Abstract
A tracking system has been developed to provide real-time feedback of skin dose and dose rate during interventional fluoroscopic procedures. The dose tracking system (DTS) calculates the radiation dose rate to the patient’s skin using the exposure technique parameters and exposure geometry obtained from the x-ray imaging system digital network (Toshiba Infinix) and presents the cumulative results in a color mapping on a 3D graphic of the patient. We performed a number of tests to verify the accuracy of the dose representation of this system. These tests included comparison of system–calculated dose-rate values with ionization-chamber (6 cc PTW) measured values with change in kVp, beam filter, field size, source-to-skin distance and beam angulation. To simulate a cardiac catheterization procedure, the ionization chamber was also placed at various positions on an Alderson Rando torso phantom and the dose agreement compared for a range of projection angles with the heart at isocenter. To assess the accuracy of the dose distribution representation, Gafchromic film (XR-RV3, ISP) was exposed with the beam at different locations. The DTS and film distributions were compared and excellent visual agreement was obtained within the cm-sized surface elements used for the patient graphic. The dose (rate) values agreed within about 10% for the range of variables tested. Correction factors could be applied to obtain even closer agreement since the variable values are known in real-time. The DTS provides skin-dose values and dose mapping with sufficient accuracy for use in monitoring diagnostic and interventional x-ray procedures.
Keywords: skin dose, dosimetry, radiation safety, cardiac fluoroscopic procedures, fluoroscopic dose, dose tracking, real-time dosimetry, fluoroscopic interventional procedures
1. INTRODUCTION
Interventional fluoroscopic procedures involve both stochastic and deterministic radiation risk to the patient. The deterministic effect of concern for most procedures is damage to the skin since this is the organ receiving the highest radiation dose, being at the entrance surface of the patient. There have been numerous reports of patient injury which has included erythema, epilation, telangiactasia and necrosis.1-4 These effects are considered to have a threshold below which the effect is not seen and above which the severity of the effect increases. Currently there is no commercially available method for assessing the risk of these effects during the procedure since there is no method to provide a real-time determination of the skin dose distribution. Surrogates for skin dose estimation include dose-area-product (DAP) measurement, reference-point or cumulative dose determination and fluoroscopic exposure-time logging. None of these are sufficient for risk estimation. DAP does not provide an indication of the dose concentration on the skin, while cumulative dose does not account for changes in beam location or source to skin distance, and exposure time has been shown to provide only very poor correlation to patient exposure.5-7
We have been developing a system to provide real-time graphic feedback to the interventionalist of the dose distribution to the patient’s skin.8,9 Such real-time feedback is important for the proper management of patient radiation risk. This paper reports on the current status of this system and on measurements made to verify its performance accuracy. Currently the system is installed in a cardiac catheterization suite and verification will focus on that application.
2. MATERIALS AND METHODS
2.1 Dose Tracking System
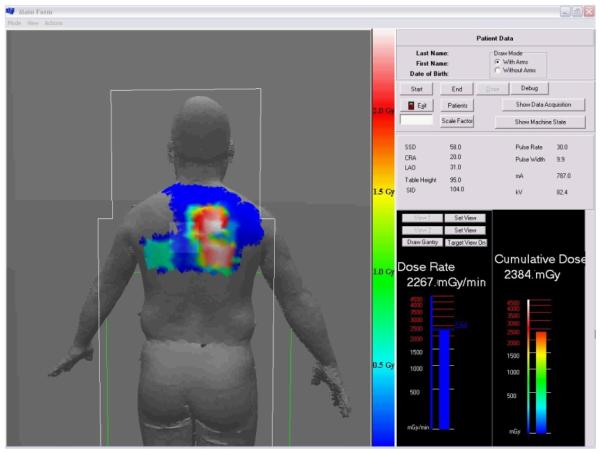
The dose tracking system (DTS) provides a graphic display showing the skin dose distribution and the skin dose rate in real time during fluoroscopic interventional procedures. The display gives a color-coded representation of the cumulative skin dose distribution on a patient graphic as well as the real-time peak skin dose rate and cumulative skin dose values at the current beam projection. Figure 1 gives an example of a screen capture of the display obtained at the end of a cardiac catheterization procedure. This display is intended to be presented in the procedure room as well as in the control room for immediate real-time feedback to the physician and technologist.
Figure 1.
DTS display at end of a cardiac procedure showing the color-coded mapping of skin dose over the back of the patient. The current virtual intersection of the x-ray beam with the patient is outlined by the lightened rectangle near the center. The current (or last exposure) peak dose rate and the peak cumulative skin dose in the current beam intersection are displayed in the bar graphs. The numerical tabulated data indicate the conditions of the last exposure.
Before the procedure begins, the patient graphic is selected from a library of models10 to obtain the closest match to the patient and its position on the table can be adjusted to correspond to the actual patient position if it differs from the default. The projection of the x-ray beam onto the patient is determined by a 3D graphic modeling of the imaging system and patient using OpenGL. The size of the beam and its projection onto the patient is obtained from the collimator position and the position of the patient relative to the x-ray tube. All geometric information relating to the position and orientation of the system gantry, collimator and patient table is obtained from a CAN bus which runs between the components of the Toshiba Infinix C-Arm imaging system which has a Varian PaxScan 2020 flat panel detector (FPD).
The DTS skin dose rate values are calculated from the product of the mA, pulse width, pulse rate and mGy per mAs. The mGy per mAs value is read from a data file that contains this value as a function of kVp for each of the three beam filters. The pulse width, mA and kVp are obtained from the CAN bus, while the pulse rate is currently not provided on the bus so it is given the default value for the protocol selected, but can be changed manually by the operator if the pulse rate is changed during the procedure. Cumulative dose is calculated by measuring the exposure time in intervals, multiplying it by the dose-rate value and summing the result. The results are summed for each element of the patient graphic that is intersected by the incident x-ray beam with individual inverse-square correction to that element. The patient graphic surface is modeled by tessellation into triangles and the cumulative values are assigned to each of the vertices.
2.2 System calibration
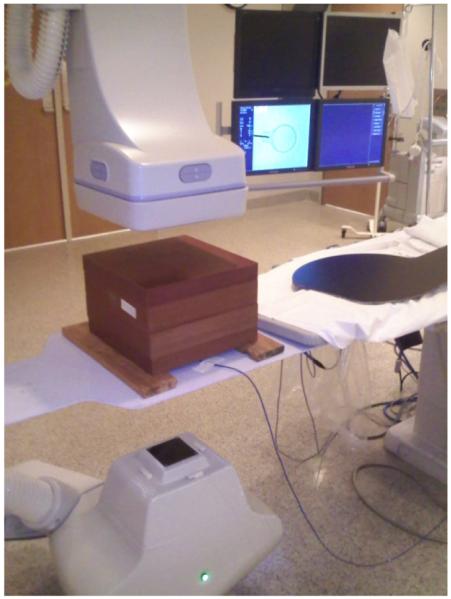
The mGy per mAs data file is determined by measurement with a PTW Unidos dosimeter and a calibrated PTW model TN34069 6 cc ionization chamber (PTW-Freiburg GmbH, Freiburg, Germany) which is placed on the tabletop under a 20 cm thick block of water equivalent plastic as shown in Figure 2. The R/min reading was measured in fluoroscopic mode with manual setting of mA, pulse width and kVp. The measured R/min value was multiplied by 9.2 to convert to skin dose in mGy. The value includes table attenuation and patient backscatter for the 7 inch mode at a position 15 cm on the tube-side of isocenter as appropriate for cardiac catheterization procedures. Separate calibrations were obtained for each of the 3 beam filters (F1 - 0.2 mm Cu, F2 - 0.3 mm Cu, F3 - 1.8 mm Al). A calibration set was also obtained for cine (DA) mode. Manual settings were not possible to generate the cine measurements as a function of kVp so the kVp was changed by changing the phantom attenuation by varying its thickness and by adding Cu sheets to obtain the higher kVp values.
Figure 2.
Setup for determination of the calibration file. The ionization chamber is placed on the tabletop under the solid water phantom 15 cm from isocenter to measure entrance skin dose.
This calibration is meant to provide the actual dose to the skin since this is the quantity that determines the deterministic risk. DAP readings and reference dose readings normally provide the air kerma without backscatter or table attenuation11 and thus do not provide an indication of skin dose even when scaled by beam area or source-to-skin distance (SSD).
2.3 Verification tests
We performed a number of tests to verify the accuracy of the dose representation of this system. These tests included comparison of system–calculated dose-rate values with ionization-chamber (6 cc PTW) measured values with change in kVp, beam filter, field size, source-to-skin distance and beam angulation.
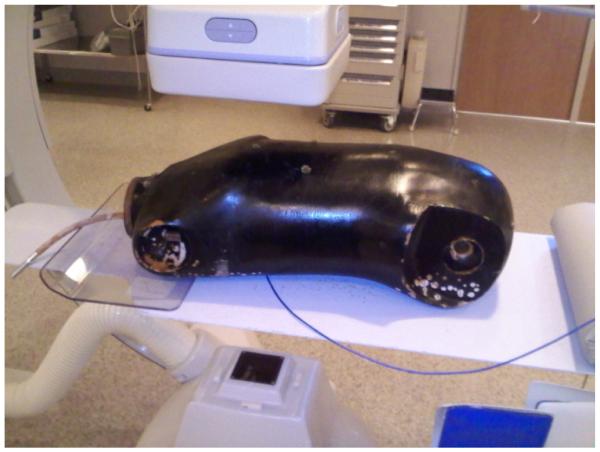
To verify the accuracy of the DTS in a simulated clinical setup, measurements of the entrance skin dose were made by placing the 6 cc PTW ionization chamber at various locations on the surface of the Alderson Rando anthropomorphic torso phantom as shown in Figure 3. The phantom was placed on the patient table with the heart at the isocenter of the Toshiba Infinix c-arm. A patient graphic was chosen from those available for the DTS that was the closest match in size to the phantom. The 7-inch mode was used for the FPD and the Toshiba system was operated in automatic technique selection mode. After the chamber was positioned and attached on the phantom, the C-arm gantry was rotated to the appropriate CRA/CAU and LAO//ROA angle to view the chamber and the heart at the center of the field of view. Imaging was performed in fluoroscopic and cine (DA at 30 frames per s) modes and the exposure rate was measured with a PTW electrometer. This value was multiplied by the exposure to dose conversion factor for soft tissue (9.2 mGy/R) to obtain the skin dose rate in mGy/min and was compared to the dose rate indicated on the DTS display. This was done for a range of projection angles normally used for a cardiac procedure.
Figure 3.
Positioning of the Alderson Rando Phantom for system verification. The ion chamber is placed on the entrance surface of the phantom in the center of the beam with the beam at various LAO/RAO and CRA/CAU orientations.
To assess the geometric accuracy of the dose distribution representation, Gafchromic® film (XR-RV3, International Specialty Products Corporation, Advanced Materials Group, Wayne, NJ) was exposed with the beam at different locations and the beam sizes and positioning was compared to the scaled DTS graphic representation. The film was read with a Canon MF5750 Scanner and a calibration curve was generated by exposing strips to different known exposures while placed on the tabletop with full solid-water backscatter.
3. RESULTS AND DISCUSSION
3.1 Calibration verification
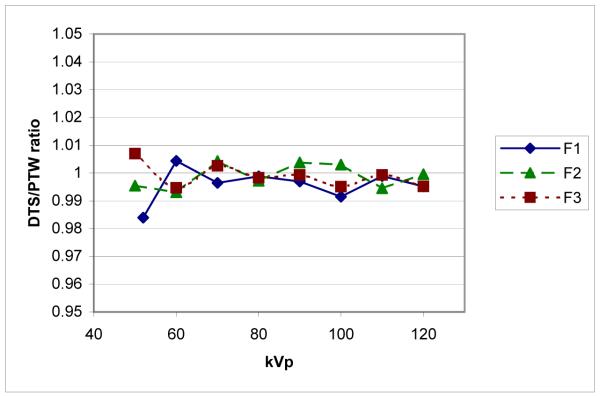
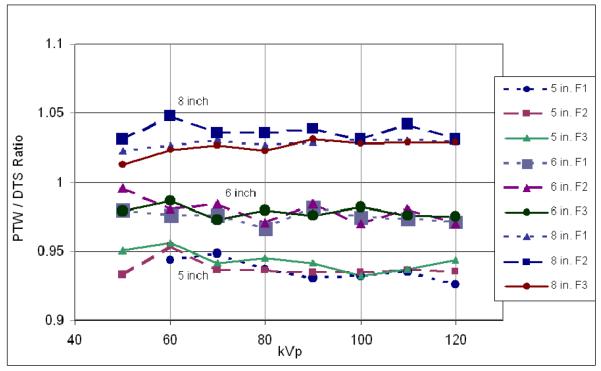
To verify that the system is able to accurately calculate the dose values, the 6 cc PTW ionization chamber was placed on the tabletop under a 20 cm block of water-equivalent plastic, with the table 15 cm tube-side of isocenter. Dose rate values were read from the DTS display with the system in ion chamber mode (i.e., giving the tabletop dose rate). The tube kilovoltage was manually varied in fluoroscopic mode from 50 to 120 kVp for all three beam filters and the measured dose-rate value was compared to that of the DTS display. The results in Figure 4 show the agreement between the DTS calculated reading and the PTW measured value to be within 1% over most of the kVp range for all filters. Excellent linearity of dose rate reading was also demonstrated with variation in mA, pulse width and pulse rate as these are multiplicative factors in the program.
Figure 4.
Agreement between DTS calculated and PTW-chamber measured dose rate vs. kVp for 3 beam filters (F1, F2 and F3) using the 7-inch mag mode.
3.2 Variation with mode
To determine the accuracy of the DTS as the FPD magnification mode is changed, the PTW ionization chamber was placed on the tabletop at 15 cm tube-side of isocenter under 20 cm of water-equivalent plastic and fluoroscopy was performed for the 5, 6, 7 and 8 inch modes. The collimated beam size was set to correspond to the selected mode. Figure 5 shows the ratio of PTW dose rate reading to the DTS reading as a function of kVp for each FPD mag mode and each beam filter. Since the DTS calculated value uses a calibration file measured for the 7-inch mag mode, the values for the 8″ mode are greater than 1 and those for the 5 and 6 inch modes are less than 1 due to differences in backscatter. It is seen that these ratios vary only a small amount as a function of kVp and filter for the same mag mode. More variation is seen between mag modes due to changes in backscatter with field size. As seen in Figure 5, the change from the 5″ to the 8″ modes was about 10% and mode specific calibration files are being implemented to reduce this difference using mode and collimator information available on the CAN bus.
Figure 5.
PTW chamber reading at each mag mode and beam filter relative to the DTS reading, which was calibrated for the 7-inch mode.
3.3 Variation with angle
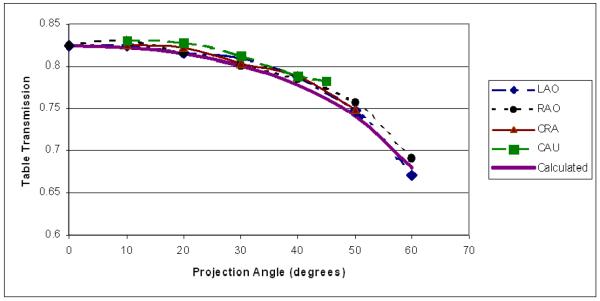
The variation of patient table attenuation with projection angle is a potential source of error. This variation was measured by placing the PTW ionization chamber at the FPD before 3 mm of Cu to reduce backscatter to the chamber and rotating the gantry so that the angle of transmission through the table was varied. Transmission measurements were made using fixed fluoroscopic techniques over RAO/LAO and CRA/CAU angles every 10 degrees. Figure 6 shows the reduction of transmission with angle for all projections at 80 kVp using filter 2. This reduction is simply the result of increased path length through the table, which varies as the inverse cosine of the incident angle. A fit was made to this data assuming exponential attenuation through the table and the calculated change of thickness with angle to obtain a value for the product of linear attenuation coefficient (μ) and thickness (T). The transmission curve obtained using this fitted μT value is shown as the “calculated” curve in the figure. This μT value is used as an angular correction in the DTS program using the compound angle derived from the RAO/LAO and CRA/CAU values. The form of the curve used for the fit assumes a simple exponential with no table beam hardening.
Figure 6.
Variation of x-ray transmission through the patient table as a function of angle measured for Filter 2, 80 kVp. The calculated curve is based on a fit of all the data to an exponential of table thickness, which varies as the inverse cosine of the projection angle.
3.4 Anthropomorphic phantom verification
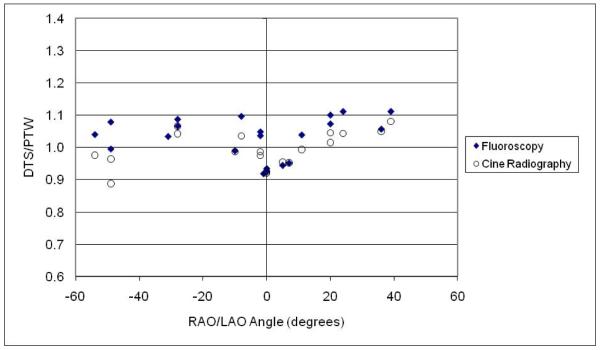
The accuracy of the DTS displayed dose/dose-rate values for a simulated procedure was evaluated by placing the ionization chamber on the surface of the Alderson phantom and comparing its reading with the DTS calculated values. Exposures were made over a range of RAO/LAO and CRA/CAU angles with the heart at isocenter and the chamber in the center of the beam. Readings were taken of both the rate and integrated dose over various intervals of exposure time using both fluoroscopy and cine radiography. Figure 7 shows the combined results taken on two different days. Plotted are the ratios of dose-rate values and integrated dose values of the DTS system to the values measured by the PTW chamber (DTS/PTW). The projection angles shown here range from 54° RAO to 36° LAO and 30° CRA to 38° CAU; values at a given LAO/RAO angle shown in the graph may have been measured at different CRA/CAU angles.
Figure 7.
Ratio of DTS dose/dose-rate reading to PTW chamber measurement at various locations on the Rando phantom and various beam projections.
Over the angles shown, the ratio of rate readings varied from 0.92 to 1.11 for the fluoro and from 0.89 to 1.05 for the cine measurements and the ratio of integrated dose readings varied from 0.93 to 1.11 for the fluoro and from .92 to 1.08 for the cine. This is good agreement considering that 1) the DTS displayed value is the highest dose (rate) reading in the field of view (this is intentional in the design of the program in order to provide the peak skin-dose values) which would be the point on the patient graphic contour with the closest SSD, while the chamber was placed to measure the exposure in the center of the field of view; 2) there is some expected variation in backscatter with location due to the differing composition of the phantom (bone, lung, soft tissue) which is not considered by the program; and 3) the contour and size of the phantom is not an exact match to the patient graphic used for the DTS calculation.
3.5 Dose distribution mapping verification
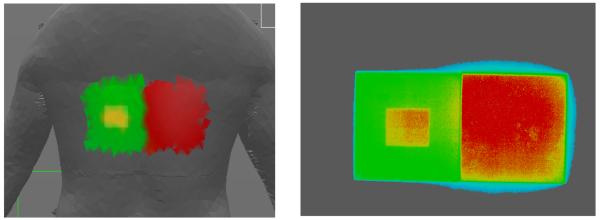
The correspondence of the dose distribution determined by the DTS system to the actual dose distribution was evaluated using Gafchromic film. Use of the film also allowed us to obtain geometric scaling factors for the x-ray beam graphics. Figure 8 gives an example of the correspondence obtained in the distribution indicated on the back of a patient graphic compared to that recorded on Gafchromic film for a fixed pattern of fluoroscopic exposures. The two larger beams were juxtaposed using the image display and the spatial positioning on the graphic was excellent. Exact matching in this comparison is not possible because of the 3D surface and the coarse resolution (large surface element size) on the patient graphic. The film was scanned and the gray levels were converted to the color scale of the DTS graphic using a calibration curve. Comparing the DTS and film distributions demonstrates excellent visual agreement within the cm-sized surface elements used for the patient graphic. The film measured distribution does show a small amount of additional penumbra and scatter at the edge of the field that is not modeled by the DTS program.
Figure 8.
Comparison of dose distribution provided on DTS graphic (left) with that recorded on Gafchromic film (right). The density values of the Gafchromic film were color-converted using a calibration curve and the color bar on the DTS graphic.The blue outline is likely due to penumbra and scatter outside the main beam that is not included on the patient graphic.
4. SUMMARY AND CONCLUSION
The DTS has been shown to be able to track entrance skin dose accurately with changes in kVp, mA, pulse width, and pulse rate. Currently, there is some variation with field size due to changes in backscatter. However, the change seen between the 5 inch and 8 inch FPD modes was less than 10% and a field-size correction factor can be incorporated to reduce this error. Good agreement was obtained between the ionization chamber readings and the DTS system for the simulated clinical setup using the Alderson Rando phantom. This agreement was not as close as the calibration verification with solid water, likely due to inexact matching of the graphic to the anthropomorphic phantom and because the peak dose indicated by the numerical display may differ from the measured central axis dose.
Overall, the DTS has been shown to provide skin-dose values and dose mapping with sufficient accuracy for use in monitoring diagnostic and interventional x-ray procedures. This method should provide a way to accurately assess skin dose and provide evidence of maintaining dose levels below the Joint Commission sentinel event level.12 Further, by providing real-time monitoring, the interventionalist can be proactive in using dose spreading13 to maintain levels below thresholds for injury. There has been increasing movement toward providing the information required for skin dose estimation using DICOM irradiation event recording.14 Such recording will require software such as described here to provide the skin-dose distribution needed to assess deterministic risk.
ACKNOWLEDGEMENTS
Support for this work was provided in part by grants R43FD0158401, R44FD0158402, R01EB002873 and R01EB0084501, and by Toshiba Medical Systems Corporation.
REFERENCES
- [1].Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high x-ray dose interventional procedures. J. Vasc. Interv. Radiol. 1994;5:71–84. doi: 10.1016/s1051-0443(94)71456-1. [DOI] [PubMed] [Google Scholar]
- [2].Shope T. Radiation induced skin injuries from fluoroscopy. Radiol. 1996;197(Supplement):449. doi: 10.1148/radiographics.16.5.8888398. [DOI] [PubMed] [Google Scholar]
- [3].Wagner LK, McNeese MD, Marx MV, Siegel EL. Severe skin reactions from interventional fluoroscopy: Case report and review of the literature. Radiol. 1999;213:773–776. doi: 10.1148/radiology.213.3.r99dc16773. [DOI] [PubMed] [Google Scholar]
- [4].Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. Am. J. Roentgenol. 2001;177:13–20. doi: 10.2214/ajr.177.1.1770003. [DOI] [PubMed] [Google Scholar]
- [5].McParland BJ. Entrance skin dose estimates derived from dose-area product measurements in interventional radiological procedures. Br. J. Radiol. 1998;71:1288–1295. doi: 10.1259/bjr.71.852.10319003. [DOI] [PubMed] [Google Scholar]
- [6].Fletcher DW, Miller DL, Balter S, Taylor MA. Comparison of four techniques to estimate radiation dose to skin during angiographic and interventional radiology procedures. J. Vasc. Interv. Radiol. 2002;13:391–397. doi: 10.1016/s1051-0443(07)61742-4. [DOI] [PubMed] [Google Scholar]
- [7].Dohatcu A, Bednarek DR, Rudin S. Analysis of dose-related data logged for fluoroscopic cardiac interventional procedures. Med. Phys. 2006;33:2210. [Google Scholar]
- [8].Dinu P, Bednarek DR, Wobschall D, Peterson R, Ionita C, Rudin S, Zeng M, Chugh K, Hoffmann KR. X-ray beam tracking system for a fluoroscopic C-arm unit. Med. Phys. 2003;30(6):1424. [Google Scholar]
- [9].Chugh K, Dinu P, Bednarek DR, Wobschall D, Rudin S, Hoffmann KR, Peterson R, Zeng M. A computer-graphic display for real-time operator feedback during interventional x-ray procedures. Proc of SPIE. 2004;5367:464. [Google Scholar]
- [10].SAE International Civilian American and European Surface Anthropometry Resource Project-CAESAR®. http://www.sae.org/standardsdev/tsb/cooperative/caesar.htm.
- [11].Bednarek DR, Rudin S. Comparison of two dose-area-product ionization chambers with different conductive surface coating for over-table and under-table tube configurations. Health Phys. 2000;78:316–321. doi: 10.1097/00004032-200003000-00010. [DOI] [PubMed] [Google Scholar]
- [12].Joint Commission . Aurora, IL: 2006. Sentinel Event Policy and Procedures. http://www.jointcommission.org. [Google Scholar]
- [13].Rudin S, Bednarek DR. Comparison of filter material and design for use in ROI angiography. Proc. SPIE. 1993;1896:354–364. [Google Scholar]
- [14].Balter S. Capturing patient doses from fluoroscopically based diagnostic and interventional systems. Health Phys. 2008;95:535–540. doi: 10.1097/01.HP.0000327650.37315.2f. [DOI] [PubMed] [Google Scholar]