Abstract
Glial-derived neurotrophic factor (GDNF) is a potential neurotrophic factor treatment of brain disorders, including Parkinson's disease. However, GDNF does not cross the blood-brain barrier (BBB). A brain-penetrating form of GDNF, which is a fusion protein of human GDNF and a chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), has been engineered for the mouse and is designated the cTfRMAb-GDNF fusion protein. The present study examined the potential toxic side effects and immune response after treatment of mice with twice-weekly cTfRMAb-GDNF fusion protein at a dose of 2 mg/kg i.v. for 12 consecutive weeks. Chronic treatment with the fusion protein caused no change in body weight, no change in 23 serum chemistry measurements, and no histologic changes in brain and cerebellum, kidney, liver, spleen, heart, or pancreas. Chronic treatment caused a low-titer immune response against the fusion protein, which was directed against the variable region of the antibody part of the fusion protein, with no immune response directed against either the constant region of the antibody or against GDNF. A pharmacokinetics and brain uptake study was performed at the end of the 12 weeks of treatment. There was no change in clearance of the fusion protein mediated by the TfR in peripheral organs, and there was no change in BBB permeability to the fusion protein mediated by the TfR at the BBB. The study shows no toxic side effects from chronic cTfRMAb-GDNF systemic treatment and the absence of neutralizing antibodies in vivo.
Introduction
Glial-derived neurotrophic factor (GDNF) is a potential treatment for Parkinson's disease, because GDNF is a trophic factor for the nigral-striatal tract in brain. However, GDNF does not cross the blood-brain barrier (BBB) (Kastin et al., 2003; Boado and Pardridge, 2009). GDNF can be made transportable through the BBB via receptor-mediated transport on an endogenous BBB peptide receptor after the reengineering of the neurotrophin as an IgG-GDNF fusion protein. The IgG part of the fusion protein is a peptidomimetic monoclonal antibody (MAb) against an endogenous BBB receptor such as the insulin receptor or the transferrin receptor (TfR). The antireceptor MAb binds an exofacial epitope on the BBB receptor, which triggers transport across the BBB, and acts as a molecular Trojan horse (MTH) to ferry into brain the fused GDNF (Boado and Pardridge, 2009). For drug delivery to the human brain, GDNF was fused to a genetically engineered MAb against the human insulin receptor (HIR) (Boado et al., 2008). However, the HIRMAb-GDNF fusion protein only cross-reacts with the insulin receptor in Old World primates such as the rhesus monkey (Pardridge et al., 1995) and cannot be tested in rodent models. There is no known MAb against the rodent insulin receptor that can be used as a MTH in rats or mice. Therefore, a surrogate MTH for the mouse, which is a chimeric MAb against the mouse TfR, was engineered and designated the cTfRMAb (Boado et al., 2009). A fusion protein of the cTfRMAb and GDNF has been engineered and designated the cTfRMAb-GDNF fusion protein. The cTfRMAb-GDNF fusion protein is a bifunctional protein and binds both to the mouse TfR and to the GDNF receptor (GFR)-α1 with high affinity and low nanomolar KD values (Zhou et al., 2010). The cTfRMAb-GDNF fusion protein is rapidly transported across the mouse BBB, and the in vivo brain uptake is 3.1% of injected dose (ID)/g brain (Zhou et al., 2010). Chronic treatment of mice with experimental Parkinson's disease with intravenous cTfRMAb-GDNF fusion protein at a dose of 1 mg/kg every other day leads to a 272% increase in striatal tyrosine hydroxylase enzyme activity and an improvement in neural deficit (Fu et al., 2010). However, the potential toxic effects of chronic administration of the cTfRMAb-GDNF fusion protein are not known. In addition, chronic administration of the cTfRMAb-GDNF fusion protein may lead to an immune response, and the formation of TfR-neutralizing antibodies (NAbs) could impair the biologic efficacy of the fusion protein in chronic treatment. Therefore, the purpose of the present study was to evaluate chronic dosing of mice with twice-weekly intravenous saline vehicle or cTfRMAb-GDNF fusion protein at a dose of 2 mg/kg per dose, or 4 mg/kg per week, for 12 consecutive weeks. To investigate potential toxicity, histology of the brain and major peripheral organs was examined, and a panel of 23 serum chemistry parameters was analyzed in the saline and cTfRMAb-GDNF fusion protein treatment groups. The immune response was analyzed with a bridging ELISA, and a potential anti-TfR NAb response was evaluated by measurement of the plasma pharmacokinetics and brain uptake of the cTfRMAb-GDNF fusion protein at the end of the 12-week treatment period. Clearance of the fusion protein by peripheral organs was used as an index of potential neutralization of the peripheral TfR, and clearance of the fusion protein by brain was used as an index of potential neutralization of the TfR at the BBB.
Materials and Methods
Production of cTfRMAb-GDNF Fusion Protein.
The cTfRMAb-GDNF fusion protein was purified by protein G affinity chromatography of serum-free medium conditioned by a stably transfected Chinese hamster ovary (CHO) line, as described previously (Zhou et al., 2010). The purity, identity, and potency of the fusion protein were verified by SDS-polyacrylamide gel electrophoresis, mouse IgG and GDNF Western blotting, TfR radioreceptor assay, and GFRα1 binding assay as described previously (Zhou et al., 2010).
Chronic Dosing of Mice.
Adult C57BL/6J mice, 10 to 12 weeks of age, were obtained from The Jackson Laboratory (Bar Harbor, ME). The treatment group included 12 males, 28 g b.wt., and 12 females, 20 g b.wt. Mice were treated twice/week with a tail vein injection of 2 mg/kg (60 μl/mouse of 1 mg/ml) of cTfRMAb-GDNF fusion protein or 60 μl/mouse of fusion protein vehicle (Tris-buffered saline, pH = 5.5). More than 500 tail vein injections were performed for the study. After 12 weeks of treatment, mice were euthanized by cervical dislocation under anesthesia, and organs were removed for histologic analysis and processed in three separate vials for fixation: 1) the entire cerebral hemisphere with cerebellum; 2) the heart, kidney, and liver; and 3) the spleen and pancreas. After 48 h of fixation in 10% buffered formalin, the tissues were embedded in paraffin, and 5-μm sections were prepared for hematoxylin and eosin staining at the UCLA Translational Pathology Core Laboratory. The terminal serum was collected and frozen, and a comprehensive metabolic panel and an anemia panel (iron and total iron-binding capacity) were analyzed at Molecular Diagnostic Services, Inc. (San Diego, CA).
Pharmacokinetics and Brain Uptake in the Mouse.
The cTfRMAb-GDNF fusion protein was tritiated with [3H]N-succinimidyl propionate (American Radiolabeled Chemicals, St. Louis, MO) as described previously (Zhou et al., 2010). The specific activity was 0.6 μCi/μg, and the trichloroacetic acid precipitability was 95.5%. At the end of the 12-week dosing with either saline or fusion protein, four mice (two males and two females) from the saline treatment group and four mice (two males and two females) from the cTfRMAb-GDNF fusion protein treatment group were tested for plasma clearance and brain uptake of the [3H]cTfRMAb-GDNF fusion protein as described previously (Zhou et al., 2010). Mice were anesthetized with 100 mg/kg ketamine i.p. and 10 mg/kg xylazine i.p. and were given intravenous injections in the tail vein with 0.1 ml (10 μCi) of [3H]cTfRMAb-GDNF fusion protein. The injection dose in each mouse of the cTfRMAb-GDNF fusion protein was 0.8 mg/kg. An aliquot (50 μl) of heparinized blood was collected from the retro-orbital vein at 0.25, 2, 5, 15, 30, and 60 min from each mouse after injection of the fusion protein. The blood was centrifuged for collection of plasma, which was analyzed for radioactivity. At 60 min after injection, the mice were euthanized without saline perfusion of organs, and major organs and the cerebral hemispheres were removed, weighed, and solubilized in Soluene-350 (PerkinElmer Life and Analytical Sciences, Waltham, MA) and analyzed for 3H radioactivity with Opti-Fluor O (PerkinElmer Life and Analytical Sciences) and a liquid scintillation counter (Tri-Carb 2100TR, PerkinElmer Life and Analytical Sciences). Brain uptake data were expressed as the percentage of ID per gram of tissue.
The plasma radioactivity in disintegrations per minute per milliliter was converted to percentage ID per milliliter, and the percentage ID per milliliter was fit to a biexponential equation.
The intercepts (A1 and A2) and the slopes (k1 and k2) were used to compute the pharmacokinetic parameters, including the mean residence time, the central volume of distribution, the steady-state volume of distribution, the area under the plasma concentration curve (AUC), and the systemic clearance. Nonlinear regression analysis used the AR subroutine of the BMDP Statistical Software (Statistical Solutions Ltd., Cork, Ireland). Data were weighted by 1/(% ID/ml)2.
The brain clearance (microliters per minute per gram), also called the BBB permeability-surface area (PS) product, is computed from the terminal brain uptake (percentage ID per gram) and the 60-min plasma AUC (percentage ID · minute per milliliter) as follows:
The brain uptake, or percentage ID per gram, was first corrected by the brain uptake in the mouse of an IgG confined to the brain vascular volume, which is 0.06% ID/g (Zhou et al., 2010).
Immunity ELISA.
The presence of anti-cTfRMAb-GDNF fusion protein antibodies in mouse serum was detected with a bridging ELISA, using the cTfRMAb-GDNF fusion protein as the capture reagent and biotinylated cTfRMAb-GDNF fusion protein as the detector reagent. As an alternative, the CHO cell-derived cTfRMAb (Boado et al., 2009), mouse IgG1κ, which is the isotype antibody for the constant regions of the fusion protein (Sigma-Aldrich, St. Louis, MO), rat 8D3 mAb against the mouse TfR, which has the same variable regions as the fusion protein (Lee et al., 2000), or human recombinant GDNF (PeproTech, Rocky Hill, NJ) was used as the capture reagent. The mouse serum was diluted in PBS. The capture reagent was plated overnight at 4°C in 96 wells at 100 μl (250 ng)/well in 0.05 M NaHCO3, pH 8.3. The wells were blocked with PBS containing 1% bovine serum albumin (PBSB), followed by the addition of 100 μl/well of the diluted mouse serum. After a 60-min incubation at 37°C, the wells were washed with PBSB and incubated with biotinylated cTfRMAb-GDNF fusion protein (12 ng/well) for 60 min. The wells were washed with PBSB, followed by incubation with 100 μl (500 ng/well) of a streptavidin-peroxidase conjugate (Vector Laboratories, Burlingame, CA) for 30 min at room temperature. The wells were washed with PBSB, and 100 μl/well of o-phenylenediamine-H2O2 developing solution (Sigma-Aldrich) was added for a 15-min incubation in the dark at room temperature. The reaction was stopped by the addition of 100 μl/well 1 M HCl, followed by the measurement of absorbance at 492 and 650 nm. The A650 was subtracted from the A492. The (A492 − A650) for the PBSB blank was then subtracted from the (A492 − A650) for the sample. Mouse serum samples were screened with the immunity ELISA at 1:50 dilutions in PBS using the cTfRMAb-GDNF fusion protein as the capture reagent. For subsequent studies and because the immunoreactivity was comparable in all fusion protein-treated mice, the terminal serum from all mice treated with the cTfRMAb-GDNF fusion protein was pooled. This pool was then diluted 1:50, 1:100 1:300, 1:1000, or 1:3000 in PBS. A mouse monoclonal GDNF-neutralizing antibody (R&D Systems, Minneapolis, MN) was tested at concentrations ranging from 0.1 to 30 μg/ml and was used as a positive control in the assay for detection of anti-GDNF antibodies in the mouse serum. The mouse dilution curves were determined for different capture reagents: the CHO-derived cTfRMAb, the hybridoma-derived rat 8D3 mAb against the mouse TfR, GDNF, or mouse IgG1κ, which is the isotype control for the constant region comprising the cTfRMAb. The cTfRMAb-GDNF fusion protein was biotinylated as described previously (Pardridge and Boado, 2009), using sulfo-biotin-LC-LC-N-hydroxysuccinimide, where LC = long-chain (Thermo Fisher Scientific, Waltham, MA). The biotinylation of the cTfRMAb-GDNF fusion protein was confirmed by SDS-polyacrylamide gel electrophoresis and Western blotting, in which the blot was probed with avidin and biotinylated peroxidase. The nonbiotinylated cTfRMAb-GDNF fusion protein gave no reaction in the Western blot, whereas the biotinylated protein was strongly visualized at the appropriate molecular size for both heavy chain and light chain.
Statistics.
Statistical differences at the p < 0.05 level were determined by Student's t test.
Results
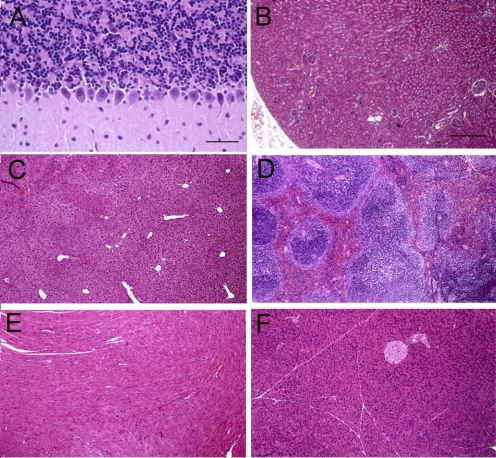
All 24 mice tolerated well the chronic treatment with twice-weekly cTfRMAb-GDNF fusion protein or saline via tail vein injection. There was no difference in body weights between the males or females of the saline- or fusion protein-treated groups (Table 1). No mice exhibited any clinical signs of immune reactions to the fusion protein, and no mice required treatment with diphenhydramine or other immune-response modifiers. There was no difference in 23 serum chemistry measurements between the saline- and fusion protein-treated mice, including no differences in serum iron or total iron-binding capacity (Table 2). No pathologic findings were observed in brain in any mice after review of sagittal sections encompassing the olfactory lobe to the cerebellum. Layers of the cerebellum, including the granular layer, the Purkinje cell layer, and the molecular layer showed normal histology (Fig. 1A). Purkinje cell dendrites were visible in the molecular layer in the fusion protein-treated mice to the same extent as in the saline-treated mice. No abnormalities were observed in peripheral organs (liver, spleen, heart, kidney, and pancreas), and representative organ histology is shown in Fig. 1 for the fusion protein-treated mice.
TABLE 1.
Body weights
Data are means ± S.D. (n = 6 mice in each of the four treatment groups).
| Weeks | cTfRMAb-GDNF |
Saline |
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| g | ||||
| 0 | 28.1 ± 2.1 | 20.2 ± 1.0 | 29.0 ± 1.2 | 19.5 ± 1.6 |
| 3 | 28.4 ± 1.9 | 20.4 ± 1.2 | 28.9 ± 0.9 | 21.1 ± 2.0 |
| 6 | 29.6 ± 1.3 | 22.4 ± 0.8 | 31.1 ± 0.9 | 22.4 ± 1.9 |
| 9 | 30.4 ± 1.5 | 22.6 ± 0.8 | 32.9 ± 0.7 | 22.7 ± 2.3 |
| 12 | 31.3 ± 2.0 | 23.4 ± 1.2 | 33.4 ± 0.6 | 23.5 ± 2.5 |
TABLE 2.
Serum metabolic panel
Data are means ± S.D. (n = 6 mice/group). No statistical differences were seen between the two groups. Males and females are combined, because there were no differences between sexes.
| Parameter | Units | Treatment Group |
|
|---|---|---|---|
| Saline | cTfRMAb-GDNF | ||
| Sodium | mEq/l | 151 ± 2 | 151 ± 2 |
| Potassium | mEq/l | 4.8 ± 0.5 | 5.1 ± 0.5 |
| Chloride | mEq/l | 125 ± 6 | 124 ± 5 |
| CO2 | mEq/l | 24 ± 4 | 23 ± 3 |
| Calcium | mg/dl | 9.7 ± 0.3 | 10.2 ± 0.3 |
| Phosphorus | mg/dl | 8.9 ± 0.7 | 9.5 ± 1.4 |
| Magnesium | mg/dl | 4.4 ± 0.2 | 4.6 ± 0.1 |
| Glucose | mg/dl | 205 ± 35 | 213 ± 38 |
| BUN | mg/dl | 22 ± 1 | 26 ± 3 |
| Creatinine | mg/dl | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Total bilirubin | mg/dl | 0.6 ± 0.3 | 0.7 ± 0.3 |
| Direct bilirubin | mg/dl | <0.1 | <0.1 |
| Total protein | g/dl | 4.8 ± 0.1 | 4.9 ± 0.3 |
| Albumin | g/dl | 3.1 ± 0.2 | 3.3 ± 0.1 |
| Globulin | g/dl | 1.7 ± 0.2 | 1.6 ± 0.3 |
| Uric acid | mg/dl | 2.4 ± 0.2 | 3.1 ± 0.8 |
| AST | IU/ml | 88 ± 24 | 98 ± 7 |
| ALT | IU/ml | 35 ± 10 | 31 ± 13 |
| ALK | IU/ml | 72 ± 26 | 77 ± 22 |
| GGT | IU/ml | <2 | <2 |
| Creatine kinase | IU/ml | 172 ± 71 | 280 ± 32 |
| Iron | μg/dl | 128 ± 11 | 132 ± 8 |
| TIBC | μg/dl | 271 ± 18 | 278 ± 10 |
BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALK, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; TIBC, total iron-binding capacity.
Fig. 1.
Hematoxylin and eosin histology for cerebellum (A), kidney (B), liver (C), spleen (D), heart (E), and pancreas (F). In the cerebellum, Purkinje cells are observed at the interface of the granular layer (top) and the molecular layer (bottom) of the section. Magnification is the same in B to F. Scale bars, 42 (A) and 210 (B) μm.
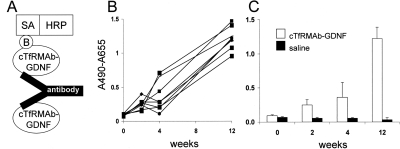
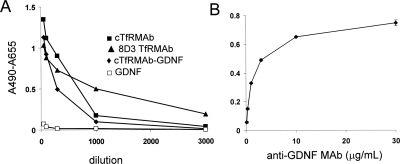
The design of the immunity bridging ELISA is shown in Fig. 2A; owing to antibody bivalency, the anti-fusion protein antibodies in mouse serum bind both the capture reagent and the biotinylated fusion protein detector reagent. There was a time-dependent increase in immune response directed against the cTfRMAb-GDNF fusion protein over the course of the 12-week treatment period in all fusion protein-treated mice (Fig. 2B). The absorbance readings at 2, 4, and 12 weeks were averaged and compared with the mean absorbance readings in the saline-treated mice, which showed no immune response against the fusion protein in the saline-treated mice (Fig. 2C). The absorbance readings shown in Fig. 2 were all determined with 1:50 dilutions of mouse sera. To determine the titer of the immune response against different portions of the fusion protein, the serum of all fusion protein-treated mice collected after 12 weeks of treatment was pooled and diluted from 1:50 to 1:3000. When the cTfRMAb-GDNF fusion protein was used as the capture reagent, the absorbance was near background at a 1:1000 dilution (Fig. 3A). The anti-fusion protein antibodies in the 12-week mouse serum pool also reacted with the original rat 8D3 TfRMAb and the cTfRMAb, but there was minimal reaction against GDNF (Fig. 3A). Mouse IgG1k is the isotype antibody for the constant region of the heavy and light chains of the fusion protein. When mouse IgG1k was used as the capture reagent, there was no immune reaction detected. To demonstrate that the bridging ELISA outlined in Fig. 2A could detect antibodies against the GDNF portion of the fusion protein, a mouse MAb against human GDNF was assayed. As shown in Fig. 3B, there is a dose-dependent and saturable immunoreactivity of this antibody in the immunity ELISA.
Fig. 2.
A, structure of the bridging ELISA for detection of antibodies against the cTfRMAb-GDNF fusion protein. The cTfRMAb-GDNF fusion protein is used as the capture reagent, and the biotinylated cTfRMAb-GDNF fusion protein is used as the detector reagent, along with a complex of streptavidin (SA) and horseradish peroxidase (HRP); the biotin moiety is designated B. B, the immune response in individual fusion protein-treated mice is plotted against the number of weeks of treatment. C, the mean immune response in the mice treated with either fusion protein or saline is plotted against the number of weeks of treatment. The capture reagent in the assays shown in B and C was the cTfRMAb-GDNF fusion protein.
Fig. 3.
A, terminal 12-week sera from all fusion protein-treated mice were pooled and diluted 1:50 to 1:3000 in PBS, and immunoreactivity was measured against four different capture reagents: cTfRMAb, 8D3 TfRMAb, cTfRMAb-GDNF fusion protein, and GDNF. B, the immunoreactivity of a mouse anti-GDNF antibody is plotted against the antibody concentration; the capture reagent in this assay was the cTfRMAb-GDNF fusion protein.
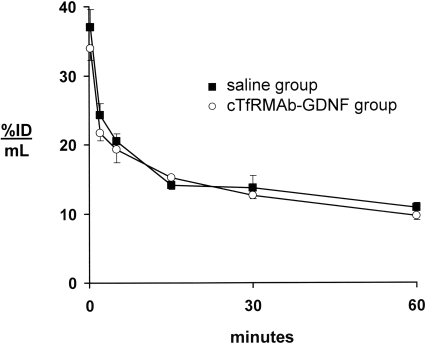
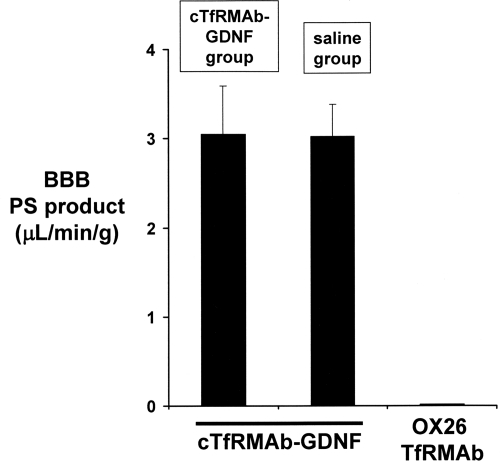
Any anti-TfR NAbs in the blood of the fusion protein-treated mice could potentially block fusion protein binding to the TfR in either peripheral organs or at the BBB. To determine whether any anti-TfR NAbs are formed, the [3H]cTfRMAb-GDNF fusion protein was injected intravenously in four of the fusion protein-treated mice (two males and two females) and four of the saline-treated mice (two males and two females) before euthanasia at the end of the 12-week treatment period. There was no change in the rate of removal of the fusion protein from blood via clearance by peripheral organs (Fig. 4). The fusion protein was metabolically stable in both treatment groups, because the plasma radioactivity at 60 min after intravenous injection was 95 ± 2% in both groups. There was no change in the plasma pharmacokinetic parameters in the saline-treated and fusion protein-treated mice (Table 3). There was no change in uptake of the fusion protein by brain or peripheral organs in the saline-treated and fusion protein-treated mice (Table 4). The brain uptake, percentage ID per gram (Table 4), and the 60-min plasma AUC (Table 3) were used to compute the BBB PS product of fusion protein, and there was no change in BBB transport of the cTfRMAb-GDNF fusion protein in the saline-treated and fusion protein-treated mice (Fig. 5).
Fig. 4.
Plasma concentration, expressed as percentage of ID per milliliter, of the [3H]cTfRMAb-GDNF fusion protein after intravenous injection in mice from either the saline treatment group or the cTfRMAb-GDNF fusion protein group. Males and females are combined, because there were no differences between sexes. Data are means ± S.E. (n = 4 mice/point).
TABLE 3.
Pharmacokinetic parameters
Data are means ± S.D. Males and females are combined, because there were no differences between sexes.
| Parameter | Units | Treatment Group |
|
|---|---|---|---|
| cTfRMAb-GDNF | Saline | ||
| A1 | % ID/ml | 18.4 ± 3.4 | 21.4 ± 3.9 |
| A2 | % ID/ml | 18.4 ± 1.3 | 16.2 ± 1.8 |
| k1 | min−1 | 0.73 ± 0.25 | 0.38 ± 0.12 |
| k2 | min−1 | 0.011 ± 0.002 | 0.0067 ± 0.0027 |
| MRT | min | 89 ± 15 | 146 ± 59 |
| Vc | ml/kg | 97 ± 9 | 95 ± 9 |
| Vss | ml/kg | 188 ± 12 | 210 ± 23 |
| AUC (60 min) | % ID · min/ml | 831 ± 27 | 859 ± 36 |
| AUCss | % ID · min/ml | 1681 ± 201 | 2479 ± 777 |
| CL | ml · min−1 · kg−1 | 2.12 ± 0.25 | 1.44 ± 0.47 |
MRT, mean residence time; Vc, central volume of distribution; Vss, steady-state volume of distribution; AUC, area under the plasma concentration curve; CL, systemic clearance.
TABLE 4.
Organ uptake of cTfRMAb-GDNF fusion protein
Data are means ± S.D. (n = 4/group). Males and females are combined, as there were no differences between sexes.
| Organ | Treatment Group |
|
|---|---|---|
| cTfRMAb-GDNF | Saline | |
| Heart | 2.00 ± 0.80 | 2.41 ± 0.70 |
| Liver | 9.76 ± 2.19 | 11.3 ± 3.4 |
| Spleen | 14.5 ± 3.7 | 13.0 ± 4.1 |
| Lung | 11.0 ± 3.5 | 10.4 ± 2.8 |
| Kidney | 4.60 ± 0.94 | 3.46 ± 0.78 |
| Brain | 2.54 ± 0.90 | 2.60 ± 0.61 |
Fig. 5.
BBB PS produce of the [3H]cTfRMAb-GDNF fusion protein measured in either the saline treatment group or the cTfRMAb-GDNF fusion protein group.
Discussion
The findings of this study are consistent with the following conclusions. First, chronic treatment of mice with intravenous cTfRMAb-GDNF fusion protein causes no toxic side effects, because there is no change in body weight (Table 1), no change in serum chemistry (Table 2), and no change in organ histology (Fig. 1). Second, chronic treatment with the fusion protein induces a time-dependent immune response (Fig. 2), which is low-titer and directed against the variable region of the cTfRMAb part of the fusion protein (Fig. 3). Third, the antibodies formed against the cTfRMAb have no functional effect, because the rate of clearance of the fusion protein mediated by the TfR in peripheral organs is unchanged (Fig. 4; Tables 3 and 4), and the clearance of the fusion protein by brain mediated by the BBB TfR is unchanged (Fig. 5).
The biologic effects of GDNF and related neurotrophins (persephin, neurturin, and artemin) are mediated by binding of the neurotrophin to the cognate receptor, which for GDNF is GFRα1. Receptor binding then triggers activation of the c-ret kinase within the target cell (Airaksinen and Saarma, 2002). GDNF, GFRα1, and the c-ret kinase are expressed in peripheral organs, as well as the central nervous system. In the mouse, GFRα1 mRNA is highly expressed in peripheral nerve, liver, and kidney, whereas the c-ret kinase mRNA is highly expressed in peripheral nerve, pituitary, heart, and skeletal muscle (Naveilhan et al., 1998). GDNF may have a role in development of the kidney (Vega et al., 1996) and the pancreas (Lucini et al., 2008). GFRα1 and c-ret are expressed in the heart and play a role in the cholinergic innervation of the heart (Hiltunen et al., 2000). There was no change in body weight (Table 1) or organ histology in kidney, liver, spleen, heart, or pancreas (Fig. 1), and there was no change in 23 serum chemistry measurements that reflect hepatic, renal, metabolic, and iron function (Table 2). The TfRMAb part of the cTfRMAb-GDNF fusion protein may potentially have effects on iron homeostasis. However, chronic treatment with the fusion protein has no effect on serum levels of iron or total iron-binding capacity (Table 2).
The chronic infusion in the brain of high doses of GDNF for 6 months in the rhesus monkey led to cerebellar degeneration (Hovland et al., 2007). However, in the present study, there was no evidence of toxicity in brain after 12 weeks of twice-weekly intravenous injections of the cTfRMAb-GDNF fusion protein (Fig. 1). There is no cerebellar degeneration in the fusion protein-treated mice, and the granule cell layer, the Purkinje cell layer, and the molecular layer of the cerebellum in the fusion protein-treated mice were indistinguishable from that of the saline-treated mice (Fig. 1A).
The fusion protein-treated mice developed a time-dependent immune response after 12 weeks of intravenous treatment (Fig. 2). However, the development of an immune response in the chronic treatment with a biologic agent is expected. What is important is the titer of the immune response and whether the antibodies formed against the fusion protein neutralize therapeutic action in vivo. The titer of the immune response is quantitated as the OD units per microliter of undiluted serum (Dickson et al., 2008). A titer of <10 is considered evidence of tolerance to the biologic agent (Dickson et al., 2008). The immunity ELISA records 1.5 OD units/100 μl of a 1:50 dilution of the mouse serum (Fig. 2), which is a titer of 0.75 OD unit/μl. The low titer of the immune response against the cTfRMAb-GDNF fusion protein is also demonstrated with the dilution curve (Fig. 3), which shows 0.09 OD unit at a dilution of 1:1000, corresponding to a titer of 0.9 OD unit/μl.
The use of different capture reagents in the immunity ELISA allows for identification of the domain of the cTfRMAb-GDNF fusion protein that accounts for the majority of the immune reactions against the fusion protein (Fig. 3). The fusion protein is composed of three domains: the variable regions of the heavy chain and the light chain, which arise from a rat IgG against the murine TfR (Boado et al., 2009), the heavy chain and light chain constant regions, which are derived from mouse IgG1 and mouse κ, respectively (Boado et al., 2009), and human GDNF (Zhou et al., 2010). The immune response against the GDNF part of the fusion protein is negligible (Fig. 3A). To confirm that the immunity ELISA could detect antibodies against the GDNF part of the fusion protein, a mouse neutralizing anti-GDNF antibody was studied, and this antibody reacted strongly in the immunity ELISA (Fig. 3B). In contrast to the minimal immune response against the GDNF part of the IgG-GDNF fusion protein in the present study, a peripheral immune response against GDNF was observed after the chronic infusion of GDNF into the brain of either rhesus monkeys (Hovland et al., 2007) or humans (Tatarewicz et al., 2007). The absence of a stronger immune response against the GDNF part of the cTfRMAb-GDNF fusion protein in the present study may be related to the presence of certain amino acid sequences, within the IgG constant region, called Tregitopes, which induce immune tolerance (De Groot et al., 2008).
The immune response against the cTfRMAb-GDNF fusion protein is primarily directed against the variable region of the cTfRMAb (Fig. 3). The variable region is composed of the framework regions and the complementarity determining regions of the antibody. If antibodies are formed against the complementarity determining region of the cTfRMAb, these could potentially neutralize antibody function in vivo by blocking cTfRMAb binding to the TfR. Neutralizing antibody assays are typically performed with cell-based bioassays in vitro. However, such an assay may not predict the process of receptor-mediated transport across the BBB in vivo via transport on the endogenous TfR. Therefore, in the present study, the pharmacokinetics and brain uptake of the [3H]cTfRMAb-GDNF fusion protein were assessed at the end of the 12-week treatment study in four mice from the saline-treated group and four mice from the fusion protein-treated group. The rate of clearance of the fusion protein from blood (Fig. 4), the pharmacokinetic parameters (Table 3), and the uptake of the fusion protein by peripheral tissues (Table 4) were unchanged in the two treatment groups. These findings indicate that there is no neutralization of the uptake of the cTfRMAb-GDNF fusion protein via the TfR in peripheral organs. Likewise, there is no change in the brain uptake of the fusion protein (Table 4) or the BBB permeability of the fusion protein (Fig. 5) in the mice treated chronically with the cTfRMAb-GDNF fusion protein. Therefore, there is no neutralization of the transport of the fusion protein via the BBB TfR in vivo.
In summary, chronic administration of the cTfRMAb-GDNF fusion protein in mice is shown to have a favorable safety profile with no histologic abnormalities in brain or peripheral organs and no change in serum chemistry. The immune response against the fusion protein generated by chronic intravenous treatment in the mouse is low-titer and has no functional consequences on the distribution of the fusion protein in brain in vivo.
Acknowledgments
We are indebted to Professor Harry Vinters (UCLA) for review of the brain histology and to Professor David Dawson (UCLA) for review of the peripheral organ histology. Winnie Tai and Phuong Tram provided technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS065917].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.038349.
- GDNF
- glial-derived neurotrophic factor
- BBB
- blood-brain barrier
- MAb
- monoclonal antibody
- TfR
- transferrin receptor
- MTH
- molecular Trojan horse
- HIR
- human insulin receptor
- HIRMAb
- engineered MAb against the HIR
- cTfRMAb
- chimeric MAb against the mouse TfR
- GFR
- GDNF receptor
- ID
- injected dose
- NAb
- TfR-neutralizing antibody
- ELISA
- enzyme-linked immunosorbent assay
- CHO
- Chinese hamster ovary
- AUC
- area under the concentration curve
- PS
- permeability-surface area
- PBS
- phosphate-buffered saline
- PBSB
- phosphate-buffered saline containing 1% bovine serum albumin.
Authorship Contributions
Participated in research design: Zhou, Boado, Hui, Lu, and Pardridge.
Conducted experiments: Zhou, Boado, Hui, Lu, and Pardridge.
Performed data analysis: Zhou, Boado, Hui, Lu, and Pardridge.
Wrote or contributed to the writing of the manuscript: Zhou, Boado, Hui, Lu, and Pardridge.
References
- Airaksinen MS, Saarma M. (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394 [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. (2009) Comparison of blood-brain barrier transport of glial-derived neurotrophic factor (GDNF) and an IgG-GDNF fusion protein in the rhesus monkey. Drug Metab Dispos 37:2299–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. (2009) Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng 102:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. (2008) GDNF fusion protein for targeted-drug delivery across the human blood-brain barrier. Biotechnol Bioeng 100:387–396 [DOI] [PubMed] [Google Scholar]
- De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, Scott DW, Martin W. (2008) Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes.” Blood 112:3303–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. (2008) Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest 118:2868–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. (2010) Intravenous treatment of experimental Parkinson's disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood-brain barrier. Brain Res 1352:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen JO, Laurikainen A, Airaksinen MS, Saarma M. (2000) GDNF family receptors in the embryonic and postnatal rat heart and reduced cholinergic innervation in mice hearts lacking ret or GFRα2. Dev Dyn 219:28–39 [DOI] [PubMed] [Google Scholar]
- Hovland DN, Jr, Boyd RB, Butt MT, Engelhardt JA, Moxness MS, Ma MH, Emery MG, Ernst NB, Reed RP, Zeller JR, et al. (2007) Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF) in rhesus monkeys. Toxicol Pathol 35:676–692 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. (2003) Glial cell line-derived neurotrophic factor does not enter normal mouse brain. Neurosci Lett 340:239–241 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. (2000) Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther 292:1048–1052 [PubMed] [Google Scholar]
- Lucini C, Maruccio L, Facello B, Cocchia N, Tortora G, Castaldo L. (2008) Cellular localization of GDNF and its GFRα1/RET receptor complex in the developing pancreas of cat. J Anat 213:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveilhan P, Baudet C, Mikaels A, Shen L, Westphal H, Ernfors P. (1998) Expression and regulation of GFRα3, a glial cell line-derived neurotrophic factor family receptor. Proc Natl Acad Sci USA 95:1295–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ. (2009) Pharmacokinetics and safety in rhesus monkeys of a monoclonal antibody-GDNF fusion protein for targeted blood-brain barrier delivery. Pharm Res 26:2227–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. (1995) Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res 12:807–816 [DOI] [PubMed] [Google Scholar]
- Tatarewicz SM, Wei X, Gupta S, Masterman D, Swanson SJ, Moxness MS. (2007) Development of a maturing T-cell-mediated immune response in patients with idiopathic Parkinson's disease receiving r-metHuGDNF via continuous intraputaminal infusion. J Clin Immunol 27:620–627 [DOI] [PubMed] [Google Scholar]
- Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR. (1996) Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA 93:10657–10661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Lu JZ, Hui EK, Pardridge WM. (2010) Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab Dispos 38:566–572 [DOI] [PMC free article] [PubMed] [Google Scholar]