels very much higher than the normal state, as they occur both above and below physiological expression levels.
While the normal function of α-synuclein is not well understood, Susan Lindquist and colleagues previously suggested that overexpression of α-synuclein causes toxicity in PD models by inhibiting the secretory pathway. This effect appeared to be mediated by loss of function of Rab1a, a key regulator of the early stages of the secretory pathway. We hypothesized that α-synuclein overexpression inhibits autophagosome synthesis by disrupting Rab1a homeostasis and secretion. This hypothesis was further fueled by studies in yeast that showed that autophagy depends on proper secretory function. We observed that α-synuclein overexpression increases Golgi fragmentation (often an indicator of secretory dysfunction) and causes a partial block in constitutive secretion as measured by a secreted fluorescent reporter. These are all phenotypes mimicked by Rab1a knockdown. We also found that knockdown of Rab1a inhibits autophagosome formation and increases the accumulation of autophagy substrates (also confirmed by complementary readouts in Drosophila models), mimicking the effects of α-synuclein overexpression. Importantly, Rab1a overexpression is also able to rescue the reduction in autophagosome numbers mediated by α-synuclein overexpression in cell culture.
The effects of α-synuclein on autophagy appear to be specific to Rab1a and Rab1a-related functions. Knockdown of Rab1a-related effectors and proteins, Sar1A and Sar1B, VDP, and Giantin inhibit secretion, decrease LC3-II levels, and increase autophagy substrate accumulation. However, loss of Rab1b or Rab2, which also regulate early secretory function, increase LC3-II levels and decrease autophagy substrate accumulation. While the trafficking routes controlled by Rab1a, Rab1b and Rab2 probably do not overlap entirely, our data indicate that some aspects of general secretory function can be uncoupled from regulation of autophagy, whereas Rab1a-specific functions contribute to autophagosome synthesis.
Atg9 is a transmembrane protein that is trafficked through the secretory pathway, where it is post-translationally modified by N-glycosylation. As depletion of Atg9 inhibits autophagosome synthesis, and proper glycosylation generally requires movement through the early secretory pathway (ER-to-Golgi trafficking), we tested if Atg9 was affected by changes in Rab1a or α-synuclein expression. Surprisingly, glycosylation of Atg9 is not overtly affected by Rab1a knockdown or α-synuclein overexpression, but localization of Atg9 is altered in both conditions. Atg9 normally localizes to the trans-Golgi network (TGN) and redistributes to autophagosomes upon autophagy induction. Knockdown of Rab1a or overexpression of α-synuclein causes decreased colocalization of Atg9 with the TGN and autophagosomes. The exact role of Atg9 in autophagosome formation is not known, but our data suggest that Atg9 plays a significant role in very early autophagosome formation. Rab1a depletion and α-synuclein overexpression decrease Atg9 colocalization with LC3-positive vesicles and reduce the number of autophagosome precursors (omegasomes). Rab1a overexpression is able to rescue the decrease in omegasome numbers caused by α-synuclein overexpression, again suggesting that Rab1a is an effector for the α-synuclein phenotype. Knockdown of Atg9 alone also reduces omegasome formation, consistent with altered Atg9 localization affecting autophagosome formation.
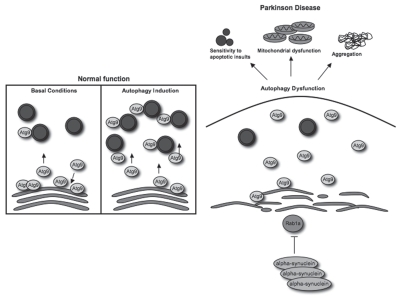
Thus, our data suggest that α-synuclein impedes autophagy by reducing autophagosome formation at a very early stage (omegasome or before). This appears to be Rab1a-dependent, but not a consequence of general inhibition of the secretory pathway, as these effects were not seen with Rab1b or Rab2 knockdown. We speculate that α-synuclein multiplications or increased expression of the wild-type protein may inhibit autophagy and contribute to many different pathologies seen in PD, including abnormal protein aggregation, mitochondrial abnormalities, increased levels of reactive oxygen species and enhanced susceptibility to cell death (Fig. 1).
Figure 1.
Autophagy inhibition by alpha-synuclein may contribute to Parkinson disease pathogenesis. In normal, healthy cells, Atg9 colocalizes with the trans-Golgi network and LC3-positive vesicles (shown in blue). Upon autophagy induction, Atg9 is mobilized away from the TGN to LC3-positive vesicles, which correlates with increased autophagosome synthesis. Our data show that overexpression of alpha-synuclein disrupts the normal localization and mobilization of Atg9 to LC3-positive vesicles, which correlates with decreased autophagosome synthesis and dysfunctional autophagy. This phenomenon may be a major contributor to PD pathogenesis, as inhibition of autophagy can increase aggregation, sensitivity to pro-apoptotic insults and mitochondrial dysfunction, all of which are associated with PD pathogenesis.
Acknowledgements
We are grateful for funding from a Wellcome Trust Senior Fellowship (DCR) and an MRC Programme grant, and the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement number 241791 (MEFOPA).
Punctum to: Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, et al. Alpha-synuclein impairs macroautophagy: implications for Parkinson's disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122.