Abstract
Bleeding complications after coronary intervention are associated with prolonged hospitalization, increased hospital costs, patient dissatisfaction, morbidity and one year mortality. Bleeding Avoidance Strategies represent a term incorporating multiple modalities that aim to reduce bleeding and vascular complications after cardiovascular catheterization. Recent improvements in the rates of bleeding complications after invasive cardiovascular procedures suggests that the clinical community has successfully embraced specific strategies and improved patient care in this area. There remains controversy regarding the efficacy, safety and/or practicality of 3 key bleeding avoidance strategies for cardiac catheterization and coronary intervention: procedural (radial artery approach, safezone arteriotomy), pharmacologic (multiple agents) and technological (vascular closure devices) approaches to improved access.
In this article, we address areas of consensus with respect to selected modalities in order to define the role of each strategy in current practice. Furthermore, we focus on areas of controversy for selected modalities in order to define key areas warranting cautious clinical approaches and the need for future randomized clinical trials in this area.
Keywords: bleeding, radial, pci, vascular closure device
Marso and colleagues summarized a percutaneous coronary intervention (PCI) related performance measure by coining the term “Bleeding Avoidance Strategies” (BAS) in their analysis of over 1.5 million patients undergoing PCI in contemporary U.S. practice (1). This analysis demonstrated that BAS incorporating vascular closure devices (VCD) and bivalirudin strategies was associated with a significantly reduced bleeding risk across a broad spectrum of patients undergoing PCI. These findings challenge the recent American Heart Association (AHA) scientific statement generating a Class III/contraindication for VCD's as a method of avoiding bleeding complications (2). This controversy is clinically relevant because major bleeding complications are associated with significant cost, transfusions, lengthened hospitalization and increased 1 year morbidity and mortality (3-7). Furthermore, implementation of best practices may improve quality of care and guideline recommendations are a component of this process (8). Thus, identification of acceptable practices in preventing bleeding complications is of paramount clinical importance.
In this article, we address this controversy by analyzing Bleeding Avoidance Strategies in the context of temporal trends in bleeding complications, recognizing that changes in multiple variables may explain these trends. We categorize BAS in 3 broad themes (Figure 1)—procedural, pharmacologic and technological--to identify areas of consensus for clinical practice as well as controversy that warrants further investigation.
Figure 1. Bleeding Avoidance Strategies classified into 3 broad categories.
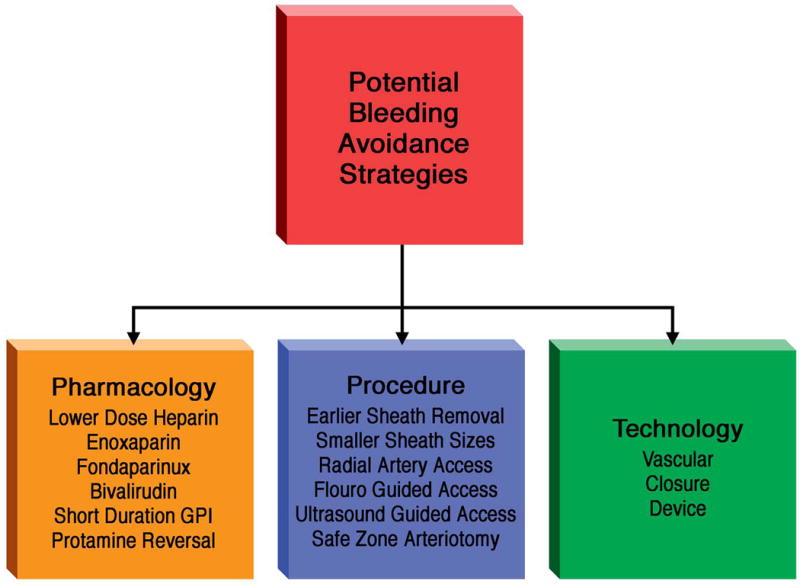
Potential improvements in bleeding complications may be related to procedural, pharmacologic and technology changes occurring over the past two decades.
Temporal Trends in Bleeding and Vascular Complications
Temporal trend studies from the CathPCI Registry, Northern New England Cardiovascular Disease Study Group, Mayo Clinic and Wake Forest University demonstrate that major bleeding complications among patients undergoing PCI have decreased over time (9-13) (Figure 2). Among > 250,000 ACS patients undergoing PCI in the CathPCI Registry, access site bleeding complications in 2005 were 1.2% and reduced to 0.78% in 2009 (P < 0.001). During this period of time, there were significant increases in the use of at least two potential PCI BAS strategies: the radial approach and bivalirudin (10). Access site bleeding improvements are not confined to low risk groups: women are higher risk than men for bleeding complications yet temporal trends in women similarly show a similar 50% reduction in bleeding and vascular complications during the past decade (12).
Figure 2. Temporal trends in bleeding complications after PCI.
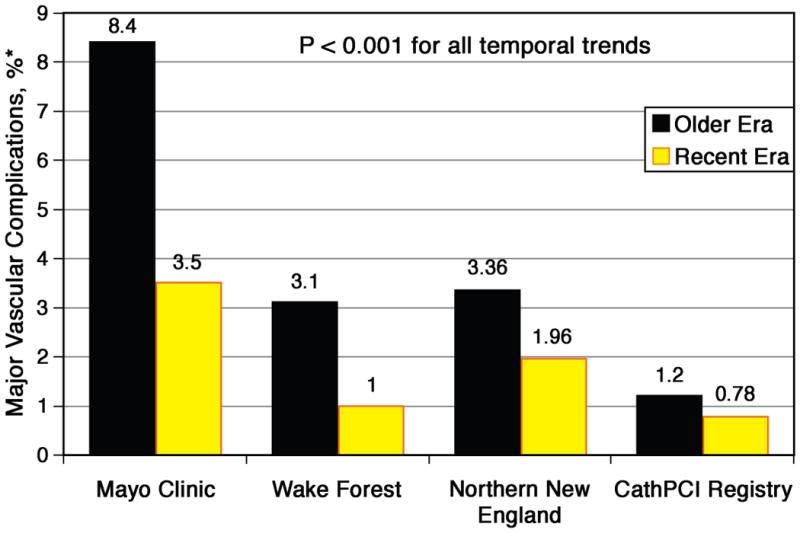
Each registry study shows a significant reduction in access site bleeding complications over time at each center or region analyzed. Bleeding definitions may vary among the registries and time periods of comparison are also different.
Bleeding complications can occur at a variety of locations. Among patients undergoing PCI, the most common site of bleeding is the vascular access site; however, in the ACS population, where there are a substantial proportion of patients treated medically or with coronary artery bypass surgery, the majority of bleeding complications are not access-site related (14). Studies indicate that gastrointestinal bleeding is the most common non-access site of hemorrhage among ACS patients and those undergoing PCI (15, 16) and is associated with significant early mortality risk(17). There are few studies that have examined site-specific trends in bleeding, but ACS registries have come to differing conclusions on trends in overall major bleeding. The GRACE investigators have shown a reduced frequency of major bleeding for ACS patients between 2000 and 2007 (2.6 to 1.8%; P < 0.0001)(18) In contrast, Roe and colleagues examined the ACTION Registry-Get With the Guidelines and found that in-hospital bleeding complications remained unchanged between 2007 and 2009 (10). In addition, among ACS patients in the NCDR CathPCI registry, gastrointestinal bleeding increased a small but significant amount between 2005 and 2009: (0.54 vs 0.67%, P < 0.0001)(10).
One confounding variable occurring throughout this discussion of bleeding trends and BAS is the variable definition of bleeding. This variability occurs across all registries as well as multiple different trial based definitions(14, 19, 20). Not only does this make inter-study comparisons difficult or impossible, the utilization of the clinically most appropriate definition of bleeding may impact conclusions regarding relative efficacy of BAS. An example of this debate is the inclusion of large hematoma (≥5 cm) in the definition of major bleeding in some trials(21, 22) or the reliance on TIMI major bleeding to define clinical significance (14, 20). Unlike other areas that have accepted uniform definitions related to important clinical endpoints(23), a unifying definition of bleeding is still being established (24).
Despite this problem with definitions, we have registry evidence that a) post PCI access site bleeding has improved, b) this improvement is seen across a broad spectrum of risk, and c) trends in non-access site bleeding are unclear and there may have been a slight increase in gastrointestinal bleeding. These temporal trend findings follow consistent evidence in randomized clinical trials for certain BAS techniques: bivalirudin (as compared with UFH/GPI)(25), fondaparinux (as compared with enoxaparin)(26) and the radial artery approach (as compared with the femoral approach) (27) decrease post PCI bleeding complications by at least 40% compared with the control strategy. For other BAS techniques, randomized clinical trial evidence is not definitive (13, 28, 29) and registry data must support or refute the temporal trend findings. For each BAS, knowledge gaps remain and thus controversy can be identified (Table 1). In order to better understand how each BAS may potentially be contributing to the positive temporal trends in bleeding complications, the ensuing sections will analyze areas of consensus and controversy for each approach.
Table 1. Selected Bleeding Avoidance Strategies: Consensus and Controversy.
| Consensus | Controversy | |
|---|---|---|
| Pharmacology | ||
| Bivalirudin | Reduction in bleeding | Mechanism of mortality benefit |
| Benefit compared to UFH alone | ||
| Benefit during radial artery PCI | ||
| Fondaparinux | Reduction in bleeding | Utilization in PCI |
| Catheter Thrombus | ||
| Enoxaparin | Predictable anticoagulation | Intravenous, subcutaneous doses |
| Monitoring | ||
| Increased, decreased or neutral bleeding complications | ||
| Technology | ||
| Vascular Closure | Improved ambulation | Increased, decreased or neutral Devices bleeding complications |
| Improved comfort | ||
| Procedural | ||
| Radial Artery | Reduction in bleeding | Operator issues and learning curve |
| Patient suitability | ||
| Prevention of radial artery occlusion | ||
| Optimized Femoral Access: | Reduction in selected | Universal applicability and efficacy bleeding complications (angiography, fluoro, ultrasound) |
Procedural Reduction in Bleeding Complications and the Radial Artery Approach
A number of procedural developments have been implemented with a goal of reducing access site related bleeding complications (Figure 1). Earlier sheath removal and use of smaller femoral artery sheaths has been associated with reduction in bleeding complications (9, 30-32). More recent procedural approaches include optimization of femoral access with the goal of reducing multiple needle punctures and non-safezone arteriotomy (puncture above the inferior epigastric artery or below the common femoral artery)(28, 33). Such optimization techniques include fluoroscopic (34) or ultrasound guided access, with superiority of the ultrasound guidance approach in a single multicenter randomized trial(35). As ultrasound guided access is not widely used, it is unlikely that this particular modality can explain the recent favorable trends in access site bleeding complications.
A procedural approach that has been consistently associated with reduced bleeding and vascular complications is transradial cardiac catheterization and PCI (27, 36, 37). Both the randomized (27, 37) and observational data (36) show a consistency in directionality of the effect of the radial approach on bleeding. From a pathophysiological standpoint, the underlying mechanisms related to the bleeding reduction with transradial PCI are straightforward – the radial artery is superficial, small in caliber, and easily compressed. The largest observational study involved over 593,000 patients in the NCDR CathPCI Registry undergoing femoral or radial procedures (36). This study demonstrated that the radial approach was associated with a 67% reduction in bleeding and vascular complications as compared to the femoral approach, without an increase in procedural failure. This is consistent with multiple randomized trials that have compared transradial PCI with non-radial access techniques(27, 37, 38).
As opposed to the CathPCI registry analysis, randomized trials have shown that there may be a higher rate of procedure failure with the radial approach, necessitating crossover to femoral access (27, 37). This discrepancy is likely the result of selection bias inherent in observational studies conducted in countries where there is low uptake of the radial approach (like the United States)(39) The success of transradial PCI may be dependent on operator experience (40-42). While a minimum number of procedures necessary to achieve competence has not been identified, the rates of procedure failure may plateau after 100 cases (43). It should be noted that crossover to femoral approach from the radial approach may be lower at centers where the primary approach is transradial; moreover, crossover from femoral to radial access also occurs but is rarely captured in registry data.
Access site bleeding is associated with significant discomfort and patient dissatisfaction. In this context, patients appear to prefer the radial to the femoral approach (44). In addition, reduction in vascular and bleeding complications is associated with cost savings from the hospital perspective(38, 41, 44, 45). Given these data, wider adoption of the radial approach to improve the safety of PCI is a reasonable objective. Whether this approach will also improve traditional efficacy measures like death or MI is the objective of an ongoing randomized trial—An International Randomized Trial of Trans-radial Versus Trans-femoral Percutaneous Coronary Intervention (PCI) Access Site Approach in Patients With Unstable Angina or Myocardial Infarction Managed With an Invasive Strategy (RIVAL) (37).
Other issues related to the radial approach that require further investigation include radiation exposure and radial artery occlusion (46). The latter appears to occur with a frequency between 0.6% and 12% (47-49). Radial artery occlusion is often asymptomatic due to the presence of collateral flow in the hand in most patients(50); however, it is not known whether transradial PCI impacts the suitability of the radial artery as a conduit for coronary artery bypass grafting. Radial artery occlusion can be minimized by the use of anticoagulation during transradial procedures, smaller catheters, and “patent hemostasis” after sheath removal(47, 49).
Despite its relatively large effects on bleeding complications, large registry studies show that transradial PCI accounts for less than 5% of U.S. PCI procedures (36); it is much more common outside the U.S. (39). Therefore, while the data for decreased bleeding complications with the radial approach are consistent, the low adoption rate of the radial approach in the U.S. makes it unlikely to be a main explanation for the decrease in bleeding complications in the U.S. Given this low adoption rate for radial mediated BAS, it is worthwhile to consider alternative (pharmacologic and mechanical) BAS strategies.
Pharmacologic Reduction in Bleeding Complications
Similar to the radial artery approach, pharmacologic developments have already passed the test of appropriate randomized clinical trials. First, the use of unfractionated heparin with and without GPI agents has changed over the past decade. Between 1991 and 1997, three trials of the use of abciximab demonstrated progressive improvements in bleeding rates (30, 51). Comparing the control arms of each study, which received heparin without a glycoprotein 2b3a inhibitor (GPI), the overall bleeding rates decreased by 79% (8.2% in EPIC vs. 1.7% in EPISTENT, p<0.001). This improvement was attributed to reductions in the dose of heparin and the lower target ACT levels in the later trials(30). The active treatment arm patients receiving abciximab also experienced a 90% reduction in vascular bleeding rates from 20.2% to 2.1% (30). Similarly, the ISAR group has recently demonstrated an association between lower heparin dosing (100units/kg) and a reduction in bleeding complications after PCI in a comparison to a historical control group (140 units/kg) (52).
More predictable anticoagulation may be achieved with low molecular weight heparins. Enoxaparin has been extensively studied and well designed trials have demonstrated reduction in bleeding complications with enoxaparin vs unfractionated heparin(53, 54). Other studies have either shown a neutral effect on bleeding with enoxaparin(55), or an increased risk of bleeding with this agent compared to unfractionated heparin (56, 57). These findings may be explained by differences in patient populations, sheath management, drug dosing and route of administration (ie, intravenous versus subcutaneous)(32, 58). Of note, enoxaparin use has increased outside the U.S in recent temporal trends studies (2000-2007) of acute coronary syndromes; during that period of time, bleeding has decreased (18). On the other hand, enoxaparin use has decreased in U.S practice and bleeding has also decreased(10). These data point to the complexity of understanding the role of any single pharmacologic, technologic or procedural approach in accounting for recent favorable trends in bleeding.
Other randomized clinical trial evidence is more consistent: the indirect factor Xa inhibitor fondaparinux significantly reduces bleeding risk as compared with enoxaparin with similar rates of ischemic complications at 9 days (59, 60). These benefits may be especially prominent in patients with renal dysfunction (61) Limited adoption of fondaparinux for PCI patients (due to concerns about catheter related thrombus (59)) make this agent unlikely to be a major component of recent favorable bleeding trends. Whether recent randomized trial data on efficacy of adjunctive low dose unfractionated heparin to prevent catheter thrombus formation impacts utilization of this agent remains to be determined (62).
Bivalirudin, a direct thrombin inhibitor, is associated with a 40-50% reduction in bleeding complications when compared with heparin-based strategies (25, 63, 64). Of note, bivalirudin does not protect against bleeding complications when used in conjunction with GPI agents (as compared to unfractionated heparin with GPI)(25). The bleeding reduction with bivalirudin compared with unfractionated heparin/GPI regimens remains significant even in the presence of lower doses of heparin: in the PROTECT-TIMI 30 trial, a heparin dose of 50u/kg was tested in conjunction with GPI and bivalirudin still maintained a significant reduction in bleeding complications (65). An even more creative way to limit the impact of unfractionated heparin dosing on GPI related bleeding effects is to reverse heparin with protamine after PCI completion: comparison of bivalirudin against this ultimate low dose heparin/GPI strategy, though, still reveals a significant reduction in bleeding complications with bivalirudin (66, 67). More recent studies have explored the use of shorter duration or intracoronary bolus only administration of GPI agents to limit bleeding side effects: whether these approaches reduce bleeding compared to bivalirudin alone has not been examined (68, 69) Lastly, the bleeding reduction seen with bivalirudin is not confined to selected clinical trial populations—large scale registry studies have similarly demonstrated significant associations between reduced bleeding complications and bivalirudin utilization (1, 12).
Many areas of controversy remain regarding implementation of bivalirudin in clinical practice: for example, upstream use of unfractionated heparin (with switching), dosing of clopidogrel and mortality reduction in STEMI trials remain areas of ongoing discussion and subgroup analysis(70-72). Even more controversial is the comparison of bivalirudin to unfractionated heparin alone (i.e, without routine use of GPI). The ISAR-REACT 3 trial compared bivalirudin against heparin alone (140 units/kg) and found that bivalirudin reduced bleeding complications (73); unlike the bivalirudin vs heparin/GPI trials (63, 64), the net efficacy of a bivalirudin strategy compared to heparin alone in this stable/unstable angina PCI population could not be demonstrated (74-76). However, the reduction in bleeding complications with bivalirudin compared with either heparin alone or heparin/GPI is consistent. Whether or not bivalirudin is superior to a lower dose heparin strategy (or heparin reversed with protamine) has not been determined. Changes in pharmacology are a plausible component of positive bleeding temporal trends: for example, utilization of bivalirudin for PCI has increased absolutely an approximate 20% in U.S. practice between 2005 and 2009 (p<0.001) with concomitant decreased use of heparin and GPI regimens(10).
Mechanical Reduction in Bleeding Complications—Vascular Closure Devices
A recent AHA Scientific Statement has issued a class III (level of evidence B) recommendation/contraindication related to VCD for the purpose of reducing vascular complications (2). Manual compression of the femoral artery access site has been the gold standard in obtaining hemostasis at the access site for the past several decades. After almost 60 years of percutaneous arterial access, hemostasis by manual compression remains unchanged; the exception is the introduction of topical hemostasis patches which have not demonstrated a reduction in major bleeding complications in trials or registries(77, 78).
In the early 1990's, early generation VCD were introduced. Koreny et al evaluated clinical outcomes from randomized clinical trials of VCD versus manual compression (79). They identified 30 studies with almost 4,000 patients and demonstrated less time to ambulation and length of hospitalization with VCD as compared to manual compression. The safety analysis was neutral: neither improvement nor reduction in the rates of vascular complications with VCD compared to manual compression could be demonstrated. This meta-analysis is often cited as evidence of “VCD risk” but this was based upon a sensitivity analysis of only two of the 30 trials in which intention to treat could be identified. Nikolsky et al, in a broader meta-analysis that included both randomized trials and registries, identified 30 studies with 37,066 patients comparing clinical outcomes after VCD versus manual compression (80). These authors observed an overall higher risk of vascular complication with VCD compared to manual compression when all studies were combined. But, the adverse risk of VCD was shown to be a result of a significantly higher rate of vascular complications particularly with the VasoSeal device compared to manual compression. Contrary to these two studies, Vaitkus et al(81) and the FDA(82) came to a different conclusion: examining 2001 data from the NCDR CathPCI Registy, the FDA observed findings similar to that of Vaitkus et al: the use of VCD were associated with a significant reduction in vascular complications as compared to manual compression, and Vasoseal was a notable exception to those positive trends (Table 2).
Table 2. Studies with 10,000 or more patients: VCD vs manual compression.
| Study | Year published | # patients | Study type | Endpoint | Complication Rates | ||
|---|---|---|---|---|---|---|---|
| VCD | MC | P Value | |||||
| Nikolsky | 2004 | 36,066 | Trial and Registry Meta-Analysis | Hematoma | OR 1.34 | CI 1.01-1.79 | P < .05 |
| Tavris | 2004 | 166,680 | National Registry (NCDR) | any VC | 1.10% | 1.70% | P<0.001 |
| Tavris | 2005 | 13,878 | National Registry (NCDR) | any VC | OR 0.99 | CI 0.77-1.28 | P=ns |
| Arora | 2007 | 12,937 | Single Center Registry | any VC | 2.40% | 4.90% | P < 0.01 |
| Ahmed | 2007 | 13,563 | Multicenter registry | Bleeding/VC | OR: 0.72 | CI 0.59-0.89 | P=0.02 |
| Applegate | 2008 | 35,016 | Single Center Registry | any VC | 1.60% | 2.10% | P=0.03 |
| Sanborn | 2009 | 11,621 | ACUITY post hoc | Access site bleeding | 2.50% | 3.30% | P=0.01 |
| Marso | 2010 | 1,522,935 | National Registry (NCDR) | Peri-procedural bleeding | OR: 0.77 | CI 0.73-0.80 | P < 0.05 |
OR= odds ratio
Several factors are relevant in examining the use of the older data to determine the current safety of VCD. First, VCD devices may have improved over time(83), especially with the removal of the Vasoseal product(82). Second, there is a learning curve with the use of VCD (84, 85); it is possible that better patient selection and knowledge of device use itself has resulted in lower rates of vascular complications. Unfortunately, the potential benefit of these incremental changes has not been absolutely proven: the equivocal and conflicting results did not spur the VCD industry to settle the question finally and definitely with a single, large randomized clinical trial.
However, since the conflicting meta-analyses of 2004, there have been at least 5 large (>10,000 patients) broadly inclusive observational and multicenter registries evaluating the safety of VCD (Table 2). Arora et al looked at rates of vascular complications in 12,937 patients from 2002 to 2005 (86)., They observed an almost 50% propensity adjusted reduction in rates of vascular complications associated with VCD utilization. Ahmed et al examined the rates of vascular complications in patients undergoing PCI from the Northern New England Cardiovascular Disease Study Group from 2002-2007(12). They observed a 28% decrease in the risk-adjusted rates of vascular complications in over 13,563 women with VCD compared to manual compression. Applegate et al evaluated rates of vascular complications in 35,016 patients over a 10-year study period, ending in 2007(11): VCD was an independent factor associated with lower rates of vascular complications. Sanborn et al performed a post hoc analysis of the ACUITY trial (87): in 11,621 patients, there was a significant 22% risk adjusted decrease in the rates of vascular complications with the use of VCD compared to manual compression. Finally, Marso et al reviewed the data from the ACC NCDR from 2004-2008 (1). Over 1.5 million patients were included in the study with a significantly lower rate of vascular complications with VCD use compared to manual compression across a broad spectrum of risk.
An appropriately powered randomized trial is needed prior to definitive conclusions (ie, Class I or Class III recommendations). The etiologies of favorable temporal trends is complex and not easily attributable to a single device or intervention: in the Mayo Clinic study of 17,901 consecutive patients between 1994 and 2005, major femoral vascular complications were reduced by 58% (from 8.4% to 3.5%, p<0.001); notably, the use of VCD comprised less than 5% of patients during the study period (9). While the Northern New England group also demonstrated a 50% reduction in bleeding complications over time, Northern New England operators utilized VCD in 43% of patients (12). The potential benefit of VCD (early ambulation, comfort (13, 88)) coupled with the inconsistent data regarding safety of VCD (80, 82) do not meet the burden of proof of harm; clinicians should be left in the appropriate gray area of Class II recommendations for this technology.
Systematic Reduction in Bleeding Complications and Cost Effectiveness
Systematic improvements in bleeding complications may require broad initiatives to address patient selection and BAS implementation. One approach is the application of a Bleeding Risk Score to individualize patient approaches with tailoring of therapies according to patient risk (21, 74, 89). Therapeutic strategies based upon risk stratification for bleeding complications though may be limited by the overlap between ischemic risk factors and bleeding risk factors(74, 89). As another example, the relative benefit of VCD as compared to manual compression may depend upon the adequacy of femoral artery access and selection of appropriate patients(28, 29, 34, 90). The consequences of VCD closure failure are not small: Bangalore reported a VCD failure rate of 2.3% in 9,853 consecutive patients demonstrating that VCD failure was associated with a 4.8 fold increased risk of vascular complication compared with successful VCD deployment in a propensity matched analysis (91). Thus, systematic attempts to optimize femoral access (including potentially fluoroscopy guided access, selected ultrasound guided access and routine femoral angiography (28, 34, 35)) in order to determine which VCD are appropriate in selected situations warrants further study.
Even if appropriately deployed BAS strategies conclusively reduce bleeding, can the incremental costs of bivalirudin/fondaparinux (compared to heparins) and VCD (as compared to manual compression) be justified? The significant economic costs of bleeding and vascular complications following PCI can provide additional incentive for increased focus on bleeding reduction strategies. A detailed analysis of the incremental costs of complications based on administrative data from 335,477 Medicare beneficiaries who underwent PCI in 2002, demonstrated an incremental cost of $6,377 and an increased length of stay of 2.8 days for patients suffering a vascular complication (92)
Exploring the ACUITY randomized clinical trial data, Pinto and colleagues determined that the use of bivalirudin was associated with a net cost savings, ostensibly through the reduction of bleeding complications. Specifically, minor bleeding events were associated with an attributable cost of $2,282 while major bleeding episodes were associated with an increased attributable cost of $8,658 (45). Similarly, a detailed attributable cost analysis of specific vascular and bleeding complications demonstrated significant incremental additional costs of hematoma ($1,399 95% CI: $700–$6,955), clinical significant bleeding ($5,440 95% CI: $2,250–$10,226) and pseudoaneursym formation ($6,357 95% CI: $4,900–$10,408) (5). Given the significant costs associated with bleeding and vascular complications following PCI, BAS may ultimately be cost effective investments of health care.
As noted previously, the radial access strategy has been found to be associated with a significant reduction of access site bleeding complications as compared with femoral access procedures. Balancing the costs and clinical advantages of VCD, bivalirudin, and radial access is complex. While radial access obviates the need for VCD use, many radial access interventionalists recommend the use of specially designed hydrophilic sheaths, wires and specially designed radial access site hemostasis devices to help improve the success and patient comfort associated with the radial artery approach. The incremental costs for these specialized radial access devices range from $55 to $75 per procedure above the costs of traditional femoral access equipment. While there are potential advantages for bivalirudin to reduce non-access site bleeding in radial artery access procedures as compared with a strategy of heparin use, lesser absolute reductions in overall bleeding complications are likely to result in lesser cost effectiveness as compared to the demonstrated cost advantages in femoral access(45, 93).
Consensus, Controversy and Practice Recommendations
BAS have emerged as an evolving and important part of cost effective, high quality clinical practice. Consensus points from randomized clinical trials and registries are robust:
Access site bleeding complication rates are less frequent now than 10 years ago in the setting of multiple pharmacologic, technologic and procedural advances
Bivalirudin, fondaparinux, and lower dose unfractionated heparin are associated with a significant reduction in bleeding complications compared with regimens incorporating higher dose UFH and/or GPI.
The radial approach reduces access site bleeding compared with the femoral approach, but the slow adoption in the U.S makes it unlikely to fully explain the falling rates of bleeding complications.
The radial artery approach and vascular closure devices allow earlier ambulation and improve patient comfort compared to femoral access/manual compression strategy.
Bleeding complications are associated with increased hospital costs, lengthened hospitalization and mortality.
On the other hand, controversy remains regarding other aspects of BAS:
Early meta-analyses and registry studies demonstrate harm, benefit and neutrality of VCD compared to manual compression depending upon analysis of overall results versus sensitivity analyses. In contrast, 5 recent large (> 10,000 patient) registries suggest a benefit for VCD compared to manual compression. Based on these registries, a large randomized trial is warranted to prove the concept that VCD decreased complications.
Are BAS related pharmacologies necessary in the setting of radial approach? Can U.S barriers to radial adoption be overcome?
Finally, while bleeding is clearly associated with 1 year death, the mechanism (ie, cessation of guideline recommended antiplatelet therapy(94)) remains speculative.
In conclusion, the coining of the term “Bleeding Avoidance Strategies” summarizes a broad multi-modality approach to quality improvement for invasive cardiovascular procedures. The trends in this area are positive indicating that clinicians are moving in the right direction. Randomized clinical trial data is robust in many areas and allows for considerable consensus. On the other hand, controversy is both expected and warranted in areas where adequate sized clinical trials have not yet been performed. In such areas, clinical judgement, patient selection and cautious utilization is consistent with other gray areas of current practice.
Reference List
- 1.Marso SP, Amin AP, House JA, et al. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–64. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Jneid H, Derdeyn CP, et al. Arteriotomy Closure Devices for Cardiovascular Procedures: A Scientific Statement From the American Heart Association. Circulation. 2010;122:1882–93. doi: 10.1161/CIR.0b013e3181f9b345. [DOI] [PubMed] [Google Scholar]
- 3.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690–7. doi: 10.1016/j.jacc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 5.Resnic FS, Arora N, Matheny M, Reynolds MR. A cost-minimization analysis of the angio-seal vascular closure device following percutaneous coronary intervention. Am J Cardiol. 2007;99:766–70. doi: 10.1016/j.amjcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 7.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–8. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–12. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 9.Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv. 2008;1:202–9. doi: 10.1016/j.jcin.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254–63. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Applegate RJ, Sacrinty MT, Kutcher MA, et al. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv. 2008;1:317–26. doi: 10.1016/j.jcin.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed B, Piper WD, Malenka D, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention: a report from the Northern New England Percutaneous Coronary Intervention Registry. Circ Cardiovasc Interv. 2009;2:423–9. doi: 10.1161/CIRCINTERVENTIONS.109.860494. [DOI] [PubMed] [Google Scholar]
- 13.Dauerman HL, Applegate RJ, Cohen DJ. Vascular closure devices: the second decade. J Am Coll Cardiol. 2007;50:1617–26. doi: 10.1016/j.jacc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Rao SV, O'Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47:809–16. doi: 10.1016/j.jacc.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 15.Nikolsky E, Stone GW, Kirtane AJ, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;54:1293–302. doi: 10.1016/j.jacc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–5. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 17.Gaglia MA, Jr, Torguson R, Gonzalez MA, et al. Correlates and consequences of gastrointestinal bleeding complicating percutaneous coronary intervention. Am J Cardiol. 2010;106:1069–74. doi: 10.1016/j.amjcard.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Fox KA, Carruthers K, Steg PG, et al. Has the frequency of bleeding changed over time for patients presenting with an acute coronary syndrome? The global registry of acute coronary events. Eur Heart J. 2010;31:667–75. doi: 10.1093/eurheartj/ehp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serebruany VL, Atar D. Assessment of bleeding events in clinical trials-proposal of a new classification. Am J Cardiol. 2007;99:288–90. doi: 10.1016/j.amjcard.2006.07.091. [DOI] [PubMed] [Google Scholar]
- 20.Steinhubl SR, Kastrati A, Berger PB. Variation in the definitions of bleeding in clinical trials of patients with acute coronary syndromes and undergoing percutaneous coronary interventions and its impact on the apparent safety of antithrombotic drugs. Am Heart J. 2007;154:3–11. doi: 10.1016/j.ahj.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–66. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 22.White HD, Aylward PE, Gallo R, et al. Hematomas of at least 5 cm and outcomes in patients undergoing elective percutaneous coronary intervention: insights from the SafeTy and Efficacy of Enoxaparin in PCI patients, an internationaL randomized Evaluation (STEEPLE) trial. Am Heart J. 2010;159:110–6. doi: 10.1016/j.ahj.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 24.Rao SV, Eikelboom J, Steg PG, et al. Standardized reporting of bleeding complications for clinical investigations in acute coronary syndromes: a proposal from the academic bleeding consensus (ABC) multidisciplinary working group. Am Heart J. 2009;158:881–6. doi: 10.1016/j.ahj.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, White HD, Ohman EM, et al. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet. 2007;369:907–19. doi: 10.1016/S0140-6736(07)60450-4. [DOI] [PubMed] [Google Scholar]
- 26.Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors or thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54:468–76. doi: 10.1016/j.jacc.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 27.Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der WR. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997;29:1269–75. doi: 10.1016/s0735-1097(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 28.Sherev DA, Shaw RE, Brent BN. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc Interv. 2005;65:196–202. doi: 10.1002/ccd.20354. [DOI] [PubMed] [Google Scholar]
- 29.Turi ZG. Optimizing vascular access: routine femoral angiography keeps the vascular complication away. Catheter Cardiovasc Interv. 2005;65:203–4. doi: 10.1002/ccd.20412. [DOI] [PubMed] [Google Scholar]
- 30.Blankenship JC, Balog C, Sapp SK, et al. Reduction in vascular access site bleeding in sequential abciximab coronary intervention trials. Catheter Cardiovasc Interv. 2002;57:476–83. doi: 10.1002/ccd.10322. [DOI] [PubMed] [Google Scholar]
- 31.Buchler JR, Ribeiro EE, Falcao JL, et al. A Randomized Trial of 5 versus 7 French Guiding Catheters for Transfemoral Percutaneous Coronary Stent Implantation. J Interv Cardiol. 2007 doi: 10.1111/j.1540-8183.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 32.Gallo R, Steinhubl SR, White HD, Montalescot G. Impact of anticoagulation regimens on sheath management and bleeding in patients undergoing elective percutaneous coronary intervention in the STEEPLE trial. Catheter Cardiovasc Interv. 2009;73:319–25. doi: 10.1002/ccd.21764. [DOI] [PubMed] [Google Scholar]
- 33.Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Interv. 2001;53:289–95. doi: 10.1002/ccd.1169. [DOI] [PubMed] [Google Scholar]
- 34.Fitts J, Ver LP, Hofmaster P, Malenka D. Fluoroscopy-guided femoral artery puncture reduces the risk of PCI-related vascular complications. J Interv Cardiol. 2008;21:273–8. doi: 10.1111/j.1540-8183.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 35.Seto AH, bu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial) JACC Cardiovasc Interv. 2010;3:751–8. doi: 10.1016/j.jcin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–86. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–40. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Mann T, Cubeddu G, Bowen J, et al. Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J Am Coll Cardiol. 1998;32:572–6. doi: 10.1016/s0735-1097(98)00288-5. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand OF, Rao SV, Pancholy S, et al. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3:1022–31. doi: 10.1016/j.jcin.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–56. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 41.Louvard Y, Lefevre T, Allain A, Morice M. Coronary angiography through the radial or the femoral approach: The CARAFE study. Catheter Cardiovasc Interv. 2001;52:181–7. doi: 10.1002/1522-726x(200102)52:2<181::aid-ccd1044>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 42.Vorobcsuk A, Konyi A, Aradi D, et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction Systematic overview and meta-analysis. Am Heart J. 2009;158:814–21. doi: 10.1016/j.ahj.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 43.Spaulding C, Lefevre T, Funck F, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39:365–70. doi: 10.1002/(SICI)1097-0304(199612)39:4<365::AID-CCD8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 44.Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999;138:430–6. doi: 10.1016/s0002-8703(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 45.Pinto DS, Stone GW, Shi C, et al. Economic evaluation of bivalirudin with or without glycoprotein IIb/IIIa inhibition versus heparin with routine glycoprotein IIb/IIIa inhibition for early invasive management of acute coronary syndromes. J Am Coll Cardiol. 2008;52:1758–68. doi: 10.1016/j.jacc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55:2187–95. doi: 10.1016/j.jacc.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 47.Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335–40. doi: 10.1002/ccd.21639. [DOI] [PubMed] [Google Scholar]
- 48.Brueck M, Bandorski D, Kramer W, Wieczorek M, Holtgen R, Tillmanns H. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009;2:1047–54. doi: 10.1016/j.jcin.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Cubero JM, Lombardo J, Pedrosa C, et al. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP) Catheter Cardiovasc Interv. 2009;73:467–72. doi: 10.1002/ccd.21900. [DOI] [PubMed] [Google Scholar]
- 50.Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der WR. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997;40:156–8. doi: 10.1002/(sici)1097-0304(199702)40:2<156::aid-ccd7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 51.Blankenship JC, Hellkamp AS, Aguirre FV, Demko SL, Topol EJ, Califf RM. Vascular access site complications after percutaneous coronary intervention with abciximab in the Evaluation of c7E3 for the Prevention of Ischemic Complications (EPIC) trial. Am J Cardiol. 1998;81:36–40. doi: 10.1016/s0002-9149(97)00796-0. [DOI] [PubMed] [Google Scholar]
- 52.Schulz S, Mehilli J, Neumann FJ, et al. ISAR-REACT 3A: a study of reduced dose of unfractionated heparin in biomarker negative patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:2482–91. doi: 10.1093/eurheartj/ehq330. [DOI] [PubMed] [Google Scholar]
- 53.Dumaine R, Borentain M, Bertel O, et al. Intravenous low-molecular-weight heparins compared with unfractionated heparin in percutaneous coronary intervention: quantitative review of randomized trials. Arch Intern Med. 2007;167:2423–30. doi: 10.1001/archinte.167.22.2423. [DOI] [PubMed] [Google Scholar]
- 54.Montalescot G, White HD, Gallo R, et al. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med. 2006;355:1006–17. doi: 10.1056/NEJMoa052711. [DOI] [PubMed] [Google Scholar]
- 55.Gibson CM, Murphy SA, Montalescot G, et al. Percutaneous coronary intervention in patients receiving enoxaparin or unfractionated heparin after fibrinolytic therapy for ST-segment elevation myocardial infarction in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol. 2007;49:2238–46. doi: 10.1016/j.jacc.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 56.White HD, Kleiman NS, Mahaffey KW, et al. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial. Am Heart J. 2006;152:1042–50. doi: 10.1016/j.ahj.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Brieger D, Van de WF, Avezum A, et al. Interactions between heparins, glycoprotein IIb/IIIa antagonists, and coronary intervention. The Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2007;153:960–9. doi: 10.1016/j.ahj.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 58.Cohen M, Levine GN, Pieper KS, et al. Enoxaparin 0.3 mg/kg IV supplement for patients transitioning to PCI after subcutaneous enoxaparin therapy for NSTE ACS: a subgroup analysis from the SYNERGY trial. Catheter Cardiovasc Interv. 2010;75:928–35. doi: 10.1002/ccd.22340. [DOI] [PubMed] [Google Scholar]
- 59.Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50:1742–51. doi: 10.1016/j.jacc.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 60.Mehta SR, Boden WE, Eikelboom JW, et al. Antithrombotic therapy with fondaparinux in relation to interventional management strategy in patients with ST- and non-ST-segment elevation acute coronary syndromes: an individual patient-level combined analysis of the Fifth and Sixth Organization to Assess Strategies in Ischemic Syndromes (OASIS 5 and 6) randomized trials. Circulation. 2008;118:2038–46. doi: 10.1161/CIRCULATIONAHA.108.789479. [DOI] [PubMed] [Google Scholar]
- 61.Fox KA, Bassand JP, Mehta SR, et al. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med. 2007;147:304–10. doi: 10.7326/0003-4819-147-5-200709040-00005. [DOI] [PubMed] [Google Scholar]
- 62.Steg PG, Jolly SS, Mehta SR, et al. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339–49. doi: 10.1001/jama.2010.1320. [DOI] [PubMed] [Google Scholar]
- 63.Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–16. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 64.Mehran R, Lansky AJ, Witzenbichler B, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374:1149–59. doi: 10.1016/S0140-6736(09)61484-7. [DOI] [PubMed] [Google Scholar]
- 65.Gibson CM, Morrow DA, Murphy SA, et al. A randomized trial to evaluate the relative protection against post-percutaneous coronary intervention microvascular dysfunction, ischemia, and inflammation among antiplatelet and antithrombotic agents: the PROTECT-TIMI-30 trial. J Am Coll Cardiol. 2006;47:2364–73. doi: 10.1016/j.jacc.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 66.Parodi G, De LG, Moschi G, et al. Safety of immediate reversal of anticoagulation by protamine to reduce bleeding complications after infarct artery stenting for acute myocardial infarction and adjunctive abciximab therapy. J Thromb Thrombolysis. 2010;30:446–51. doi: 10.1007/s11239-010-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parodi G, Migliorini A, Valenti R, et al. Comparison of bivalirudin and unfractionated heparin plus protamine in patients with coronary heart disease undergoing percutaneous coronary intervention (from the Antithrombotic Regimens aNd Outcome [ARNO] trial) Am J Cardiol. 2010;105:1053–9. doi: 10.1016/j.amjcard.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Fung AY, Saw J, Starovoytov A, et al. Abbreviated infusion of eptifibatide after successful coronary intervention The BRIEF-PCI (Brief Infusion of Eptifibatide Following Percutaneous Coronary Intervention) randomized trial. J Am Coll Cardiol. 2009;53:837–45. doi: 10.1016/j.jacc.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 69.Gu YL, Fokkema ML, Kampinga MA, et al. Intracoronary versus intravenous abciximab in ST-segment elevation myocardial infarction: rationale and design of the CICERO trial in patients undergoing primary percutaneous coronary intervention with thrombus aspiration. Trials. 2009;10:90. doi: 10.1186/1745-6215-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White HD, Chew DP, Hoekstra JW, et al. Safety and efficacy of switching from either unfractionated heparin or enoxaparin to bivalirudin in patients with non-ST-segment elevation acute coronary syndromes managed with an invasive strategy: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol. 2008;51:1734–41. doi: 10.1016/j.jacc.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 71.Dangas G, Mehran R, Guagliumi G, et al. Role of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2009;54:1438–46. doi: 10.1016/j.jacc.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Parodi G, Antoniucci D, Nikolsky E, et al. Impact of bivalirudin therapy in high-risk patients with acute myocardial infarction: 1-year results from the HORIZONS-AMI (Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2010;3:796–802. doi: 10.1016/j.jcin.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Kastrati A, Neumann FJ, Mehilli J, et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359:688–96. doi: 10.1056/NEJMoa0802944. [DOI] [PubMed] [Google Scholar]
- 74.Iijima R, Ndrepepa G, Mehilli J, et al. Profile of bleeding and ischaemic complications with bivalirudin and unfractionated heparin after percutaneous coronary intervention. Eur Heart J. 2009;30:290–6. doi: 10.1093/eurheartj/ehn586. [DOI] [PubMed] [Google Scholar]
- 75.Schulz S, Mehilli J, Ndrepepa G, et al. Bivalirudin vs. unfractionated heparin during percutaneous coronary interventions in patients with stable and unstable angina pectoris: 1-year results of the ISAR-REACT 3 trial. Eur Heart J. 2010;31:582–7. doi: 10.1093/eurheartj/ehq008. [DOI] [PubMed] [Google Scholar]
- 76.Kastrati A, Neumann FJ, Mehilli J, et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359:688–96. doi: 10.1056/NEJMoa0802944. [DOI] [PubMed] [Google Scholar]
- 77.Nader RG, Garcia JC, Drushal K, Pesek T. Clinical evaluation of SyvekPatch in patients undergoing interventional, EPS and diagnostic cardiac catheterization procedures. J Invasive Cardiol. 2002;14:305–7. [PubMed] [Google Scholar]
- 78.Applegate RJ, Sacrinty MT, Kutcher MA, et al. Propensity score analysis of vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention using thrombin hemostatic patch-facilitated manual compression. J Invasive Cardiol. 2007;19:164–70. [PubMed] [Google Scholar]
- 79.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350–7. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 80.Nikolsky E, Mehran R, Halkin A, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44:1200–9. doi: 10.1016/j.jacc.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 81.Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J Invasive Cardiol. 2004;16:243–6. [PubMed] [Google Scholar]
- 82.Tavris DR, Dey S, brecht-Gallauresi B, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use - phase II. J Invasive Cardiol. 2005;17:644–50. [PubMed] [Google Scholar]
- 83.Applegate RJ, Sacrinty M, Kutcher MA, et al. Vascular complications with newer generations of angioseal vascular closure devices. J Interv Cardiol. 2006;19:67–74. doi: 10.1111/j.1540-8183.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 84.Balzer JO, Scheinert D, Diebold T, Haufe M, Vogl TJ, Biamino G. Postinterventional transcutaneous suture of femoral artery access sites in patients with peripheral arterial occlusive disease: a study of 930 patients. Catheter Cardiovasc Interv. 2001;53:174–81. doi: 10.1002/ccd.1144. [DOI] [PubMed] [Google Scholar]
- 85.Warren BS, Warren SG, Miller SD. Predictors of complications and learning curve using the Angio-Seal closure device following interventional and diagnostic catheterization. Catheter Cardiovasc Interv. 1999;48:162–6. doi: 10.1002/(sici)1522-726x(199910)48:2<162::aid-ccd8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 86.Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153:606–11. doi: 10.1016/j.ahj.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 87.Sanborn TA, Ebrahimi R, Manoukian SV, et al. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:57–62. doi: 10.1161/CIRCINTERVENTIONS.109.896704. [DOI] [PubMed] [Google Scholar]
- 88.Chevalier B, Lancelin B, Koning R, et al. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv. 2003;58:285–91. doi: 10.1002/ccd.10431. [DOI] [PubMed] [Google Scholar]
- 89.Pocock SJ, Mehran R, Clayton TC, et al. Prognostic modeling of individual patient risk and mortality impact of ischemic and hemorrhagic complications: assessment from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2010;121:43–51. doi: 10.1161/CIRCULATIONAHA.109.878017. [DOI] [PubMed] [Google Scholar]
- 90.Ellis SG, Bhatt D, Kapadia S, Lee D, Yen M, Whitlow PL. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;67:541–5. doi: 10.1002/ccd.20671. [DOI] [PubMed] [Google Scholar]
- 91.Bangalore S, Arora N, Resnic FS. Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ Cardiovasc Interv. 2009;2:549–56. doi: 10.1161/CIRCINTERVENTIONS.109.877407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kugelmass AD, Cohen DJ, Brown PP, Simon AW, Becker ER, Culler SD. Hospital resources consumed in treating complications associated with percutaneous coronary interventions. Am J Cardiol. 2006;97:322–7. doi: 10.1016/j.amjcard.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 93.Hamon M, Rasmussen LH, Manoukian SV, et al. Choice of arterial access site and outcomes in patients with acute coronary syndromes managed with an early invasive strategy: the ACUITY trial. EuroIntervention. 2009;5:115–20. doi: 10.4244/eijv5i1a18. [DOI] [PubMed] [Google Scholar]
- 94.Wang TY, Xiao L, Alexander KP, et al. Antiplatelet therapy use after discharge among acute myocardial infarction patients with in-hospital bleeding. Circulation. 2008;118:2139–45. doi: 10.1161/CIRCULATIONAHA.108.787143. [DOI] [PubMed] [Google Scholar]


