Abstract
5-Methoxy-N,N,-dimethyltryptamine (5-MeO-DMT), an abused serotonergic indolealkylamine drug, was placed into Schedule I controlled substance status in the United States as of January 19, 2011. In previous studies, we have shown the impact of monoamine oxidase A and cytochrome P450 2D6 enzymes on 5-MeO-DMT metabolism and pharmacokinetics. The aim of this study was to investigate 5-MeO-DMT pharmacokinetic properties after intravenous or intraperitoneal administration of three different doses (2, 10, and 20 mg/kg) to CYP2D6-humanized (Tg-CYP2D6) and wild-type control mice. Systemic exposure [area under the curve (AUC)] to 5-MeO-DMT was increased nonproportionally with the increase in dose. The existence of nonlinearity in serum 5-MeO-DMT pharmacokinetics was clearly manifested by dose-normalized AUC values, which were approximately 1.5- to 2.0-fold (intravenous) and 1.8- to 2.7-fold (intraperitoneal) higher in wild-type or Tg-CYP2D6 mice dosed with 10 and 20 mg/kg 5-MeO-DMT, respectively, than those in mice treated with 2 mg/kg 5-MeO-DMT. Furthermore, a two-compartment model including first-order absorption, nonlinear (Michaelis-Menten) elimination, and CYP2D6-dependent linear elimination from the central compartment was developed to characterize the intravenous and intraperitoneal pharmacokinetic data for 5-MeO-DMT in wild-type and Tg-CYP2D6 mice. In addition, 5-MeO-DMT was readily detected in mouse brain after drug treatment, and brain 5-MeO-DMT concentrations were also increased nonproportionally with the increase of dose. The results establish a nonlinear pharmacokinetic property for 5-MeO-DMT in mice, suggesting that the risk of 5-MeO-DMT intoxication may be increased nonproportionally at higher doses.
Introduction
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT), a psychoactive indolealkylamine derivative, is present in a variety of plant and animal preparations used for social or recreational purposes (Ott, 2001; Brandt et al., 2004; McKenna, 2004; Yu, 2008; Shen et al., 2010a; McIlhenny et al., 2011). As a potent, fast-acting hallucinogen with short duration, 5-MeO-DMT produces psychedelic effects in humans after different routes of administration, e.g., inhalation, intravenous injection, sublingual or intranasal insufflation, or oral administration with an inhibitor of monoamine oxidase A (MAO-A). Human self-experiments have revealed that the visionary threshold can be induced by insufflation or sublingual ingestion of 10 mg of 5-MeO-DMT free base (equal to 0.14 mg/kg) (Ott, 2001). The hallucination begins at 3 to 4 min, peaks approximately 35 to 40 min, and ends approximately 60 to 70 min after insufflation of 5-MeO-DMT free base. It is noteworthy that 5-MeO-DMT has been a controlled substance in European countries for years, whereas it became a Schedule I controlled substance in the United State as of January 19, 2011 (DEA-2010-0024, Schedules of Controlled Substances: Placement of 5-Methoxy-N,N-Dimethyltryptamine Into Schedule I of the Controlled Substances Act, http://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr1220.htm). Before that, 5-MeO-DMT was available on the Internet as a natural product or synthetic compound. 5-MeO-DMT intoxications were also documented (Brush et al., 2004; Sklerov et al., 2005), and the symptoms included extremely high body temperature and heart rate, as well as combative hallucinating without vital signs. Particular attention (Sklerov et al., 2005; Callaway et al., 2006) was drawn to a fatal case in which a strikingly high concentration of 5-MeO-DMT was found in the decedent's heart blood.
The toxicity of 5-MeO-DMT has been studied in different animal models including mouse, rat, sheep, and monkey (Benington et al., 1965; Gillin et al., 1976). After treatment with 5-MeO-DMT, animals often show hyperthermia, ataxia, mydriasis, tremor, convulsion, shivering, and salivation. Although the LD50 of 5-MeO-DMT ranges from 48 to 278 mg/kg for different routes of administration to mice (Benington et al., 1965; Gillin et al., 1976), exposure to 1 mg/kg 5-MeO-DMT causes severe toxic effects to sheep (Gillin et al., 1976). The species difference may be attributed to the differences in its metabolism and pharmacokinetics in individual animal models in addition to animal physiology, which hampers the prediction of 5-MeO-DMT toxicity in humans. Therefore, an understanding of its pharmacokinetic properties across species is critical for proper correlation of drug exposure with drug effect and, ultimately, extrapolation of animal data to humans (Rhomberg, 1995; Green et al., 2009; Jiang et al., 2011).
Unfortunately, there has been no report on 5-MeO-DMT pharmacokinetics in humans. Limited pharmacokinetic studies using mouse or rat models suggest that 5-MeO-DMT is rapidly absorbed and eliminated (Sitaram et al., 1987a,b; Shen et al., 2009, 2010b). The low urinary recovery and biliary excretion of substrate drug indicate that 5-MeO-DMT is predominantly eliminated through metabolism (Agurell et al., 1969; Sitaram et al., 1987a). In vitro and in vivo studies show that MAO-A-mediated deamination is the major metabolic pathway for 5-MeO-DMT, whereas other metabolic pathways such as N-oxygenation, N-demethylation, and O-demethylation were also reported (Agurell et al., 1969; Squires, 1975; Sitaram et al., 1987a,b; Shen et al., 2009, 2010b). The O-demethylation mediated by CYP2D6 produces an active metabolite, bufotenine (Yu et al., 2003a). In addition, each study on the metabolism and disposition of 5-MeO-DMT was limited to a single dose (Agurell et al., 1969; Sitaram et al., 1987a,b; Barker et al., 2001; Shen et al., 2009, 2010b). Therefore, in this study we investigated 5-MeO-DMT pharmacokinetics in mice at multiple dose levels and through different routes of administration, which cover the range from nontoxic to toxic dose levels, aiming to define the pharmacokinetic properties of 5-MeO-DMT, delineate the impact of CYP2D6 on systemic clearance of 5-MeO-DMT, and develop a pharmacokinetic model to quantitatively characterize serum concentration-time profiles of 5-MeO-DMT.
Materials and Methods
Chemical and Materials.
5-MeO-DMT oxalate and 5-methyl-N,N-dimethyltryptamine (5-Me-DMT) were purchased from Sigma-Aldrich (St. Louis, MO). Saline was bought from Henry-Schein (Melville, NY). All other reagents or organic solvents were either analytical or high-performance liquid chromatography grade (VWR, Radnor, PA).
Animals.
Wild-type FVB/N and CYP2D6-humanized (Tg-CYP2D6) mice (Corchero et al., 2001) were housed under controlled temperature (20 ± 2°C), relative humidity (50–60%), and 12-h light/dark cycles with food and water provided ad libitum. Age-matched male adult mice (8 weeks old) weighing 25 to 30 g were used in the experiments. All animal procedures were approved by the institutional animal care and use committee at University at Buffalo, The State University of New York (Buffalo, NY).
Pharmacokinetic Studies.
5-MeO-DMT oxalate was dissolved in saline to a 10 mg/ml drug stock solution and further diluted with saline to appropriate concentrations that were used at 10 μl/g b.wt. for intraperitoneal doses and 5 μl/g b.wt. for intravenous doses, respectively. Wild-type or Tg-CYP2D6 mice were treated intraperitoneally or intravenously with 2, 10, or 20 mg/kg 5-MeO-DMT. Blood samples were collected from individual mice at different time points (0–180 min, n = 3 or 4 per time point) after the administration of 5-MeO-DMT. Serum was isolated with a serum separator (BD, Franklin Lakes, NJ) and stored at −80°C before analysis.
Brain Drug Distribution.
After intraperitoneal treatment with 2, 10, or 20 mg/kg 5-MeO-DMT, wild-type or Tg-CYP2D6 mice were sacrificed at 20, 30, or 60 min. Brain tissues were excised, rinsed, and homogenized with ice-cold saline. The homogenate were stored at −80°C for less than 1 year before analysis.
LC-MS/MS Quantification.
A simple protein precipitation method was used for the processing of serum samples. Ice-cold acetonitrile (60 μl) containing 50 nM 5-Me-DMT (internal standard) was added to the serum sample (20 μl) for protein precipitation. After centrifugation at 14,000 rpm for 10 min, the supernatant was injected for LC-MS/MS analysis.
The brain samples were subjected to liquid-liquid extraction. Brain homogenate (50 μl) was mixed with 10 μl of 5-Me-DMT (100 nM) and 5 μl of sodium hydroxide (1 M) and extracted with 1 ml of ethyl acetate. After centrifugation at 14,000 rpm for 5 min, 900 μl of supernatant was transferred to a new vial and evaporated to dryness under a stream of air. The residue was reconstituted with 50 μl of 50% methanol and centrifuged at 14,000 rpm for 5 min. The supernatant was injected for LC-MS/MS analysis.
LC-MS/MS quantification of 5-MeO-DMT in mouse serum and brain samples was performed using a Shimadzu prominence high-performance liquid chromatograph (Kyoto, Japan) coupled to an API 3000 TurboIonSpray ionization triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). 5-MeO-DMT was separated from the internal standard on a 3-μm phenyl-hexyl column (50 × 4.6 mm; Phenomenex, Torrance, CA), and quantified with a validated method (Shen et al., 2009). Brain 5-MeO-DMT concentrations were converted to nanograms per gram on the basis of the weights of individual mouse brain tissues.
Pharmacokinetic Modeling.
Noncompartmental analysis was conducted using composite mean serum concentration-time data obtained from wild-type or Tg-CYP2D6 mice at each dose level with WinNonlin (version 5.3; Pharsight, Mountain View, CA). The maximal serum drug concentration (Cmax) and the time it occurred (Tmax) after intraperitoneal administration were recorded as observed. The area under the serum drug concentration versus time curve up to the last measured time point (AUC0→t) was calculated by the trapezoidal rule, and the AUC0→∞ was estimated by extrapolating the AUC0→t to infinity using the last measured concentration and the terminal slope (λ) of linear regression from the semilogarithmic drug concentration versus time curve. The elimination half-life (t1/2) was determined by the relationship of 0.693/λ. The clearance (CL) was calculated by dose/AUC0→∞, and the volume of distribution at steady state (Vss) was determined as CL times AUMC over AUC, where AUMC is the area under the first moment curve. Oral bioavailability (F) was estimated as the ratio of AUC0→∞ values after intraperitoneal and intravenous administration.
A compartmental model (Fig. 1) was proposed to describe 5-MeO-DMT data. Serum 5-MeO-DMT concentration-time data from wild-type and Tg-CYP2D6 mice after intravenous and intraperitoneal administration were fit simultaneously to this model (Fig. 1) with ADAPT V (Biomedical Simulations Resource, University of South California, Los Angeles, CA) by a naive-pooled population analysis. The initial estimates of model parameters were obtained from noncompartmental analysis. Model parameters were estimated with the maximum likelihood estimation method. Different models with or without a peripheral compartment and with linear or nonlinear distribution or elimination were also tested. The final model (Fig. 1) was selected on the basis of the goodness-of-fit criteria that included the Akaike information criterion, an estimation criterion value for the maximum likelihood method, and visual inspection of fitted profiles.
Fig. 1.
The pharmacokinetic model developed to describe serum 5-MeO-DMT concentration-time profiles in mice after intravenous and intraperitoneal administration. Xa, amount of drug at the absorption site; ka, absorption rate constant; F, bioavailability; XC and XT, amount of drug in the central and peripheral compartment, respectively; VC and VT, volume of distribution of 5-MeO-DMT in the central and peripheral compartment, respectively; Km and Vmax, Michaelis-Menten parameters for the saturable elimination of 5-MeO-DMT from the central compartment; CLCYP2D6, clearance of 5-MeO-DMT from the central compartment by CYP2D6-dependent metabolism, which only occurs in Tg-CYP2D6 mice; CLD, distribution clearance of 5-MeO-DMT between the central and peripheral compartments.
The differential equations of the final model were as follows:
 |
The initial condition (IC) for each equation is provided in the parentheses. Equations 1, 2, and 6 were used to fit the intraperitoneal data from wild-type mice. Equations 1, 3, and 6 were used to fit the intraperitoneal data from Tg-CYP2D6 mice. Equations 4 and 6 were used to fit the intravenous data from wild-type mice, and eqs. 5 and 6 were used to fit the intravenous data from Tg-CYP2D6 mice. In the equations, Xa represents the amount of 5-MeO-DMT at the absorption site, and XC and XT represent the amount of 5-MeO-DMT in the central and peripheral compartment, respectively. ka is the absorption rate constant at the absorption site, and F is the bioavailability. CLD is the distribution clearance. VC and VT represent the volume of distribution of 5-MeO-DMT in the central and peripheral compartment, respectively. Km is the Michaelis-Menten constant of the nonlinear elimination from the central compartment, and Vmax is the maximal velocity. CLCYP2D6 represents the additional linear clearance of 5-MeO-DMT from the central compartment that is dependent on CYP2D6 protein and thus occurs only in Tg-CYP2D6 mice.
Statistical Analyses.
Statistical analysis was conducted using Student's t test or one-way analysis of variance followed by Bonferroni post hoc test (GraphPad Prism 5; GraphPad Software Inc., San Diego, CA). Differences were considered statistically significant when p < 0.05.
Results
LC-MS/MS Method Was Developed for Quantification of Serum and Brain 5-MeO-DMT Concentrations.
A LC-MS/MS method coupled with protein precipitation (Shen et al., 2009) was developed and validated for quantification of 5-MeO-DMT in serum samples. This method was optimized for the measurement of brain 5-MeO-DMT in this study. In particular, a liquid-liquid extraction method instead of protein precipitation was used for brain sample preparation. The recovery of 5-MeO-DMT was in the range of 62 to 70%. The lowest limit of quantification of 5-MeO-DMT was 4.1 nM, and the standard curve ranged from 4.1 to 3000 nM with a linear regression coefficient greater than 0.99. The interday accuracy and precision were 99 to 106% and 3.7 to 9.6% for quality control samples at concentrations of 12.3, 111, and 1000 nM. Similar to the method for quantification of serum samples, the analysis of 5-MeO-DMT or 5-Me-DMT (internal standard) in brain samples did not show any ion suppression or enhancement caused by the coeluted matrix (data not shown). In addition, our pilot study showed that 5-MeO-DMT in brain homogenate was stable at −80°C for more than 1 year, which was longer than the storage time of the brain samples. The methods were selective and sensitive and were able to quantify blood and brain 5-MeO-DMT concentrations in mice treated with drug, whereas endogenous 5-MeO-DMT in untreated mice was below the quantification limit.
Blood 5-MeO-DMT Showed Nonlinear Pharmacokinetics After Intravenous Bolus Administration.
After quantification of 5-MeO-DMT, serum 5-MeO-DMT concentration-time curves were established (Fig. 2, A and B). Noncompartmental analyses were first conducted to estimate 5-MeO-DMT pharmacokinetic parameters in wild-type (Table 1) and Tg-CYP2D6 (Table 2) mice. 5-MeO-DMT pharmacokinetic parameters (e.g., AUC0→∞, CL, Vss, and t1/2) were not significantly different between wild-type (47.6 μmol · min/l, 0.19 l · min−1 · kg−1, 2.27 l/kg, and 9.2 min, respectively) and Tg-CYP2D6 (49.1 μmol · min/l, 0.19 l · min−1 · kg−1, 2.55 l/kg, and 8.4 min, respectively) mice, indicating a limited role for CYP2D6 in systemic clearance of 5-MeO-DMT. Of interest, the change in AUC0→∞ was greater than the proportional increase in either genotype (Tables 1 and 2). When the doses were increased to 10 and 20 mg/kg, the AUC0→∞ values were increased to 487 and 920 μmol · min/l in wild-type mice and to 369 and 866 μmol · min/l in Tg-CYP2D6 mice, respectively. Although the dose-normalized AUC0→∞ values for 10 and 20 mg/kg were comparable, each was much higher than that for the 2 mg/kg dose (2.0- and 1.9-fold higher in wild-type mice and 1.5 and 1.8-fold higher in Tg-CYP2D6 mice, respectively). The systemic clearance (CL) of 5-MeO-DMT at a higher dose (10 or 20 mg/kg) was consistently approximately 50% lower than that at a low dose (2 mg/kg), and the half-life (t1/2) at a higher dose (10 or 20 mg/kg) was 30 to 63% longer than that at a low dose (2 mg/kg) in wild-type and Tg-CYP2D6 mice (Tables 1 and 2). The existence of nonlinearity in serum 5-MeO-DMT pharmacokinetics was also manifested by the dose-normalized serum drug concentration-time curves of 2 mg/kg doses (Fig. 2, C and D), which did not superimpose with those of 10 and 20 mg/kg doses in either wild-type or Tg-CYP2D6 mice.
Fig. 2.
Serum 5-MeO-DMT concentration versus time curves in wild-type (A) and Tg-CYP2D6 mice (B) after intravenous administration. Existence of nonlinear pharmacokinetics is manifested by the dose-normalized serum drug concentration versus time profiles in both wild-type (C) and Tg-CYP2D6 mice (D). Mice were dosed intravenously with 2 (●), 10 (■), or 20 (▲) mg/kg 5-MeO-DMT. Values are means ± S.D. (n = 3 at each time point).
TABLE 1.
Pharmacokinetic parameters of 5-MeO-DMT in wild-type mice after intravenous and intraperitoneal administration determined by noncompartmental analyses
| Intravenous Administration |
Intraperitoneal Administration |
|||||
|---|---|---|---|---|---|---|
| 2 mg/kg | 10 mg/kg | 20 mg/kg | 2 mg/kg | 10 mg/kg | 20 mg/kg | |
| AUC0→∞ (μmol · min/l) | 47.6 | 487 | 920 | 24.9 | 231 | 654 |
| AUC0→∞/dose (kg · min/l) | 5.19 | 10.6 | 10.0 | 2.71 | 5.03 | 7.13 |
| CL (l · min−1 · kg−1) | 0.19 | 0.094 | 0.10 | |||
| Vss (l/kg) | 2.27 | 2.08 | 1.84 | |||
| Tmax (min) | 3 | 5 | 5 | |||
| Cmax (μmol/l) | 1.31 | 7.00 | 15.8 | |||
| Cmax/dose (kg/l) | 0.14 | 0.15 | 0.17 | |||
| t1/2 (min) | 9.2 | 12 | 15 | |||
| F (%) | 52.2 | 47.3 | 71.0 | |||
TABLE 2.
Pharmacokinetic parameters of 5-MeO-DMT in Tg-CYP2D6 mice after intravenous and intraperitoneal administration determined by noncompartmental analyses
| Intravenous Administration |
Intraperitoneal Administration |
|||||
|---|---|---|---|---|---|---|
| 2 mg/kg | 10 mg/kg | 20 mg/kg | 2 mg/kg | 10 mg/kg | 20 mg/kg | |
| AUC0→∞ (μmol · min/l) | 49.1 | 369 | 866 | 20.9 | 225 | 569 |
| AUC0→∞/dose (kg · min/l) | 5.36 | 8.10 | 9.45 | 2.28 | 4.92 | 6.20 |
| CL (l · min−1 · kg−1) | 0.19 | 0.12 | 0.11 | |||
| Vss (l/kg) | 2.55 | 1.68 | 2.18 | |||
| Tmax (min) | 3 | 5 | 5 | |||
| Cmax (μmol/l) | 1.01 | 8.93 | 18.3 | |||
| Cmax/dose (kg/l) | 0.11 | 0.19 | 0.20 | |||
| t1/2 (min) | 8.4 | 12 | 12 | |||
| F (%) | 42.5 | 61.0 | 65.6 | |||
It should be noted that the use of oxalate salt did not affect the pharmacokinetics of 5-MeO-DMT in mice. This result was confirmed by our pilot studies, showing that similar pharmacokinetic profiles of 5-MeO-DMT were observed in mice receiving 5-MeO-DMT free base and 5-MeO-DMT oxalate (data not shown).
Nonlinear Pharmacokinetics Was Also Observed for Blood 5-MeO-DMT after Intraperitoneal Administration.
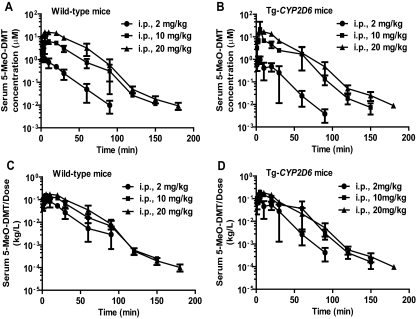
After intraperitoneal administration, 5-MeO-DMT was readily absorbed with a Cmax of approximately 3 to 5 min in wild-type and Tg-CYP2D6 mice (Fig. 3, A and B; Tables 1 and 2). The AUC0→∞ values were 24.9, 231, and 654 μmol · min/l for 2, 10, and 20 mg/kg 5-MeO-DMT in wild-type mice, respectively, and 20.9, 225 and 569 μmol · min/l for 2, 10, and 20 mg/kg 5-MeO-DMT in Tg-CYP2D6 mice, respectively. Consistent with the finding from intravenous administration, the increase in AUC0→∞ with dose was greater than the proportional change. In particular, the dose-normalized AUC0→∞ values at the 10 and 20 mg/kg doses were 1.9- and 2.6-fold higher, respectively, than that for the 2 mg/kg dose in wild-type mice and 2.2- and 2.7-fold higher, respectively, than that for the 2 mg/kg dose in Tg-CYP2D6 mice. Furthermore, the dose-normalized Cmax at a higher dose (10 or 20 mg/kg) was 7 to 80% higher than that at 2 mg/kg in mice (Tables 1 and 2). In addition, the dose-normalized serum drug concentration-time curve of 2 mg/kg dose did not superimpose with those of higher doses (10 and 20 mg/kg) in either wild-type or Tg-CYP2D6 mice (Fig. 3, C and D). The results indicated that 5-MeO-DMT pharmacokinetics in mice was nonlinear.
Fig. 3.
Serum 5-MeO-DMT concentration versus time curves in wild-type (A) and Tg-CYP2D6 mice (B) after intraperitoneal administration. Nonlinear pharmacokinetics is obvious from the dose-normalized serum drug concentration versus time profiles in wild-type (C) and Tg-CYP2D6 mice (D). Mice were treated intraperitoneally with 2 (●), 10 (■), or 20 (▲) mg/kg 5-MeO-DMT. Values represent means ± S.D. (n = 4 at each time point).
A Compartmental Model Was Developed to Describe 5-MeO-DMT Concentration-Time Profiles.
To better understand 5-MeO-DMT pharmacokinetic properties, the serum 5-MeO-DMT concentration-time data after intravenous and intraperitoneal administration were simultaneously analyzed with ADAPT V. A number of different pharmacokinetic models, including one or two compartments with linear or Michaelis-Menten elimination, were first tested for the intravenous data. A one-compartment model with linear elimination failed to capture the 5-MeO-DMT data. The change from linear to Michaelis-Menten elimination or the addition of a peripheral compartment underpredicted the concentrations of 5-MeO-DMT at early time points in mice receiving 10 and 20 mg/kg doses (data not shown). A two-compartment model with Michaelis-Menten elimination plus a CYP2D6-dependent linear elimination from the central compartment provided the best fit, according to the goodness-of-fit criteria. Therefore, a compartmental model (Fig. 1) was developed for simultaneous fit of the intravenous and intraperitoneal pharmacokinetic data, which included a dosing compartment with a linear first-order absorption rate. This model nicely captured the serum drug concentration-time data at different intravenous and intraperitoneal doses in both wild-type and Tg-CYP2D6 mice (Fig. 4), which is also evident from goodness-of-fit plots (Fig. 5). The estimated compartmental pharmacokinetic parameters for 5-MeO-DMT are shown in Table 3. The ka value was 0.13 min−1, suggesting that 5-MeO-DMT was rapidly absorbed after intraperitoneal administration. According to the Vmax and Km values (2.76 μmol · min−1 · kg−1 and 13.2 μM, respectively), intrinsic clearance (CLint) of 5-MeO-DMT from the central compartment would be 0.21 l · min−1 · kg−1, which was close to the systemic clearance value at the 2 mg/kg dose obtained by noncompartmental analyses (Tables 1 and 2) and agreed with a linear elimination of 5-MeO-DMT at the low dose. In contrast, a low CLCYP2D6 value (0.0256 l · min−1 · kg−1) in Tg-CYP2D6 mice supported the fact that CYP2D6-mediated O-demethylation was a minor pathway in systemic elimination of 5-MeO-DMT. Overall, this compartmental model (Fig. 1) provided a reasonable description of 5-MeO-DMT pharmacokinetic data (Fig. 4) in mice.
Fig. 4.
Simultaneous fitting of intravenous and intraperitoneal pharmacokinetic data in both wild-type (A and C) and Tg-CYP2D6 (B and D) mice using the proposed compartmental model (Fig. 1). Values are means ± S.D. (n = 3–4 at each time point). Lines represent the predicted serum 5-MeO-DMT concentrations.
Fig. 5.
Goodness-of-fit plots of a two-compartment model versus a one-compartment model. Shown are model-predicted versus observed drug concentrations of the one-compartment model (A) and the final two-compartment model (B) and standard residuals versus model predictions of the one-compartment model (C) and the final two-compartment model (D).
TABLE 3.
Pharmacokinetic parameters of 5-MeO-DMT after intravenous and intraperitoneal administration determined by compartmental analyses (Fig. 1)
| Parameter | Unit | Estimated Value | CV% |
|---|---|---|---|
| Vmax | μmol · min−1 · kg−1 | 2.76 | 0.028 |
| Km | μM | 13.2 | 0.034 |
| CLD | l · min−1 · kg−1 | 0.280 | 0.006 |
| CLCYP2D6 | l · min−1 · kg−1 | 0.0256 | 0.012 |
| VC | l/kg | 1.34 | 0.005 |
| VT | l/kg | 1.91 | 0.011 |
| ka | min−1 | 0.131 | 0.005 |
| F | % | 81.3 | 0.004 |
5-MeO-DMT Exhibited Nonlinear Accumulation in Mouse Brain after Intraperitoneal Drug Administration.
Because 5-MeO-DMT acts on the central nervous system, we further measured brain 5-MeO-DMT concentrations at 20, 30, and 60 min after intraperitoneal drug administration. Compared with serum drug concentrations at the same time point, brain 5-MeO-DMT concentrations were much higher. For instance, the brain/serum ratios of 5-MeO-DMT concentrations ranged from 1.8 to 4.1 in wild-type mice and from 1.2 to 4.2 in Tg-CYP2D6 mice treated with 10 mg/kg drug. When the dose was 20 mg/kg 5-MeO-DMT, the ratios were 2.7 to 4.3 and 3.2 to 5.2 in wild-type and Tg-CYP2D6 mice, respectively. Most importantly, 5-MeO-DMT concentrations increased nonproportionally in brain with the increase in dose, similar to the nonlinear pharmacokinetics observed for blood 5-MeO-DMT (Figs. 2 and 3). Therefore, the dose-normalized brain 5-MeO-DMT concentrations were calculated (Fig. 6) and were approximately 3- to 11-fold higher at 20 min, 3- to 14-fold higher at 30 min, and 2- to 16-fold higher at 60 min, respectively, in mice dosed with 10 or 20 mg/kg drug than in those dosed with 2 mg/kg drug. The results indicated a nonlinear accumulation of 5-MeO-DMT in mouse brain.
Fig. 6.
Dose-normalized brain 5-MeO-DMT concentrations at 20 min (A), 30 min (B), and 60 min (C) after intraperitoneal administration of 2, 10, or 20 mg/kg drug in wild-type and Tg-CYP2D6 mice. Data are means ± S.D. (n = 4 in each group). *, p < 0.05, compared with 2 mg/kg treatment for the same genotype of mice; #, p < 0.05, compared with wild-type mice treated with the same dose of drug.
Discussion
The current study reveals nonlinear pharmacokinetics for 5-MeO-DMT, a new Schedule I controlled substance in the United States (DEA-2010-0024, Schedules of Controlled Substances: Placement of 5-Methoxy-N,N-Dimethyltryptamine Into Schedule I of the Controlled Substances Act, http://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr1220.htm), in a mouse model after intravenous and intraperitoneal drug administration (Figs. 2 and 3). Although the experiments were not designed for studying toxicological effects, we did observe significant behavior changes and toxic syndromes in mice receiving higher doses (10 and 20 mg/kg) of 5-MeO-DMT. The decrease (∼50%) in systemic clearance at higher doses indicates the presence of nonlinearity in 5-MeO-DMT elimination. Given the facts that metabolic elimination is the major elimination pathway for 5-MeO-DMT and MAO-A-mediated deamination is the main metabolic pathway for 5-MeO-DMT (Agurell et al., 1969; Squires, 1975; Suzuki et al., 1981; Sitaram et al., 1987b; Shen et al., 2009, 2010b), saturation of MAO-A-mediated metabolic elimination is presumably the cause of nonlinear clearance of 5-MeO-DMT.
Nonlinear pharmacokinetics has been documented for other drugs of abuse in different species. An amphetamine drug of abuse, 3,4-methylenedioxymethamphetamine (“Ecstasy”), exhibits nonlinear pharmacokinetics in rodents, nonhuman primates, and humans, which may influence the likelihood and severity of 3,4-methylenedioxymethamphetamine toxicities (de la Torre et al., 2000; Mueller et al., 2008; Baumann et al., 2009). The current study clearly demonstrates nonlinear pharmacokinetics for 5-MeO-DMT in mice (Figs. 2 and 3), which may complicate the prediction of 5-MeO-DMT toxicity. In general, nonlinear pharmacokinetics leads to a much narrower range between safe and toxic doses and makes extrapolation of the dose effect or dose-toxicity relationship more difficult (Tonn et al., 2009; Lledó-García et al., 2010). Thus, development of an appropriate quantitative model may offer better understanding of pharmacokinetic properties in support of pharmacological and toxicological investigations.
The calculated systemic clearance for 5-MeO-DMT (0.094–0.19 l · min−1 · kg−1 from noncompartmental analyses) (Tables 1 and 2) is close to or higher than hepatic blood flow in mice (90 ml · min−1 · kg−1) (Davies and Morris, 1993), suggesting the existence of extrahepatic elimination. Indeed, MAO-A is expressed in many extrahepatic tissues, e.g., gut, kidney, lung, and myocardium (Thorpe et al., 1987; Saura et al., 1996), which probably contributes to the metabolic elimination of 5-MeO-DMT. The Vss of 5-MeO-DMT in mice is 1.68 to 2.55 l/kg, which is much higher than the volume of blood and total body water of a mouse with a body weight of 20 g (0.81 l/kg) (Davies and Morris, 1993), indicating extensive binding of 5-MeO-DMT to tissues. Accumulation of 5-MeO-DMT in animal tissues is supported by its high lipophilicity (partition coefficient = 3.30) (Glennon and Gessner, 1979), and it is also evident from previous studies in rats (Sitaram and McLeod, 1990).
The two-compartment pharmacokinetic model (Fig. 1) developed for 5-MeO-DMT in the current study provides good rationalization of the intravenous and intraperitoneal data in mice (Figs. 4 and 5). The capacity-limited elimination of 5-MeO-DMT (Km and Vmax) from the central compartment explains well the nonlinear profiles after intravenous and intraperitoneal administration (Figs. 2 and 3), and the simulated clearance values (Supplemental Fig. 1) show a clear tread of decrease in drug clearance with the increase in dose in both wild-type and transgenic mice, supporting nonlinear pharmacokinetics for 5-MeO-DMT. The estimated Km value (13.2 μM) is close to the reported Km value for deamination of tryptamine analogs (including 5-MeO-DMT) by MAO-A (Squires, 1975; Suzuki et al., 1981; Yu et al., 2003b). Because mouse serum 5-MeO-DMT concentrations after a 2 mg/kg i.v. dose are much lower than the estimated Km value, MAO-mediated elimination of drug at this dose should still be in the linear range. Therefore, 5-MeO-DMT at dose levels lower than 2 mg/kg could be in a linear kinetic range, which is supported by simulated results (data not shown). In contrast, serum 5-MeO-DMT concentrations at earlier time points after higher doses (10 or 20 mg/kg) of drug (Figs. 2 and 3) are much higher than the Km value, and thus MAO-mediated elimination could be saturated, leading to nonlinear drug elimination, compared with lower (e.g., 2 mg/kg) doses. On the other hand, CYP2D6 was shown to play a minor role in elimination of 5-MeO-DMT in vivo, as indicated by the CLCYP2D6 value that is only approximately 10% of the intrinsic clearance (Vmax/Km) (Table 3). Nevertheless, it is not known whether CYP2D6-mediated metabolism (Yu et al., 2003a) would compensate for the clearance of 5-MeO-DMT when MAO-A activity is inhibited.
5-MeO-DMT is a nonselective serotonin (5-HT) receptor agonist. 5-HT1A and 5-HT2 receptors have been shown to determine the complex pharmacological and toxicological actions of 5-MeO-DMT in the central nervous system (Winter et al., 2000; Krebs-Thomson et al., 2006; Halberstadt et al., 2008). Understanding brain 5-MeO-DMT concentrations would be helpful. Our pilot study has shown that 5-MeO-DMT concentrations in different mouse brain regions, e.g., hippocampus, hypothalamus, striatum, and cortex, are comparable (data not shown). Therefore, this study focuses on measurement of 5-MeO-DMT concentrations in the whole brain after intraperitoneal administration. Consistent with previous findings (Barker et al., 2001), 5-MeO-DMT is capable of penetrating the blood-brain barrier and accumulating within animal brains. The most significant finding is the greater than proportional increase of brain 5-MeO-DMT concentration with the increase in dose (Fig. 6). In agreement with the nonlinear pharmacokinetics of blood 5-MeO-DMT (Figs. 2 and 3), the nonlinear accumulation of 5-MeO-DMT in brain tissue illustrates an increased risk of 5-MeO-DMT intoxication at higher doses.
In summary, 5-MeO-DMT exhibits nonlinear pharmacokinetics in mice after intravenous and intraperitoneal administration. A two-compartment pharmacokinetic model with linear absorption and capacity-limited elimination and minor metabolic elimination by CYP2D6 well characterize the pharmacokinetic profiles of 5-MeO-DMT in wild-type and Tg-CYP2D6 mice. These findings may serve as a basis for further investigation of the relationship between 5-MeO-DMT drug exposure and toxicity, particularly at doses when saturation of metabolic elimination occurs. In addition, 5-MeO-DMT is accumulated at a level greater than proportional in mouse brain, which increases the potential of 5-MeO-DMT toxicity. The results provide important insights into the risk of 5-MeO-DMT intoxication from the pharmacokinetic aspect.
Supplementary Material
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA021172].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.039107.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- 5-MeO-DMT
- 5-methoxy-N,N-dimethyltryptamine
- MAO-A
- monoamine oxidase-A
- 5-Me-DMT
- 5-methyl-N,N-dimethyltryptamine
- Tg-CYP2D6
- CYP2D6-humanized
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- 5-HT
- serotonin.
Authorship Contributions
Participated in research design: Shen and Yu.
Conducted experiments: Shen and Jiang.
Performed data analysis: Shen, Jiang, and Yu.
Wrote or contributed to the writing of the manuscript: Shen, Jiang, and Yu.
References
- Agurell S, Holmstedt B, Lindgren JE. (1969) Metabolism of 5-methoxy-N,N-dimethyltryptamine-14C in the rat. Biochem Pharmacol 18:2771–2781 [DOI] [PubMed] [Google Scholar]
- Barker SA, Littlefield-Chabaud MA, David C. (2001) Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: application of a new solid-phase extraction LC-APcI-MS-MS-isotope dilution method. J Chromatogr B Biomed Sci Appl 751:37–47 [DOI] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. (2009) Effects of dose and route of administration on pharmacokinetics of (+ or −)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos 37:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benington F, Morin RD, Clark LC., Jr (1965) 5-Methoxy-N,N-dimethyltryptamine, a possible endogenous psychotoxin. Ala J Med Sci 2:397–403 [PubMed] [Google Scholar]
- Brandt SD, Freeman S, McGagh P, Abdul-Halim N, Alder JF. (2004) An analytical perspective on favoured synthetic routes to the psychoactive tryptamines. J Pharm Biomed Anal 36:675–691 [DOI] [PubMed] [Google Scholar]
- Brush DE, Bird SB, Boyer EW. (2004) Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J Toxicol Clin Toxicol 42:191–195 [DOI] [PubMed] [Google Scholar]
- Callaway JC, Grob CS, McKenna DJ, Nichols DE, Shulgin A, Tupper KW. (2006) A demand for clarity regarding a case report on the ingestion of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in an Ayahuasca preparation. J Anal Toxicol 30:406–407; author reply 407 [DOI] [PubMed] [Google Scholar]
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. (2001) The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4α on the disposition of debrisoquine in the mouse. Mol Pharmacol 60:1260–1267 [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. (2000) Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 49:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin JC, Tinklenberg J, Stoff DM, Stillman R, Shortlidge JS, Wyatt RJ. (1976) 5-Methoxy-N,N-dimethyltryptamine: behavioral and toxicological effects in animals. Biol Psychiatry 11:355–358 [PubMed] [Google Scholar]
- Glennon RA, Gessner PK. (1979) Serotonin receptor binding affinities of tryptamine analogues. J Med Chem 22:428–432 [DOI] [PubMed] [Google Scholar]
- Green AR, Gabrielsson J, Marsden CA, Fone KC. (2009) MDMA: on the translation from rodent to human dosing. Psychopharmacology (Berl) 204:375–378 [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. (2008) Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 201:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XL, Gonzalez FJ, Yu AM. (2011) Drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. Drug Metab Rev 43:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. (2006) The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl) 189:319–329 [DOI] [PubMed] [Google Scholar]
- Lledó-García R, Nácher A, Casabó VG, Merino-Sanjuán M. (2010) A pharmacokinetic model for evaluating the impact of hepatic and intestinal first-pass loss of saquinavir in the rat. Drug Metab Dispos 39:294–301 [DOI] [PubMed] [Google Scholar]
- McIlhenny EH, Riba J, Barbanoj MJ, Strassman R, Barker SA. (2011) Methodology for and the determination of the major constituents and metabolites of the Amazonian botanical medicine ayahuasca in human urine. Biomed Chromatogr doi:10.1002/bmc.1551 [DOI] [PubMed] [Google Scholar]
- McKenna DJ. (2004) Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther 102:111–129 [DOI] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Maurer HH, McCann UD, Ricaurte GA. (2008) Nonlinear pharmacokinetics of (+/−)3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327:38–44 [DOI] [PubMed] [Google Scholar]
- Ott J. (2001) Pharmepéna-Psychonautics: human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs 33:403–407 [DOI] [PubMed] [Google Scholar]
- Rhomberg L. (1995) Use of quantitative modelling in methylene chloride risk assessment. Toxicology 102:95–114 [DOI] [PubMed] [Google Scholar]
- Saura J, Nadal E, van den Berg B, Vila M, Bombi JA, Mahy N. (1996) Localization of monoamine oxidases in human peripheral tissues. Life Sci 59:1341–1349 [DOI] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Winter JC, Yu AM. (2010a) Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metab 11:659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Yu AM. (2009) Development of a LC-MS/MS method to analyze 5-methoxy-N,N-dimethyltryptamine and bufotenine, and application to pharmacokinetic study. Bioanalysis 1:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Wu C, Jiang XL, Yu AM. (2010b) Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochem Pharmacol 80:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, Blackman GL, McLeod WR. (1987a) Urinary excretion of 5-methoxy-N,N-dimethyltryptamine, N,N-dimethyltryptamine and their N-oxides in the rat. Biochem Pharmacol 36:2235–2237 [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR. (1987b) In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem Pharmacol 36:1509–1512 [DOI] [PubMed] [Google Scholar]
- Sitaram BR, McLeod WR. (1990) Observations on the metabolism of the psychotomimetic indolealkylamines: implications for future clinical studies. Biol Psychiatry 28:841–848 [DOI] [PubMed] [Google Scholar]
- Sklerov J, Levine B, Moore KA, King T, Fowler D. (2005) A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J Anal Toxicol 29:838–841 [DOI] [PubMed] [Google Scholar]
- Squires RF. (1975) Evidence that 5-methoxy-N,N-dimethyl tryptamine is a specific substrate for MAO-A in the rat: implications for the indoleamine dependent behavioural syndrome. J Neurochem 24:47–50 [DOI] [PubMed] [Google Scholar]
- Suzuki O, Katsumata Y, Oya M. (1981) Characterization of eight biogenic indoleamines as substrates for type A and type B monoamine oxidase. Biochem Pharmacol 30:1353–1358 [PubMed] [Google Scholar]
- Thorpe LW, Westlund KN, Kochersperger LM, Abell CW, Denney RM. (1987) Immunocytochemical localization of monoamine oxidases A and B in human peripheral tissues and brain. J Histochem Cytochem 35:23–32 [DOI] [PubMed] [Google Scholar]
- Tonn GR, Wong SG, Wong SC, Johnson MG, Ma J, Cho R, Floren LC, Kersey K, Berry K, Marcus AP, et al. (2009) An inhibitory metabolite leads to dose- and time-dependent pharmacokinetics of (R)-N-{1-[3-(4-ethoxy-phenyl)-4-oxo-3,4-dihydro-pyrido[2,3-d]pyrimidin-2-yl]-ethyl}-N-pyridin-3-yl-methyl-2-(4-trifluoromethoxy-phenyl)-acetamide (AMG 487) in human subjects after multiple dosing. Drug Metab Dispos 37:502–513 [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. (2000) The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav 65:75–82 [DOI] [PubMed] [Google Scholar]
- Yu AM. (2008) Indolealkylamines: biotransformations and potential drug-drug interactions. AAPS J 10:242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Herraiz T, Küpfer A, Gonzalez FJ. (2003a) Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics 13:307–319 [DOI] [PubMed] [Google Scholar]
- Yu AM, Granvil CP, Haining RL, Krausz KW, Corchero J, Küpfer A, Idle JR, Gonzalez FJ. (2003b) The relative contribution of monoamine oxidase and cytochrome P450 isozymes to the metabolic deamination of the trace amine tryptamine. J Pharmacol Exp Ther 304:539–546 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.