Abstract
During development of the central nervous system, stem and progenitor cell proliferation and differentiation are controlled by complex inter- and intracellular interactions that orchestrate the precise spatiotemporal production of particular cell types. Within the embryonic retina, progenitor cells are located adjacent to the retinal pigment epithelium (RPE), which differentiates prior to the neurosensory retina and has the capacity to secrete a multitude of growth factors. We found that secreted proteinaceous factors in human prenatal RPE conditioned media (RPE CM) prolonged and enhanced the growth of human prenatal retinal neurospheres. The growth-promoting activity of RPE CM was mitogen-dependent and associated with an acute increase in transcription factor phosphorylation. Expanded populations of RPE CM-treated retinal neurospheres expressed numerous neurodevelopmental and eye specification genes and markers characteristic of neural and retinal progenitor cells, but gradually lost the potential to generate neurons upon differentiation. Misexpression of Mash1 restored the neurogenic potential of long term cultures, yielding neurons with phenotypic characteristics of multiple inner retinal cell types. Thus, a novel combination of extrinsic and intrinsic factors was required to promote both progenitor cell proliferation and neuronal multipotency in human retinal neurosphere cultures. These results support a pro-proliferative and anti-apoptotic role for RPE in human retinal development, reveal potential limitations of human retinal progenitor culture systems, and suggest a means for overcoming cell fate restriction in vitro.
Introduction
Mammalian retinal histogenesis follows a well-conserved progression of events, beginning with the differentiation of the retinal pigment epithelium (RPE) from the outer layer of the bilayered optic cup [1–5]. Cells within the inner layer of the optic cup then undergo proliferation to form a dense neuroblastic layer containing undifferentiated retinal progenitor cells (RPCs). The apical surfaces of the RPE and RPC layers lie in direct apposition to one another, separated only by a potential space that is contiguous with the early ventricular system of the developing brain [3]. Differentiation of RPCs and formation of the normal laminar structure of the retina occur in a precise spatiotemporal order, with long projection neurons (ganglion cells) appearing early, followed by photoreceptors and retinal interneurons and, ultimately, Müller glia cells [6–16]. Lineage tracing studies have shown that these cells are all derived from a common progenitor [17,18] whose competency to generate particular retinal cell types changes with time [6,9,10,19].
The importance of RPE in the development of the neurosensory retina has been established by multiple studies [20–24]. A specific role for RPE-secreted proteins in rat retinal development was investigated using medium conditioned by primary and transformed cultures of rat RPE. In these studies, rat RPE conditioned medium (RPE CM) was shown to promote RPC and Müller glia proliferation, retinal cell neurite extension, and photoreceptor cell maturation and survival [5,25–29]. Similarly, re-aggregated embryonic chick retinal cells exhibited sustained proliferation and enhanced laminar organization when cultured in the presence of RPE [30–32]. The diffusible RPE factor(s) involved in these effects have not been definitively identified, although an approximately 67-kDa protein has been implicated [27]. It is known, however, that RPE has the capacity to secrete a multitude of proteins, including numerous growth factors and neurotrophins, which could potentially affect developing and mature retinal cells [1,5,33,34].
In addition to providing insight into the role of RPE during retinal histogenesis, information from studies on rodent and chick RPE CM could be used to optimize strategies to propagate RPCs in vitro. In particular, RPCs harvested from pre- and postnatal human neural retina have proven difficult to maintain and expand for extended periods in standard neurosphere culture [35–39]. Neurospheres are spherical aggregates of neural stem and progenitor cells grown in suspension, typically using defined medium supplemented with FGF2 and/or EGF [13,39–42]. Enhancement of human cortical neural progenitor cell (hNPCctx) proliferation was observed when neurospheres were passaged using a mechanical chopping method, which avoids exposure of cell surface proteins to proteolytic enzymes and maintains potentially important intercellular contacts within the sphere structure [42]. However, application of this technique to prenatal human RPC (hRPC) neurospheres only supported growth for approximately one month [35]. Moreover, in both retinal and cortical neurospheres, the competency of progenitor cells to produce neurons declined over time in culture while the propensity to yield glia increased [35,43].
In an effort to improve the growth potential of hRPC neurospheres derived from human embryonic retina, we utilized CM obtained from serum-free, monolayer cultures of human embryonic RPE [33]. Compared to untreated retinal neurospheres, RPE CM-treated neurospheres displayed a greatly enhanced growth period lasting up to one year in culture. The RPE CM-mediated effect was mitogen-dependent and selective for retinal neurospheres. Examination of the cell fate potential of differentiated, RPE CM-treated neurosphere cultures confirmed a time-dependent decline in neurogenesis. However, following transduction with a lentiviral vector expressing the basic helix-loop-helix (bHLH) protein Mash1, the capacity of late-passage retinal neurosphere cultures to produce neurons was restored.
Methods
Retinal neurosphere culture
Human retina and RPE were isolated from post mortem fetal eye tissue with a mean gestational age of 91 ± 14 days as described [33,35]. The method of collection conformed to the NIH guidelines for the collection of such tissues, as well as the IRB requirements for the University of Wisconsin. Briefly, after removal of the anterior portion of the eye cup and vitreous, the retina was carefully detached without disturbing the underlying RPE. Wide margins of anterior retina near the ora serrata and posterior retina surrounding the optic nerve were excluded from the dissection. After sectioning the tissue into 200 µm cubes with a McIlwain® tissue chopper, it was seeded into T75 flasks and cultured as neurospheres in standard medium consisting of DMEM/HAMS F12 (3:1), 1% antibiotic-antimycotic, 2% B27 (Invitrogen, Carlsbad, CA), 20 ng/ml EGF (Sigma-Aldrich, St. Louis, MO), 20 ng/ml FGF2 (R&D Systems, Minneapolis, MN) and 5 µg/ml heparin (Sigma-Aldrich), or in supplemented conditioned medium (CM) collected from monolayers of fetal human RPE [33]. Neurospheres were passaged by chopping as described previously [35,42] and half the medium was exchanged every 1 to 2 days.
Conditioned media production
Monolayer cultures were established from human fetal RPE and lens after plating onto 10 µg/ml laminin-coated flasks containing standard medium as described [33]. Conditioned medium was collected daily, sterile-filtered through a 0.2 µm membrane, and supplemented with 20 ng/ml EGF, 20 ng/ml FGF2 and 5 µg/ml heparin before use. Conditioned medium from human prenatal cortical neurosphere cultures [42] was collected and processed in an identical fashion.
Growth studies
Increases in neurosphere volume were used as an index of growth as previously described for neural [42] and retinal [35] progenitor cultures. For growth assays examining the effects of EGF receptor (EGFR) and FGF2 receptor (FGFR1) blocking agents, a subset of retinal neurospheres (culture passage 12 and 13) were placed in RPE CM with 20 ng/ml EGF, 20 ng/ml FGF2, 5 µg/ml heparin, 10 µM EGFR inhibitor AG1517 and 10 µM FGFR1 inhibitor SU5402 (Calbiochem, La Jolla, CA) for the duration of the experiment. For 5-bromo-2’-deoxyuridine (BrdU) incorporation assays, neurospheres cultured in RPE CM (≥ 25 passages) were pulsed with 0.2 µM BrdU (Sigma-Aldrich) for 14 hours before dissociation and plating onto coverslips coated with 0.01% poly-L-lysine and 10 µg/ml laminin for 1 hour. Additional neurospheres from parallel cultures were grown in standard medium for 48 hours prior to being pulsed with BrdU. Following fixation, cells were stained with 1:300 anti-BrdU (Roche, Indianapolis, IN) according to manufacturer’s instructions. Data were expressed as mean ± SEM and were analyzed using one-way ANOVA with Newman-Keuls post-hoc test.
Immunocytochemistry
Proliferating retinal neurospheres were dissociated into single cell suspensions with Accutase® for 10 min at 37°C and 30,000–50,000 cells were plated on coated glass coverslips in standard medium for 4 hours or allowed to differentiate for 7 days. Cells were fixed with 4% paraformaldehyde and processed for immunocytochemistry with primary antibodies (Supplemental Table 1). After rinsing with PBS, cells were incubated for 30 min with secondary antibodies conjugated to Alexa 488 or Alexa 546 (Molecular Probes, Carlsbad, CA). Hoechst 33258 (1:10,000 in PBS) was added for 5 min to visualize nuclei. For TUNEL assays, 50,000 cells from dissociated neurosphere cultures (≥19 passages) were plated per laminin- and poly-L-lysine-coated coverslip in RPE CM for 24 hr, washed 3 times with standard medium and placed in 500 µl standard medium or RPE CM for 48 hrs before being fixed and assessed for percentage of TUNEL positive cells as directed by the manufacturer (Promega, Madison WI).
Cell counts
Cell counts were performed using a Nikon fluorescence microscope (40X objective) and Metamorph Imaging software (Universal Imaging Corporation, Downington, PA). Quantification of cells was based on counting the number of Hoechst-stained nuclei and the specified immuno-markers in at least 5 independent fields containing at least 60 cells per field (total area > 25 mm2) from a minimum of 3 coverslips.
PhosphoCREB assay
Retinal neurospheres were maintained for ≥ 5 passages in either standard medium (naïve group) or mitogen-supplemented RPE CM (RPE CM group). After Accutase® dissociation, single cell suspensions were diluted to 1000 cells/µl, and 50,000 cells were plated onto coated glass coverslips and starved of EGF and FGF2 by placing them in either 2% B27 only (naïve group) or RPE CM supplemented with 2% B27 (RPE CM group). After 24 hours, all media was removed and the cultures were challenged for 7 minutes by the addition of 500 µl of either: 1) vehicle (DMEM/F12 with 2% B27 only), 2) standard medium (DMEM/F12 with 2% B27, 20 ng/ml EGF, 20 ng/ml FGF2 and 5µg/ml heparin), 3) RPE CM (RPE CM with 2% B27) or 4) mitogen-supplemented RPE CM (RPE CM with 2% B27, 20 ng/ml EGF, 20 ng/ml FGF2 and 5µg/ml heparin). Following fixation in 4% paraformaldehyde, the cells were immunostained for phosphorylated CREB (pCREB), and the percentage of labeled cells was determined.
RT-PCR
RNA isolation and cDNA synthesis was performed as previously described [33]. The cDNA templates were diluted 1:40 and added to PCR reactions containing Master Mix™ (Promega, Madison, WI) and 10 µM each of the appropriate forward and reverse primers (Supplemental Table 2). Samples were initially denatured at 95°C for 5 minutes followed by 30 cycles of PCR amplification (95°C for 15 sec, 60°C for 30 sec, 72°C for 1 min) and a final extension at 72°C for 10 min. PCR products were visualized on a 1.5% agarose gel containing 0.1% ethidium bromide. PCR reactions were repeated using at least three different retinal neurosphere cultures to ensure reproducibility. Quantitative RT-PCR (40 cycles) was also performed as described [33] using primer pairs that span at least one intron (Supplemental Table 3), SYBR Green® 2X PCR Master Mix (Applied Biosystems) and the Opticon 2 DNA Engine and Opticon Monitor 2.02 software (MJ Research).
Lentiviral infection
A self-inactivating lentiviral construct containing a mouse phosphoglycerate kinase-1 internal promoter [44] driving the human gene encoding Mash1 (Supplemental Methods) was used to generate constitutive Mash1-expressing retinal progenitor cells. Transducing units (TU)/ml were determined by infecting HeLa cells and analyzing proviral insertion number by quantitative PCR. Retinal neurospheres were dissociated and resuspended in RPE CM at 1000 cells/µl and mixed with virus (3 TU/106 cells). 50,000 cells per coverslip were plated onto coated glass coverslips for 24 hours in supplemented RPE CM. Thereafter, RPE CM was withdrawn and cells were allowed to differentiate in the absence of mitogens for an additional 5 days prior to fixation.
Results
Secreted Proteins from RPE CM Selectively Prolong and Enhance the Growth of Human Retinal Neurospheres
In an earlier study, we observed that human prenatal retinal neurospheres cultured in defined, serum-free medium supplemented with B27, EGF, FGF2 and heparin (standard medium) stopped growing after approximately one month in vitro (equivalent to approximately 2 passages) [35]. In an effort to improve the growth potential of human retinal neurospheres, we examined the effects of Neurobasal® medium and leukemia inhibitory factor, each of which has been shown to promote long term growth of cortical neurospheres [41,43]. In addition, primary prenatal retinal cells were grown as dissociated, monolayer cultures on laminin-coated flasks in standard medium. However, these interventions failed to support significant culture expansion beyond one month (data not shown).
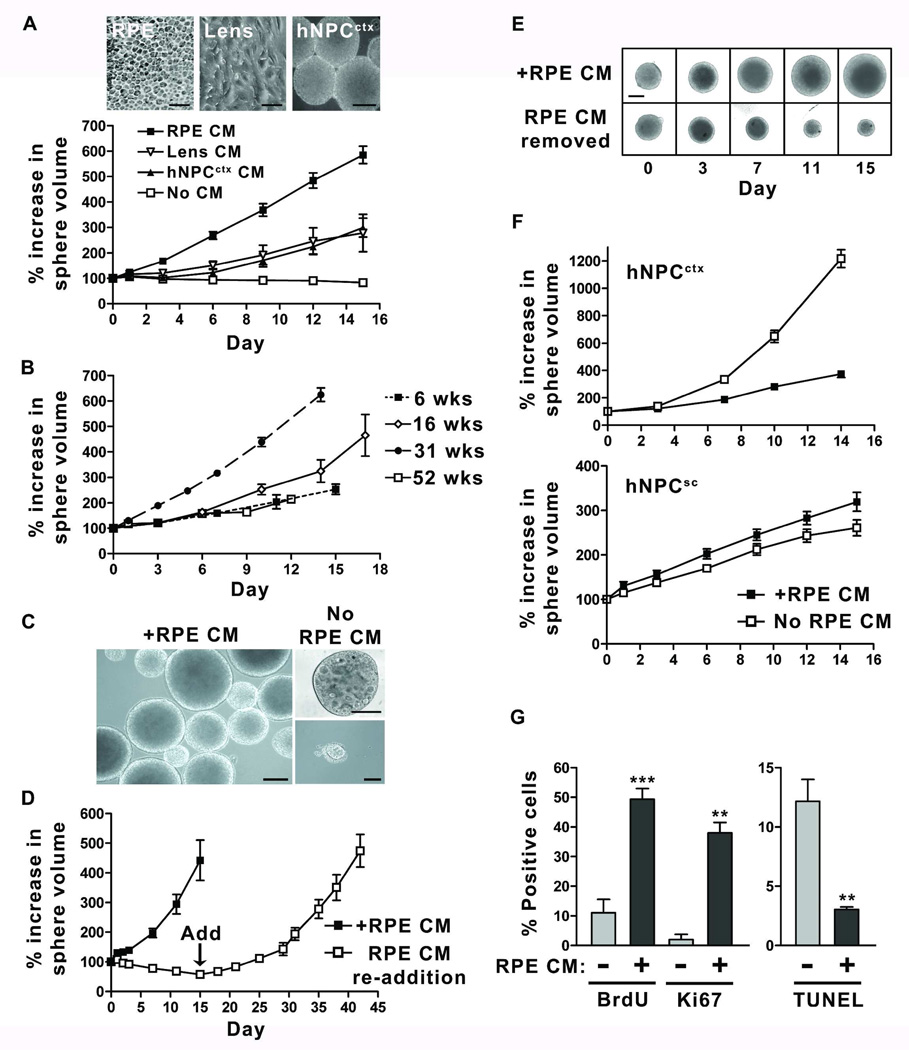
We then investigated the effect of mitogen-supplemented conditioned medium (CM) from different cell sources on retinal neurosphere growth. We chose to examine CM from human prenatal RPE [33] and lens [45], as these tissues are well positioned to influence retinal progenitor cells (RPCs) during development [34,46–48]. In addition, we tested the effect of CM from neurosphere cultures of human cortical neural progenitor cells (hNPCctx) [42], which are known to secrete factors important for their own expansion. In all independent cultures tested (n=13, passage 4–42), retinal neurosphere growth assays revealed a dramatic growth-promoting effect of RPE CM (Figure 1A). Lens CM and hNPCctx CM also improved growth of retinal neurosphere cultures (n=3) to a lesser extent, but standard medium did not support neurosphere growth after one month in culture. The addition of 5 or 10% fetal calf serum to mitogen-supplemented standard medium likewise failed to promote human retinal neurosphere growth (data not shown). The RPE CM-mediated enhancement of retinal neurosphere growth continued for up to 52 weeks (42 passages), but in all cases culture expansion rates eventually declined and growth ceased (Figure 1B). Morphologically, human retinal neurospheres cultured in standard medium would reduce in size, extrude cells and become vacuolated after one month in vitro, while those propagated long term in supplemented RPE CM maintained a uniform golden color and spherical structure with small, pilocytic surface processes similar to human hNPCctx neurosphere cultures (Figure 1C) [42]. When RPE CM was replaced with standard medium, retinal neurospheres abruptly began to decrease in size (Figure 1D and E), a process that could be completely reversed by the re-introduction of RPE CM (n=4 independent cultures, passage 10–30) (Figure 1D). Furthermore, the growth-promoting effect of RPE CM was relatively specific for embryonic retinal neurospheres, since human embryonic cortical neurosphere cultures (n=3) underwent a significant growth reduction in response to supplemented RPE CM, and growth of human embryonic spinal cord neurosphere cultures (n=3) was largely unaffected by its presence (Figure 1F). Retinal neurospheres obtained from postnatal human eyes did not demonstrate growth with or without RPE CM (data not shown).
Figure 1.
RPE CM selectively enhances growth of retinal neurospheres. (A) Representative photomicrographs of cell sources used for conditioned medium collection (upper panels). Comparison of neurosphere growth curves measuring increases in individual sphere volume over 15 days for retinal neurospheres supplemented with CM from human prenatal RPE, lens monolayer cultures or cortical neural progenitor (hNPCctx) neurosphere cultures (lower panel); scale bars = 20 µm (RPE and lens) or 200 µm (hNPCctx). (B) Growth of retinal neurospheres in RPE CM at 6, 16, 31 and 52 weeks in culture (4, 12, 24 and 42 passages, respectively). (C) Sphere morphology from a retinal neurosphere culture grown for two months (6 passages) in standard medium supplemented with RPE CM (left panel) compared to those in standard medium only (right panels); scale bars = 200 µm. (D) Sphere growth assay comparing retinal neurospheres maintained continuously in RPE CM versus those switched to standard medium only at day 0 with subsequent re-addition of RPE CM at day 15. (E) Fifteen day time course showing changes in the size of representative retinal neurospheres maintained either in RPE CM (upper panels) or standard medium following withdrawal of RPE CM (lower panel); scale bar = 200 µm. (F) Growth assays comparing the effect of RPE CM on neurospheres derived from human fetal cortex (hNPCctx) (upper panel) or spinal cord (hNPCsc) (lower panel). (G) Quantification of cellular proliferation by BrdU incorporation and Ki67 immunodetection (left panel) or apoptotic cell death by TUNEL staining (right panel) in retinal neurospheres grown in RPE CM or standard medium following withdrawal of RPE CM. Values are expressed as percentage ± SEM (n≥ 3). ** p<0.01, *** p<0.001.
We next sought to determine if the improved growth of RPE CM-treated retinal neurospheres resulted from an increase in cellular proliferation and/or a reduction in cell death. In these experiments (n≥ 3, passage 19–26), retinal neurospheres exposed to supplemented RPE CM exhibited greater cell proliferation capacity than parallel cultures maintained in standard medium without CM, as determined by BrdU incorporation (49.3 ± 8.1% vs. 11.1 ± 9.0%, p<0.0003) and Ki67 expression (38.0 ± 6.1% vs. 2.0 ± 2.5%, p<0.005) (Figure 1G). In addition, apoptotic cell death was reduced in the presence of RPE CM from 12.2 ± 3.2% in untreated cultures to 3.0 ± 0.4% in RPE CM-treated cultures (p<0.008), as determined by TUNEL staining (Figure 1G). Together, these results demonstrate that diffusible factor(s) secreted by RPE selectively prolong and enhance human embryonic retinal neurosphere growth through a combination of pro-proliferative and anti-apoptotic mechanisms.
To ascertain whether the growth-promoting effect of RPE CM on human retinal neurospheres was mediated by secreted proteins, CM was incubated with trypsin, followed by inactivation with soybean trypsin inhibitor and supplementation with B27 and mitogens. This treatment abolished the majority of its activity in all cultures tested (n=6, passage 13–16) (Supplemental Figure S1A), indicating that secreted proteins are largely responsible for the retinal neurosphere growth enhancement observed with RPE CM. We then subjected RPE CM to protein fractionation to examine whether its growth-promoting activity would segregate into a particular molecular weight (MW) class. Protein fractions of CM were generated using size-exclusion filtration devices with 100-, 50-, 30- and 3-kDa MW cutoffs and compared with unfractionated CM in retinal neurosphere growth assays. In all retinal neurosphere cultures examined (n=5, passage 9–38), fractions that excluded higher MW proteins (100-kDa cutoff fraction) retained full growth-promoting activity, while all other fractions (50-kDa cutoff and below) exhibited significantly reduced activity (Supplemental Figure S1B). Coomassie-stained SDS-PAGE revealed a number of major and minor bands in unfractionated, concentrated CM (without B27), but also showed that the 50-kDa MW cutoff fraction eliminated most proteins greater than or equal to 15-kDa (Supplemental Figure S1C). This result was confirmed with silver stained gels (data not shown). Thus, the majority of the RPE CM growth-promoting effect can be assigned to one or more soluble proteins or protein complexes of mid-range MW. However, by virtue of the residual growth effect still present in the 3-kDa cutoff fraction, very small molecular weight protein(s) and/or non-proteinaceous factor(s) likely possess lesser abilities to enhance human retinal neurosphere growth.
The Growth-Promoting Activity of RPE CM is Mitogen-Dependent
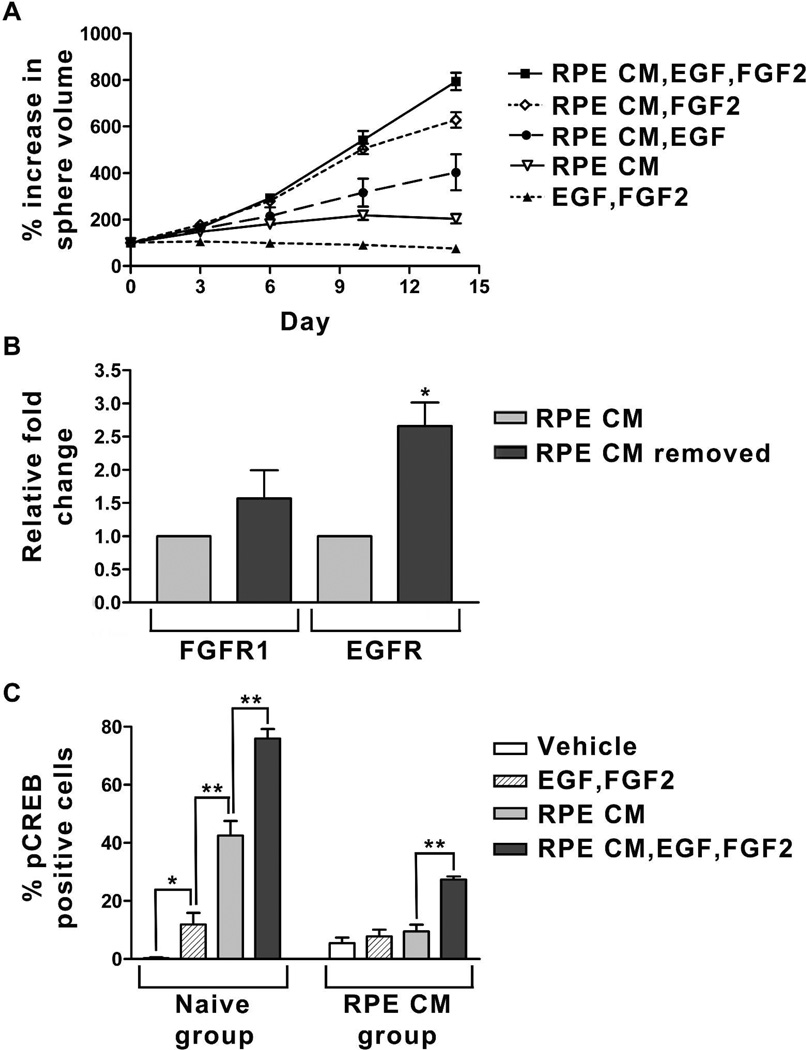
A commonly observed feature of undifferentiated neural stem and progenitor cells is their dependency on EGF and/or FGF2 for continued expansion [41,42,49,50]. To determine if RPE CM-treated human retinal neurospheres are similarly dependent on exogenous EGF and FGF2, retinal neurosphere growth assays were performed on 6 independent cultures (passage 9–13) in the presence and absence of these mitogens. Exogenous EGF or FGF2 was required to observe substantial RPE CM-mediated retinal neurosphere growth, and a synergistic effect was seen in the presence of both growth factors (Figure 2A). To confirm that the growth effect of RPE CM was dependent on mitogen receptor activation, EGF receptor (EGFR) and FGF2 receptor (FGFR1) autophosphorylation was suppressed with the specific tyrosine kinase inhibitors AG1517 and SU5402, respectively. Addition of these two compounds to medium containing RPE CM, EGF and FGF2 prohibited growth and led to retinal neurosphere volume loss (Supplemental Figure S2). Therefore, RPE CM acted to restore and enhance the mitogen-responsiveness of retinal neurospheres. Potential mechanisms for this effect could involve EGF and FGF2 receptor expression and/or signaling pathways linked to cell proliferation. To investigate the first possibility, we performed real-time PCR to quantify FGFR1 and EGFR gene expression in RPE CM-treated and untreated retinal neurosphere cultures. In these experiments (n=3, passage 11–21), there was no clear effect (<1 standard deviation difference) on FGFR1 gene expression four days after removal of RPE CM (Figure 2B). Interestingly, EGFR gene expression was upregulated (>2 standard deviations) in the absence of RPE CM over the same time period. This finding suggests that the growth-promoting effect of RPE CM was not due to enhanced mitogen receptor expression.
Figure 2.
Mitogens are required for RPE CM-dependent retinal neurosphere growth. (A) Retinal neurophere growth assay comparing the effects of standard medium, RPE CM without mitogens, or RPE CM with 20 ng/ml EGF and/or 20 ng/ml FGF2. (B) Changes in EGFR and FGFR1 gene expression in retinal neurosphere cultures after withdrawal of RPE CM for 4 days, as determined by quantitative RT-PCR. Values are expressed as fold change in gene expression relative to retinal neurospheres maintained continuously in RPE CM. *>2 standard deviation difference. (C) Quantification of phosphoCREB immunolabeled cells following 7 minute challenge with either vehicle alone, vehicle + 20 ng/ml EGF and 20 ng/ml FGF2, RPE CM without mitogens, or RPE CM + 20 ng/ml EGF and 20 ng/ml FGF2. Cultures were maintained in either standard medium (naïve group) or RPE CM (RPE CM group) prior to challenge. Values are expressed as percentage ± SEM (n=3). *p<0.03, ** p<0.003.
To investigate whether RPE CM had an effect on intracellular EGF or FGF2 signaling, we examined phosphorylation of cAMP response element binding protein (CREB) after mitogen challenge in the presence and absence of RPE CM (n=3 individual cultures). CREB is a transcription factor involved in cell proliferation that is activated in response to both EGF and FGF2 stimulation [51]. In cells dissociated from neurosphere cultures (≥ 5 passages) that had not been previously exposed to RPE CM (naïve group), re-addition of EGF and FGF2 resulted in a significant increase in the percentage of cells immunopositive for phosphorylated CREB (pCREB) compared to controls treated with vehicle alone (11.9 ± 3.9% vs. 0.3 ± 0.3%, p<0.03) (Figure 2C). The effect of RPE CM alone was greater than that of EGF and FGF2 (42.8 ± 5.1% vs. 11.9 ± 3.9%, p<0.003); however, the combined addition of RPE CM, EGF and FGF2 yielded the highest percentage of pCREB-positive cells (76.0 ± 3.3%, p<0.0015). In parallel cultures grown in RPE CM (RPE CM group), re-addition of either EGF and FGF2 or RPE CM alone did not significantly increase the percentage of pCREB-positive cells over vehicle-treated controls (7.8 ± 2.4% (EGF and FGF2) and 9.5 ± 2.4% (RPE CM) vs. 5.5 ± 1.9% (vehicle), p=0.48 and 0.23, respectively) (Figure 2C). By contrast, simultaneous re-addition of RPE CM, EGF and FGF2 to this group produced a significant increase in CREB phosphorylation compared to vehicle alone (27.4 ± 1.1%, p<0.0001). These results demonstrated that the effect of RPE CM on retinal neurosphere growth was mediated at least in part through mitogen-dependent signal transduction pathways.
Differentiation Potential of RPE CM-Treated Retinal Neurospheres
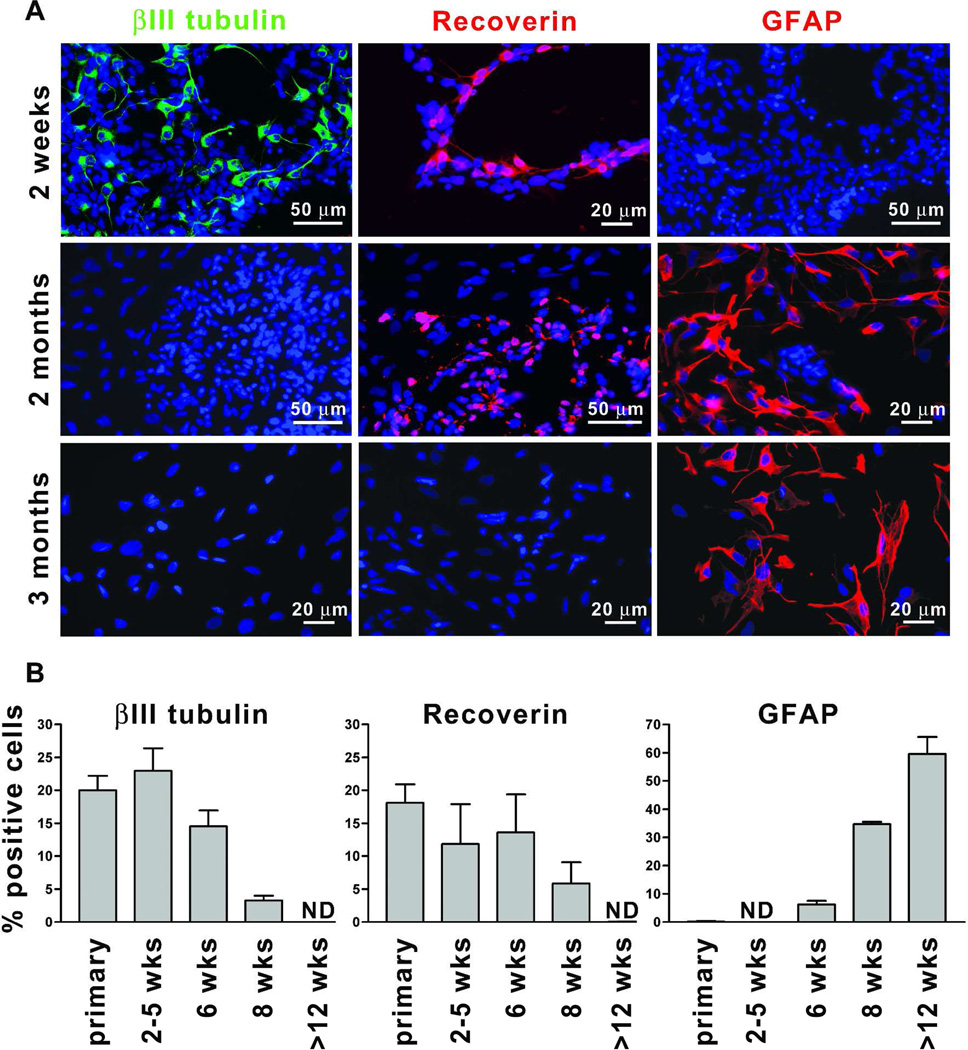
We previously reported that early-passage human prenatal retinal neurospheres lost their neurogenic potential after two months in culture under standard, serum-free conditions [35]. To determine the fate potential of retinal neurospheres grown in RPE CM, we quantified the expression of βIII tubulin (an early neuronal marker), recoverin (a marker for photoreceptors and rare cone bipolar cells) and glial fibrillary acidic protein (GFAP, a glial marker) in cells prompted to differentiate at various times in vitro (n≥ 3 individual cultures). At time points up to 6 weeks (3–5 passages), no significant difference was seen in the percentage of cells expressing βIII tubulin or recoverin when compared to primary cultures (Figure 3A and B). Broad, flat cells expressing GFAP were rarely observed in differentiated retinal neurosphere cultures until 6 weeks in vitro, but thereafter the percentage steadily increased. Under differentiating conditions, these GFAP-expressing cells were immunonegative for mature markers of Müller glia such as cellular retinaldehyde binding protein (CRALBP) and glutamine synthetase (data not shown). Over a similar time period, the percentage of βIII tubulin-positive and recoverin-positive cells declined to undetectable levels. No RPE CM-treated cultures tested (n=9) retained the capacity to produce neurons or photoreceptors beyond 3 months (≥ 9 passages) in culture. Thus, although RPE CM has the ability to maintain robust growth of human retinal neurospheres long term, it does not prevent the loss of neurogenic potential.
Figure 3.
Retinal neurospheres cultured in RPE CM lose neurogenic potential over time. (A) Retinal neurosphere cultures were expanded in RPE CM for 2 weeks (top panels), 2 months (middle panels) or 3 months (lower panels), dissociated, plated as monolayers and differentiated in the absence of mitogens and RPE CM for 7 days. Representative photomicrograph images are shown following immunolabeling with anti-βIII tubulin (green, left panels), anti-recoverin (red, middle panels) and anti-GFAP (red, right panels). Nuclei were visualized with Hoechst dye. (B) Quantification of anti-βIII tubulin, anti-recoverin and anti-GFAP immunolabeled cells from primary cultures or cultures grown for 2–5 wks (1–2 passages), 6 wks (3–4 passages), 8 wks (5–6 passages) and ≥12 wks (≥ 8 passages) prior to differentiation. Values are expressed as percentage ± SEM (n≥ 3). ND: not detectable.
Undifferentiated, RPE CM-Treated Retinal Neurospheres Express Markers and Genes Characteristic of Retinal and Neural Progenitor Cells
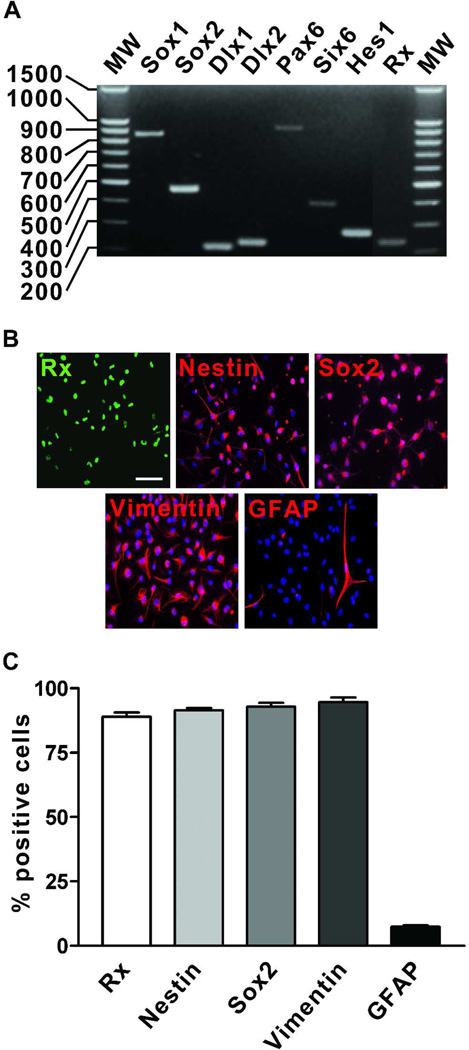
We next examined the gene and protein expression profile of undifferentiated human retinal neurosphere cultures maintained long term (> 3 months or ≥ 9 passages) in RPE CM, to determine whether they retained characteristics of progenitors despite their loss of neurogenic potential. In prior reports, undifferentiated neurospheres derived from mammalian embryonic and adult retina were shown to express nestin, a marker of neural precursor cells, along with various neurodevelopmental and eye specification genes indicative of retinal progenitor cells [1,13,37–39,46,52–54]. In all cultures tested (n=9), PCR detected the expression of Sox1, Sox2, Dlx1 and Dlx2, genes involved in neural and retinal development (Figure 4A) [39,54–58]. Furthermore, multiple homeodomain transcription factor genes expressed during neural and retinal development were present, including Pax6, Six6 and Rx [10,11,39,53–55,59–64]. In contrast to the range of homeodomain gene transcripts present in these cultures, the only basic helix-loop-helix (bHLH) transcription factor gene consistently detected was Hes1, which is involved in the maintenance of retinal progenitor proliferation and the production of Müller glia [11]. Basic HLH genes indicative of neurogenic retinal progenitors, such as Math5, NeuroD and Mash1, were not expressed at detectable levels in long term cultures (data not shown).
Figure 4.
Retinal neurospheres grown long term in RPE CM display characteristics of retinal and neural progenitors. (A) Representative RT-PCR analysis of retinal- and neural-specific gene expression in a retinal neurosphere culture maintained in RPE CM for 10 months (37 passages). (B) Photomicrograph images of a retinal neurosphere culture grown long term (25 passages) in RPE CM and immunolabeled with antibodies directed against the retinal progenitor cell marker Rx, the neural stem and progenitor cell markers nestin, Sox2 and vimentin, and the glial marker GFAP. (C) Quantification of immunocytochemistry results across ≥ 3 individual cultures. Values are expressed as percentage ± SEM. Scale bar = 50 µm.
Expression of selected proteins in undifferentiated retinal neurosphere cultures (n≥ 3) was quantified by immunocytochemistry. A large majority of cells were positive for the retinal progenitor cell marker Rx (88.9 ± 4.7%) and the neural and retinal progenitor markers nestin (91.5 ± 4.1%), Sox2 (92.9 ± 2.5%) and vimentin (94.6 ± 3.7%) (Figure 4B and C). These proteins have also been associated with the development of Müller glia and/or the maintenance of stem cell characteristics of human Müller glia cell lines [11,14,65]. However, mature Müller glia markers (CRALBP and glutamine synthetase) were not detected by immunocytochemistry (data not shown). GFAP expression, which is a feature of neural (but not retinal) progenitors [35,43], was only present in a small percentage of cells in undifferentiated neurosphere cultures (7.4 ± 2.7%) (Figure 4B and C). These GFAP-positive cells did not coexpress markers of undifferentiated retinal progenitor cells (data not shown), however, suggesting that they had undergone spontaneous differentiation. Together with their observed lack of neurogenic potential, these results suggest that human retinal neurospheres cultured for extended periods in RPE CM are composed of glial-restricted retinal progenitors.
Restoration of the Neurogenic Potential of Retinal Neurosphere Cultures by Misexpression of Mash1
In an attempt to re-establish the potential of long term retinal neurosphere cultures to produce neurons, we first tried varying the differentiation conditions. However, changing the cell plating density [66,67] or exposing differentiating cells to selected factors (e.g., FGF2 or NT4) [50,68–70] did not alter the fate potential (data not shown). Since manipulation of the cell environment was unsuccessful, we next sought to modify the intrinsic gene expression profile by misexpressing Mash1, a proneural bHLH transcription factor known to play an important role in the differentiation of multiple neuronal cell types within the brain and retina, including bipolar cells and photoreceptors [11,71–76].
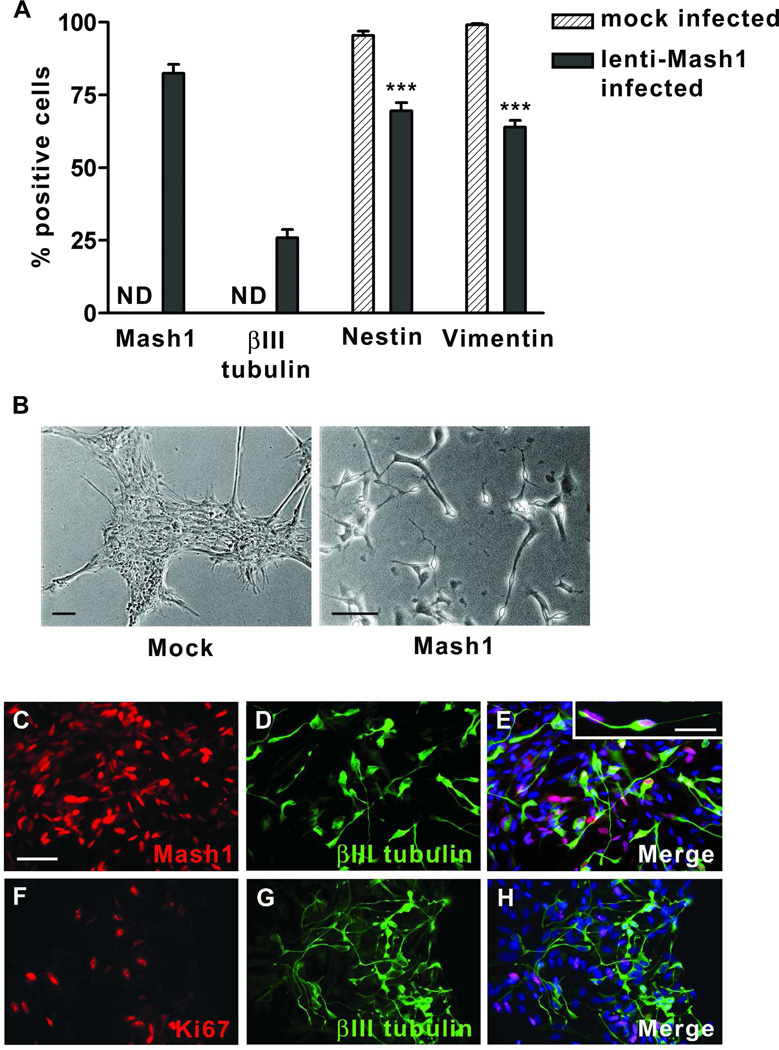
Misexpression of Mash1 in long term RPE CM-treated retinal neurosphere cultures (≥ 3 months, 8–26 passages) was achieved with high efficiency (82.5 ± 3.1%) using lentiviral vector delivery (Figure 5A) (n≥ 4) [44]. Following transduction, 25.9 ± 2.9% of cells expressed βIII tubulin (Figure 5A) and assumed the morphology of neurons with phase-bright cell bodies and long, thin processes (Figure 5B). Indeed, transduced cultures maintained for an extended period (12 days) in RPE CM contained occasional neurons with processes extending over 300 µm (Supplemental Figure S3). Mock-infected cultures (n≥ 4) failed to express βIII tubulin or display neuronal morphology (Figure 5A and B).
Figure 5.
Misexpression of Mash1 restores neurogenic potential to retinal neurosphere cultures grown long term (≥ 26 passages) in RPE CM. (A) Quantification of Mash1, βIII tubulin, nestin and vimentin immunolabeled cells in mock- and lenti-Mash1 infected cells after differentiation for 5 days. Values are expressed as percentage ± SEM; ***p<0.0001. ND: not detected. (B) Phase photomicrograph images of mock- (left panel) and lenti-Mash1 infected (right panel) cells after differentiation for 5 days; scale bars = 50 µm. (C–H) Photomicrographs of differentiated lenti-Mash1 infected cells after immunostaining with either anti-Mash1 counterstained with anti-βIII tubulin (C–E) or anti-Ki67 counterstained with anti-βIII tubulin (F–H). Nuclei were visualized with Hoechst dye; scale bars = 50 µm (C–H) or 20 µm (inset).
Immunostaining of transduced cultures also revealed that all βIII tubulin-positive cells were Mash1-positive and Ki67-negative, the latter indicating that these cells had exited the cell cycle (Figure 5C–H). Furthermore, the re-appearance of βIII tubulin-positive neurons was associated with a proportionate decrease in the percentage of cells expressing nestin and vimentin when compared to mock-infected cells (nestin: 69.6 ± 7.4% vs. 95.5 ± 3.2%; vimentin: 64.0 ± 5.1% vs. 99.1 ± 1.2%, respectively, p<0.0001) (Figure 5A). Consistent with this finding, no βIII tubulin-positive cells were found to coexpress nestin or vimentin (data not shown). Thus, Mash1 succeeded in re-directing a substantial portion of glial-restricted retinal progenitor cells from long term, RPE CM-treated retinal neurosphere cultures toward a neuronal fate.
Mash1-Infected Retinal Neurosphere Cultures Express Markers of Multiple Inner Retinal Cell Types
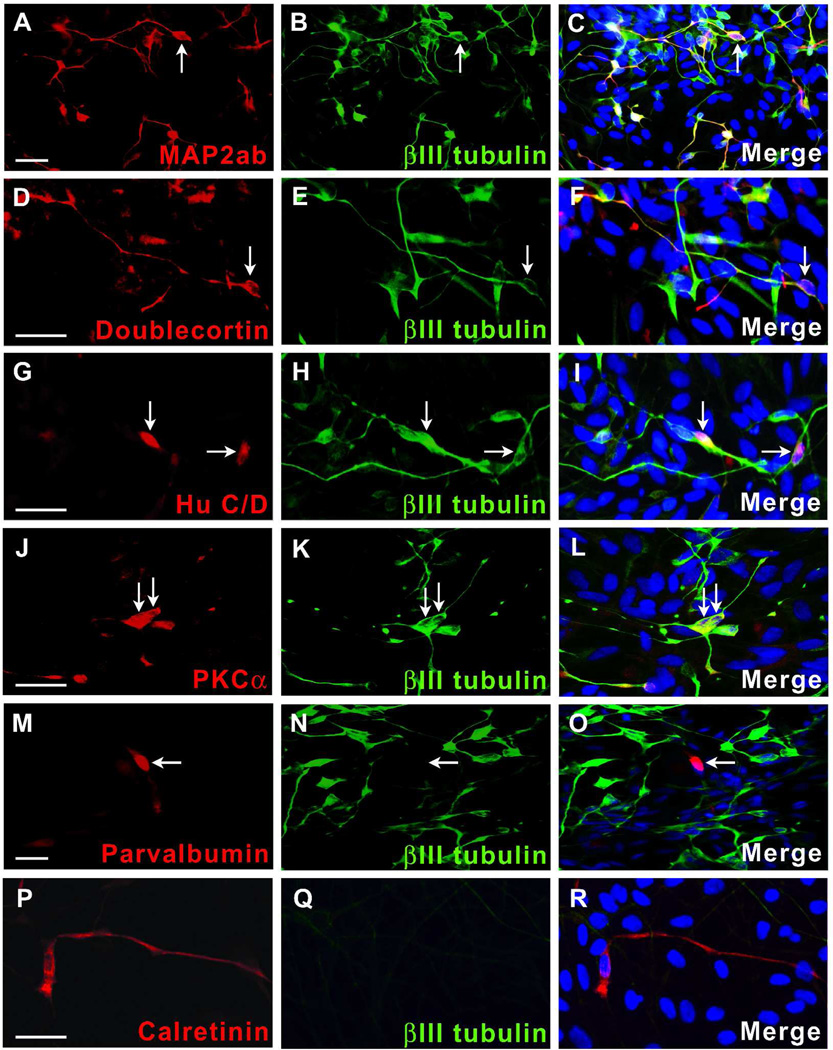
The retina consists of 6 major classes of neurons and one indigenous class of glia, all of which are derived from a common progenitor [6,19]. To better define the neurons being produced in our cultures following Mash1 misexpression, we performed immunocytochemistry using a panel of retinal cell type-selective antibodies. Subpopulations of βIII tubulin-positive neurons expressed MAP2ab (57.8 ± 4.1%), doublecortin (7.0 ± 1.2%) or HuC/D (56.8 ± 4.1%), markers known to be expressed in many types of neurons, including retinal ganglion and/or amacrine cells (Figure 6A–I and Table 1) [65,73,77–82]; however, staining for another ganglion cell marker, Brn3a [83], was not definitive in these cultures. An antibody directed against protein kinase Cα (PKCα), which labels bipolar cells in the retina [38,84–86], was found in a small percentage of differentiated neurons (6.0 ± 3.5%) (Figure 6J–L and Table 1). None of these markers was present in αIII tubulin-negative cells. Two additional markers of amacrine cells in the retina, parvalbumin and calretinin [38,84,87], were found in 1.6 ± 0.4% and 1.0 ± 0.1% of total cells, respectively, although these cells did not coexpress αIII tubulin (Figure 6M–R and Table 1). None of the preceding markers of retinal neurons was present in mock-infected or uninfected control cultures. Antibodies used to identify horizontal cells (anti-calbindin), photoreceptors (anti-recoverin) or glia (anti-GFAP) did not demonstrate immunoreactivity in lenti-Mash1 infected cells. These results suggest that misexpression of Mash1 in long term human retinal neurosphere cultures results in the generation of cells with characteristics of distinct classes of inner retinal neurons.
Figure 6.
Numerous inner retinal markers are expressed in lenti-Mash1 infected retinal neurosphere cultures. Photomicrographs of differentiated, lenti-Mash1 infected cells immunolabeled for anti-βIII-tubulin and either anti-MAP2ab (A–C) or anti-doublecortin (D–F) (to identify maturing and early migrating neurons, respectively), anti-HuC/D (G–I), anti-PKCα, (J–L), anti-parvalbumin (M–O) or anti-calretinin (P–R). Nuclei were visualized with Hoechst dye. White arrows designate representative labeled cells. Scale bars = 20 µm.
Table 1.
Quantification of cell-selective immunomarkers in human retinal progenitors following Mash1 missexpression.
| Antibody | % Total cells | % βIII tubulin-labeled cells |
|---|---|---|
| βIII tubulin | 25.8 ± 2.9 | (100) |
| MAP2ab | 14.2 ± 1.9 | 57.8 ± 4.1 |
| Doublecortin | 1.3 ± 0.2 | 7.0 ± 1.2 |
| HuC/D | 9.2 ± 1.4 | 56.8 ± 4.1 |
| PKCα | 0.7 ± 0.3 | 6.0 ± 3.5 |
| Parvalbumin | 1.6 ± 0.4 | ND |
| Calretinin | 1.0 ± 0.1 | ND |
Human retinal progenitor cells were infected with lenti-Mash1, differentiated for 5 days, and immunostained to identify neuronal cell subtypes. Immunopositive cells were quantified either as the percentage of total cells or, if co-labeled with βIII tubulin, as the percentage of βIII tubulin-positive cells. Values are expressed as mean percentage ± SEM. ND: not detected.
Discussion
In this study, we sought to overcome the growth limitations of human prenatal retinal neurosphere cultures by modifying a method originally developed to expand human prenatal cortical neurospheres [35,42]. Previously described techniques for culturing human prenatal retinal cells maintained them as dissociated monolayers for at least seven [88] or eight [38] passages, but growth declined at later passages depending on culture conditions. Using our standard medium, which included EGF and FGF2, both dissociated and neurosphere cultures of human prenatal retinal cells failed to demonstrate significant expansion.
Given the importance of RPE during early neuroretinal development [5,20–24] and its pro-proliferative effects on rodent and chick RPC cultures [5,27,30,31], we hypothesized that RPE CM would improve prenatal human retinal neurosphere growth as well. However, the degree to which RPE CM could selectively halt retinal neurosphere volume loss and restore a robust growth pattern was surprising. This effect may be mediated by multiple soluble factors, some of which are likely not exclusive to RPE, as shown by the lesser growth-promoting abilities of cortical neurosphere and lens CM. It is also important to note that RPE CM could not improve growth of neurospheres derived from other human embryonic CNS tissues, and in fact had a detrimental effect on cortical neurosphere growth. These regional differences in response to RPE CM are being further investigated.
A recent examination of the growth factors and other proteinaceous components secreted by RPE cultures revealed a number of molecules with pro-proliferative activity in the developing brain and retina, including vascular endothelial growth factor and cystatin C [33]. In addition, RPE CM contains high levels of pigment epithelium-derived factor [33], a molecule that promotes cell survival in the retina [89,90] and neural stem cell self-renewal in the subventricular zone of the brain [91]. Inorganic compounds present in RPE CM may also play a role in the expansion of retinal neurosphere cultures, since CM protein fractions restricted to elements less than 3-kDa independently sustained modest neurosphere growth. One such candidate compound is ATP, which has recently been shown to support mouse RPC growth [20]. Efforts are ongoing to identify the RPE CM factors involved in the observed retinal neurosphere growth enhancement.
While the secreted RPE factor(s) responsible for retinal neurosphere growth remain to be delineated, present evidence does suggest a mechanism for the effect. Our results show that RPE CM confers mitogen responsiveness to retinal neurospheres, a property that neurospheres from other CNS regions innately possess [41,42,50]. The rapid phosphorylation of CREB following CM challenge suggests that RPE CM likely acts in part by potentiating existing cellular EGF and FGF2 signaling pathways and/or relieving potential inhibitory influence(s) present in self-conditioned retinal neurosphere medium.
Although RPE CM is effective in promoting the long term expansion of retinal neurosphere cultures, it does not prevent the progressive cell fate restriction observed in its absence [35]. Specifically, in both RPE CM-treated and untreated cultures, retinal neurospheres invariably lost their neurogenic potential after 2–3 months in vitro and became increasingly gliogenic. This pattern of cell fate determination is reminiscent of normal mammalian cortical and retinal development, during which progenitor cells initially give rise to long projection neurons, followed by interneurons and/or photoreceptors, and finally glia [6,10–13,19,38,43,80,92]. In the developing retina, Müller cells are the sole type of glia derived from RPCs [6,10] and have been shown to retain progenitor characteristics after differentiation [65,82,93,94]. However, the absence of mature markers suggests that our long term cultures do not consist of fully differentiated Müller glia. Other GFAP-positive glia found in the retina originate from the optic nerve, but these astrocytes are unlikely to be present in our cultures given the gestational ages used [38,95,96], the method of dissection [35], and the gene and protein expression profile of the cells [10,35,38,56,68,95,96].
An examination of the transcription factor profile of undifferentiated, long term cultures of retinal neurospheres revealed a potential cause for their loss of neurogenic potential. Pro-neural, bHLH transcription factors such as Mash1 and NeuroD were absent by PCR, whereas transcription factors favoring Müller glia production (Hes1 and Rx) were present [6,11,19,72,97], along with a host of genes and proteins expressed by neural and retinal progenitors. The expression of Hes1 in all of our cultures was particularly intriguing, since it also plays a prominent role in the maintenance of a proliferating pool of retinal progenitors [6,11,59,62,98,99].
Taken together, our findings suggest that the fate potential of long term retinal neurosphere cultures is governed in large part by intrinsic limitations rather than extrinsic influences. Consistent with this hypothesis, culture modifications that enhanced neurogenesis in rodent RPCs failed to alter cell fate potential in late passage human retinal neurospheres [66,68,69]. Therefore, we misexpressed the pro-neural bHLH transcription factor Mash1 in an attempt to overcome Hes signaling and push the hRPCs toward a neuronal fate. Mash1 was chosen because of its demonstrated efficacy in rat [71] and human (unpublished observations) embryonic cortical neurosphere cultures and its role in neuron and photoreceptor production in the retina [11,72,75,76,100]. Although lenti-Mash1 infected hRPCs were immunonegative for photoreceptor-specific proteins, they did express a number of markers indicative of inner retinal neurons, including ganglion cells, amacrine cells and bipolar cells. However, the majority of lenti-Mash1 infected cells remained nestin-positive and failed to demonstrate characteristics of either neurons or glia. A possible explanation is that low levels of Mash1 are sufficient to halt proliferation and thwart default gliogenesis in our hRPC population, but higher levels are needed to restore neurogenesis [71]. Further manipulation of the transgene expression levels may improve the yield of neurons from these cultures.
Conclusion
We have shown that extrinsic factors secreted by human RPE can profoundly influence proliferation of hRPCs, but that the profile of intrinsic factors expressed within these hRPCs restricts their competency to produce neuronal cell types. Future efforts will examine the effect of misexpression of combinations of pro-neural and retina-specific transcription factors on the cell fate potential of these cultures. Using this approach, it may be possible to produce significant populations of individual human retinal cell types for in vitro and in vivo study. This in turn could lead to further insight into the molecular events underlying human retinogenesis and the development of cell replacement therapies for retinal degenerative diseases.
Supplementary Material
Acknowledgements
DMG laboratory was supported by grants from the National Eye Institute (K08EY015138), Foundation Fighting Blindness (Walsh Retinal Stem Cell Consortium), Heckrodt Foundation, Lincy Foundation and the Retina Research Foundation. DMG is a recipient of a Research to Prevent Blindness Robert E. McCormick Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NEI or NIH.
Abbreviations
- bHLH
basic helix-loop-helix transcription factor
- CM
conditioned medium
- CREB
cAMP response element binding protein
- EGF
epidermal growth factor
- EGFR
EGF receptor
- FGF2
fibroblast growth factor 2
- FGFR1
FGF2 receptor
- GFAP
glial fibrillary acidic protein
- hNPCctx
human cortical neural progenitor cell
- hNPCsc
human spinal cord progenitor cell
- PEDF
pigment epithelium-derived factor
- PKC
protein kinase C
- RPE
retinal pigment epithelium
- RPC
retinal progenitor cell
- VEGF
vascular endothelial growth factor
Footnotes
Author Contributions:
DMG: Conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
LSW: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
EC: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
RLS: Collection and assembly of data, data analysis
JSM: Collection and assembly of data, data analysis
HJK: Collection of data, manuscript writing, final approval of manuscript
BLS: Collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript
JNM: Collection of data, data analysis, final approval of manuscript
CNS: Conception and design, financial support, data interpretation, final approval of manuscript
References
- 1.Sheedlo HJ, Heath A, Brun AM, et al. Microscopic characterization of rat retinal progenitor cells. Brain Res. 2007;1185:59–67. doi: 10.1016/j.brainres.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 2.Braekevelt CR, Hollenberg MJ. Comparative electron microscopic study of development of hyaloid and retinal capillaries in albino rats. Am J Ophthalmol. 1970;69:1032–1046. doi: 10.1016/0002-9394(70)91053-6. [DOI] [PubMed] [Google Scholar]
- 3.Barishak YB. Embryology of the Eye and its Adnexa. Basel, Switzerland: Karger; 2001. pp. 7–15. [Google Scholar]
- 4.Bharti K, Nguyen MT, Skuntz S, et al. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006;19:380–394. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheedlo HJ, Turner JE. Influence of a retinal pigment epithelial cell factor(s) on rat retinal progenitor cells. Dev Brain Res. 1996;93:88–99. doi: 10.1016/0165-3806(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 6.Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends Neurosci. 2006;29:563–570. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Rapaport DH, Wong LL, Wood ED, et al. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- 8.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 9.Donovan SL, Dyer MA. Regulation of proliferation during central nervous system development. Semin Cell Dev Biol. 2005;16:407–421. doi: 10.1016/j.semcdb.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 11.Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol. 2004;15:83–99. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Reh TA, Fischer AJ. Retinal stem cells. Methods Enzymol. 2006;419:52–73. doi: 10.1016/S0076-6879(06)19003-5. [DOI] [PubMed] [Google Scholar]
- 13.James J, Das AV, Rahnenführer J, et al. Cellular and molecular characterization of early and late retinal stem cells/progenitors: differential regulation of proliferation and context dependent role of Notch signaling. J Neurobiol. 2004;61:359–376. doi: 10.1002/neu.20064. [DOI] [PubMed] [Google Scholar]
- 14.Walcott JC, Provis JM. Müller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Experiment Ophthalmol. 2003;31:246–249. doi: 10.1046/j.1442-9071.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen AM, Raff MC. Continuous observation of multipotential retinal progenitor cells in clonal density culture. Dev Biol. 1997;188:267–279. doi: 10.1006/dbio.1997.8645. [DOI] [PubMed] [Google Scholar]
- 16.Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci U S A. 2006;103:18998–19003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cepko CL, Austin CP, Yang X, et al. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 19.Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- 20.Pearson RA, Dale N, Llaudet E, et al. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- 22.Ilia M, Jeffery G. Retinal cell addition and rod production depend on early stages of ocular melanin synthesis. J Comp Neurol. 2000;420:437–444. [PubMed] [Google Scholar]
- 23.Jeffery G. The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci. 1997;20:165–169. doi: 10.1016/s0166-2236(96)10080-1. [DOI] [PubMed] [Google Scholar]
- 24.Jensen AM, Walker C, Westerfield M. Mosaic eyes: a zebrafish gene required in pigmented epithelium for apical localization of retinal cell division and lamination. Development. 2001;128:95–105. doi: 10.1242/dev.128.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Sheedlo HJ, Turner JE. Effects of retinal pigment epithelial cell-secreted factors on neonatal rat retinal explant progenitor cells. J Neurosci Res. 1996;44:519–531. doi: 10.1002/(SICI)1097-4547(19960615)44:6<519::AID-JNR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Sheedlo HJ, Nelson TH, Lin N, et al. RPE secreted proteins and antibody influence photoreceptor cell survival and maturation. Dev Brain Res. 1998;107:57–69. doi: 10.1016/s0165-3806(97)00219-8. [DOI] [PubMed] [Google Scholar]
- 27.Sheedlo HJ, Brun-Zinkernagel AM, Oakford LX, et al. Rat retinal progenitor cells and a retinal pigment epithelial factor. Dev Brain Res. 2001;127:185–187. doi: 10.1016/s0165-3806(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 28.Sheedlo HJ, Bartosh TJ, Wang Z, et al. RPE-derived factors modulate photoreceptor differentiation: a possible role in the retinal stem cell niche. In Vitro Cell Dev Biol Anim. 2007;43:361–370. doi: 10.1007/s11626-007-9051-3. [DOI] [PubMed] [Google Scholar]
- 29.Gaur VP, Liu Y, Turner JE. RPE conditioned medium stimulates photoreceptor cell survival, neurite outgrowth and differentiation in vitro. Exp Eye Res. 1992;54:645–659. doi: 10.1016/0014-4835(92)90020-s. [DOI] [PubMed] [Google Scholar]
- 30.Layer PG, Rothermel A, Willbold E. From stem cells towards neural layers: a lesson from re-aggregated embryonic retinal cells. Neuroreport. 2001;12:A39–A46. doi: 10.1097/00001756-200105250-00001. [DOI] [PubMed] [Google Scholar]
- 31.Layer PG, Rothermel A, Hering H, et al. Pigmented epithelium sustains cell proliferation and decreases expression of opsins and acetylcholinesterase in reaggregated chicken retinospheroids. Eur J Neurosci. 1997;9:1795–1803. doi: 10.1111/j.1460-9568.1997.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer G, Layer PG. An in vitro model of proliferation and differentiation of the chick retina: coaggregates of retinal and pigment epithelial cells. J Neurosci. 1986;6:1885–1896. doi: 10.1523/JNEUROSCI.06-07-01885.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamm DM, Melvan JN, Shearer RL, et al. A novel serum-free method for culturing human prenatal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:788–799. doi: 10.1167/iovs.07-0777. [DOI] [PubMed] [Google Scholar]
- 34.Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamm DM, Nelson AD, Svendsen CN. Human retinal progenitor cells grown as neurospheres demonstrate time-dependent changes in neuronal and glial cell fate potential. Ann N Y Acad Sci. 2005;1049:107–117. doi: 10.1196/annals.1334.011. [DOI] [PubMed] [Google Scholar]
- 36.Mayer EJ, Carter DA, Ren Y, et al. Neural progenitor cells from postmortem adult human retina. Br J Ophthalmol. 2005;89:102–106. doi: 10.1136/bjo.2004.057687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu G, Seiler MJ, Arai S, et al. Alternative culture conditions for isolation and expansion of retinal progenitor cells. Curr Eye Res. 2004;28:327–336. doi: 10.1076/ceyr.28.5.327.28679. [DOI] [PubMed] [Google Scholar]
- 38.Yang P, Seiler MJ, Aramant RB, et al. In vitro isolation and expansion of human retinal progenitor cells. Exp Neurol. 2002;177:326–331. doi: 10.1006/exnr.2002.7955. [DOI] [PubMed] [Google Scholar]
- 39.Klassen H, Ziaeian B, Kirov II, et al. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J Neurosci Res. 2004;77:334–343. doi: 10.1002/jnr.20183. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 41.Wachs FP, Couillard-Despres S, Engelhardt M, et al. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest. 2003;83:949–962. doi: 10.1097/01.lab.0000075556.74231.a5. [DOI] [PubMed] [Google Scholar]
- 42.Svendsen CN, ter Borg MG, Armstrong RJ, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 43.Wright LS, Prowse KR, Wallace K, et al. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006;312:2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Déglon N, Tseng JL, Bensadoun JC, et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Hum Gene Ther. 2000;11:179–190. doi: 10.1089/10430340050016256. [DOI] [PubMed] [Google Scholar]
- 45.Nagineni CN, Bhat SP. Lens fiber cell differentiation and expression of crystallins in co-cultures of human fetal lens epithelial cells and fibroblasts. Exp Eye Res. 1992;54:193–200. doi: 10.1016/s0014-4835(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 46.Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48:1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- 47.Jablonski MM, Tombran-Tink J, Mrazek DA, et al. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao S, Chen Q, Hung FC, et al. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- 49.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caldwell MA, He X, Wilkie N, et al. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19:475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- 51.Ciccolini F, Svendsen CN. Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: identification of neural precursors responding to both EGF and FGF-2. J Neurosci. 1998;18:7869–7880. doi: 10.1523/JNEUROSCI.18-19-07869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- 53.Zilinski C, Brownell I, Hashimoto R, et al. Expression of FoxE4 and Rx visualizes the timing and dynamics of critical processes taking place during initial stages of vertebrate eye development. Dev Neurosci. 2004;26:294–307. doi: 10.1159/000082271. [DOI] [PubMed] [Google Scholar]
- 54.Klassen HJ, Ng TF, Kurimoto Y, et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- 55.Le Rouedec RD, Rayner K, Rex M, et al. The transcription factor cSox2 and Neuropeptide Y define a novel subgroup of amacrine cells in the retina. J Anat. 2002;200:51–56. doi: 10.1046/j.0021-8782.2001.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taranova OV, Magness ST, Fagan BM, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Melo J, Qiu X, Du G, et al. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- 58.de Melo J, Du G, Fonseca M, et al. Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development. 2005;132:311–322. doi: 10.1242/dev.01560. [DOI] [PubMed] [Google Scholar]
- 59.Furukawa T, Kozak CA, Cepko CL. Rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U S A. 1997;94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engelhardt M, Wachs FP, Couillard-Despres S, et al. The neurogenic competence of progenitors from the postnatal rat retina in vitro. Exp Eye Res. 2004;78(5):1025–1036. doi: 10.1016/j.exer.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Akimoto M. Transcriptional factors involved in photoreceptor differentiation. Semin Ophthalmol. 2005;20:25–30. doi: 10.1080/08820530590921864. [DOI] [PubMed] [Google Scholar]
- 62.Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Retin Eye Res. 2003;22:567–577. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28:135–142. [PubMed] [Google Scholar]
- 64.López-Ríos J, Tessmar K, Loosli F, et al. Six3 and Six6 activity is modulated by members of the groucho family. Development. 2003;130:185–195. doi: 10.1242/dev.00185. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 66.Kholodenko IV, Buzdin AA, Kholodenko RV, et al. Mouse retinal progenitor cell (RPC) cocultivation with retinal pigment epithelial cell culture affects features of RPC differentiation. Biochemistry (Mosc) 2006;71:767–774. doi: 10.1134/s0006297906070091. [DOI] [PubMed] [Google Scholar]
- 67.Ezeonu I, Wang M, Kumar R, et al. Density-dependent differentiation in nontransformed human retinal progenitor cells in response to basic fibroblast growth factor- and transforming growth factor-alpha. DNA Cell Biol. 2003;22:607–620. doi: 10.1089/104454903770238085. [DOI] [PubMed] [Google Scholar]
- 68.Merhi-Soussi F, Angénieux B, Canola K, et al. High yield of cells committed to the photoreceptor fate from expanded mouse retinal stem cells. Stem Cells. 2006;24:2060–2070. doi: 10.1634/stemcells.2005-0311. [DOI] [PubMed] [Google Scholar]
- 69.Qiu G, Seiler MJ, Mui C, et al. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res. 2005;80:515–525. doi: 10.1016/j.exer.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Wu P, Tarasenko YI, Gu Y, et al. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- 71.Kim HJ, Sugimori M, Nakafuku M, et al. Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol. 2007;203:394–405. doi: 10.1016/j.expneurol.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 72.Tomita K, Nakanishi S, Guillemot F, et al. Mash1 promotes neuronal differentiation in the retina. Genes Cells. 1996;1:765–774. doi: 10.1111/j.1365-2443.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 73.Philips GT, Stair CN, Young Lee H, et al. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev Biol. 2005;279:308–321. doi: 10.1016/j.ydbio.2004.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berninger B, Costa MR, Koch U, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai L, Morrow EM, Cepko CL. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development. 2000;127:3021–3030. doi: 10.1242/dev.127.14.3021. [DOI] [PubMed] [Google Scholar]
- 76.Fadool JM. Understanding retinal cell fate determination through genetic manipulations. Prog Brain Res. 2001;131:541–554. doi: 10.1016/s0079-6123(01)31042-7. [DOI] [PubMed] [Google Scholar]
- 77.Lee EJ, Kim IB, Lee E, et al. Differential expression and cellular localization of doublecortin in the developing rat retina. Eur J Neurosci. 2003;17:1542–1548. doi: 10.1046/j.1460-9568.2003.02583.x. [DOI] [PubMed] [Google Scholar]
- 78.Ekström P, Johansson K. Differentiation of ganglion cells and amacrine cells in the rat retina: correlation with expression of HuC/D and GAP-43 proteins. Dev Brain Res. 2003;145:1–8. doi: 10.1016/s0165-3806(03)00170-6. [DOI] [PubMed] [Google Scholar]
- 79.Farah MH. Neurogenesis and cell death in the ganglion cell layer of vertebrate retina. Brain Res Rev. 2006;52:264–274. doi: 10.1016/j.brainresrev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Engelhardt M, Wachs FP, Couillard-Despres S, et al. The neurogenic competence of progenitors from the postnatal rat retina in vitro. Exp Eye Res. 2004;78:1025–1036. doi: 10.1016/j.exer.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- 82.Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 83.Liu W, Khare SL, Liang X, et al. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127:3237–3247. doi: 10.1242/dev.127.15.3237. [DOI] [PubMed] [Google Scholar]
- 84.Engelsberg K, Ehinger B, Ghosh F. Early development of retinal subtypes in long-term cultures of human embryonic retina. Curr Eye Res. 2008;33:185–191. doi: 10.1080/02713680701843784. [DOI] [PubMed] [Google Scholar]
- 85.Zahir T, Klassen H, Young MJ. Effects of ciliary neurotrophic factor on differentiation of late retinal progenitor cells. Stem Cells. 2005;23:424–432. doi: 10.1634/stemcells.2004-0199. [DOI] [PubMed] [Google Scholar]
- 86.Haverkamp S, Haeseleer F, Hendrickson A. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Vis Neurosci. 2003;20:589–600. doi: 10.1017/s0952523803206015. [DOI] [PubMed] [Google Scholar]
- 87.Hendrickson A, Yan YH, Erickson A, et al. Expression patterns of calretinin, calbindin and parvalbumin and their colocalization in neurons during development of Macaca monkey retina. Exp Eye Res. 2007;85:587–601. doi: 10.1016/j.exer.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 88.Kelley MW, Turner JK, Reh TA. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Invest Ophthalmol Vis Sci. 1995;36:1280–1289. [PubMed] [Google Scholar]
- 89.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 91.Ramírez-Castillejo C, Sánchez-Sánchez F, Andreu-Agulló C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 92.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 93.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 94.Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Müller glia in the 302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 95.Zahir T, Klassen H, Tomita M, et al. Sorbitol causes preferential selection of Muller glial precursors from late retinal progenitor cells in vitro. Mol Vis. 2006;12:1606–1614. [PubMed] [Google Scholar]
- 96.Seiler MJ, Aramant RB. Photoreceptor and glial markers in human embryonic retina and in human embryonic retinal transplants to rat retina. Dev Brain Res. 1994;80:81–95. doi: 10.1016/0165-3806(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 97.Furukawa T, Mukherjee S, Bao ZZ, et al. Rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 98.Lee HY, Wroblewski E, Philips GT, et al. Multiple requirements for Hes 1 during early eye formation. Dev Biol. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kageyama R, Ohtsuka T, Hatakeyama J, et al. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. Epub 2005. [DOI] [PubMed] [Google Scholar]
- 100.Ishibashi M, Ang SL, Shiota K, et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.