Summary
In the absence of EBF1, B cell development is arrested at an uncommitted progenitor stage that exhibits increased lineage potentials. Previously, we investigated the roles of EBF1 (E) and its DNA binding partner Runx1 (R) by evaluating B lymphopoiesis in single (Ehet and Rhet) and compound haploinsufficent (ERhet) mice. Here, we demonstrate that reduced Ebf1 gene dosage results in the inappropriate expression of NK cell lineage-specific genes in B cell progenitors. Moreover, prolonged expression of Ly6a/Sca-1 suggested the maintenance of a relatively undifferentiated phenotype. These effects were exacerbated by reduced expression of Runx1 and occurred despite expression of Pax5. Repression of inappropriately expressed genes was restored in most pre-B and all immature B cells of ERhet mice. Enforced EBF1 expression repressed promiscuous transcription in pro-B cells of ERhet mice and in Ebf1−/−Pax5−/− fetal liver cells. Together, our studies suggest that normal levels of EBF1 are critical for maintaining B cell identity by directing repression of non-B cell-specific genes.
Introduction
Lineage specification is the process by which cells acquire fates that will be retained in their descendant cells [1]. As one of the most important drivers of B lineage specification, EBF1 (EBF/O/E-1/COE1) is essential for B lineage specification and development [2, 3]. EBF1 can direct cells towards the B cell fate at the expense of other cell lineages and promotes B cell development in the absence of upstream regulators, including IL-7 and the transcription factors PU.1, Ikaros or E2A [4-10]. Recent evidence suggests that EBF1 controls B lymphopoiesis in at least two ways by: 1) activating genes that encode essential components of the B lineage-specific program and 2) reinforcing B lineage commitment (together with Pax5) by repressing genes of other hematopoietic programs [10-13].
Previously, we demonstrated the synergistic activation of early B cell-specific genes by EBF1 and its regulatory partner Runx1 [14]. To determine the importance of interactions between these two factors in vivo, we analyzed the phenotypes of Ebf1 (Ehet), Runx1 (Rhet), or compound (ERhet) haploinsufficient mice. B cell numbers were reduced significantly in bone marrow and spleens and the progression of B cell development was impeded in Ehet and ERhet mice [15]. Here, we demonstrate that a significant fraction of Ehet and ERhet pro-B (and some pre-B) cells express NK cell-associated genes, including Cd244 (CD244/2B4), Cd160 (CD160/BY55) and Klrb1c (NK1.1). Promiscuous expression of NK cell-specific genes was observed in the presence of Pax5; therefore, Pax5 expression alone is insufficient for the maintenance of B cell identity in the presence of reduced EBF1. Repression of NK cell-specific transcripts was restored by EBF1 in ERhet pro-B cells and Ebf1−/−Pax5−/− fetal liver cells. Together, these data confirm the importance of EBF1 in B cell commitment.
Results and Discussion
Promiscuous expression of NK cell-specific genes in Ehet and ERhet mice
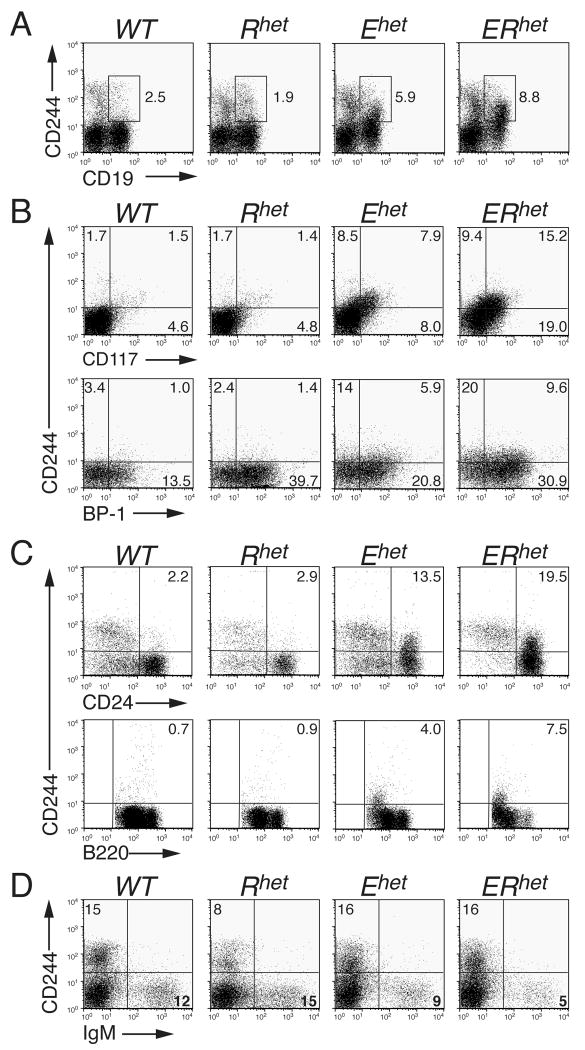
To characterize differences between gene expression patterns of B cell progenitors from WT and ERhet mice we utilized Affymetrix DNA microarray analysis with B220+CD2+mIgM− pre-B cells as a source of mRNA. The results indicated that multiple genes, including many that are normally expressed in NK cells, were expressed in the cells from ERhet mice (Supplemental Table 1); however, the data may have been influenced by co-purified B220+CD2+ NK cell progenitors [16]. Therefore, we assessed bone marrow cells for the co-expression of NK cell-specific surface proteins and CD19, the B cell ‘commitment marker’ [17]. CD244 (2B4) is expressed on some multipotent progenitors and on mature and progenitor NK cells [18, 19]. Flow cytometry of bone marrow-derived cells of WT and Rhet mice detected distinct CD19+ B cell and CD244+ NK cell populations as well as infrequent, diffuse CD19+CD244+ double-positive cells (Fig. 1A). In contrast, significant, well-defined populations of CD19+CD244+ cells were detected in Ehet (5.9%) and ERhet (8.8%) mice, indicating the mixing of lineage markers.
FIGURE 1.
Loss of B cell identity in haploinsufficient mice. (A) Detection of the NK cell marker CD244 (2B4) and the B cell commitment marker CD19 together on haploinsufficent bone marrow cells. (B) Detection of CD244 on various stages of developing B cells in the bone marrow. Cells were pre-gated on CD19+ cells. (C) Expression of CD244 on B220+CD43+-gated CD24+ bone marrow cells (upper panel); expression of CD244 on B220+CD43−-gated B220lo bone marrow cells (lower panel). (D) Lack of CD244 expression on CD19+-gated mIgM+ cells from the bone marrow. All data are representative of greater than four independent experiments.
We examined the extent of co-expression of CD19 and CD244 together with the stage-specific markers of B cell development CD117 and BP-1. As expected [15], percentages of CD19-gated CD117+ pro-B cells were increased in Ehet and ERhet mice relative to WT and Rhet mice (Fig. 1B, upper panel). Moreover, CD19-gated CD117+CD244+ cells increased from 1.5% to 15.2% in WT versus ERhet bone marrow. BP-1 is a marker of late pro-B cells of normal mice [20]. CD19-gated CD244+BP-1+ cells were nearly undetectable (1.0%) in WT mice, but they were increased substantially in Ehet (5.9%) and ERhet (9.6%) mice (Fig. 1B, lower panel). The presence of CD24 on B220+CD43+ bone marrow-derived cells is indicative of pro-B cells (Fr. B-C′) as well [20]. The expression of CD244 on B220+CD43+-gated cells increased from 2.2% in WT mice to 13.5% and 19.5% in Ehet and ERhet mice, respectively (Fig. 1C, upper panel). CD244 expression on B220+CD43−-gated B220lo pre-B cells (Fr. D) from WT and Rhet mice was minimal and was not detected as a defined population (Fig. 1C, lower panel). In contrast, CD43−-gated CD244+B220lo bone marrow populations resolved well in Ehet and ERhet mice (4.0% and 7.5%, respectively), but expression of CD244 was less than that observed at the pro-B cell stage. In the bone marrow, CD25 is a marker of pre-B cells [21]. We demonstrated previously that CD19-gated CD25+ pre-B cells were reduced greatly in Ehet and ERhet bone marrow [15]. CD244 was not detected on CD19-gated CD25+ cells in the mutant mice (Supporting Information Fig. 1). CD244 was virtually absent on B220+CD43−-gated B220med (Fr. E, immature B cells) and B220hi (Fr. F, mature B cells) cells from mice of all four genotypes (Fig.1C, lower panel). Moreover, CD244 was not detected on live-gated mIgM+ bone marrow cells (Fig.1D) or mIgM+ splenocytes (Supporting Information Fig. 2A) of any of the mice. Similar results for Fr. B-F cells were obtained when the expression of NK1.1 was evaluated (Supporting Information Fig. 3). NK1.1+CD244+ NK cells were present at normal percentages in spleens of mice of each genotype (Supporting Information Fig. 2B). Taken together, CD244 expression is detected on early progenitors of Ehet and ERhet mice up to and including some Fr. D pre-B cells, but CD244 is not detected on most Fr. D cells, CD25+ pre-B cells or at later stages of B cell development.
Quantitation of NK cell-specific gene expression in developing B cells
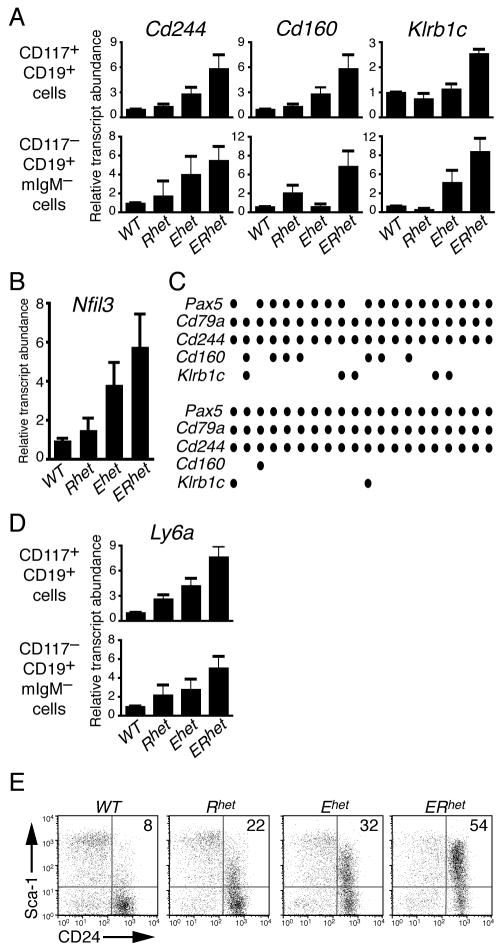
CD244 is expressed on a subset of transiently reconstituting, multipotent hematopoietic progenitors, as well as NK cells [19]. Therefore, we evaluated whether genes activated at later stages of the NK cell program were expressed in B cell progenitors from the haploinsufficient mice. Quantities of specific transcripts in CD117+CD19+ (pro-B) and CD117−CD19+mIg− (late pro-B/pre-B) cells were analyzed using qRT-PCR. ERhet pro-B cells (Fig. 2A, upper panel) expressed increased Cd244 transcripts relative to WT cells (5.2-fold; p=0.019). Interestingly, we detected similarly increased transcripts of the NK-specific Cd160 gene (encoding CD160) when comparing WT and ERhet pro-B cells (5.9-fold; p=0.018). Transcripts of Klrb1c (encoding NK1.1) were increased 2.6-fold in pro-B cells from ERhet bone marrow (p<0.0001). Significantly elevated Cd244, Cd160, and Klrb1c transcripts were detected in late pro-B/pre-B cells of ERhet versus WT mice (Fig. 2A, lower panel). Klrb1c transcripts were increased in Ehet (4.7-fold; p=0.099) and ERhet (9.6-fold; p=0.0063) cells relative to those of WT (and Rhet) mice. The increases in these transcripts are largely due to BP-1+ B cell progenitors, which are present in similar numbers in mice with the four genotypes [15].
FIGURE 2.
Quantitation of NK cell-, B cell- and progenitor-specific transcripts in bone marrow-derived B cells of haploinsufficient mice. All transcript levels were normalized to Hprt transcripts (mean±SEM). qT-PCR data are representative of three or more independent experiments and three to six mice per genotype. (A) qRT-PCR analysis of NK cell-specific transcripts in pro-B (upper panel) and late pro-B/pre-B cells (lower panel). (B) qRT-PCR analysis of Nfil3 transcripts in CD117+CD19+ bone marrow cells. (C) Single-cell multiplex amplification of cDNA derived from individual CD117+CD19+CD244+ bone marrow cells. Circles indicate positive signals for each RT-PCR probe set. (D) qRT-PCR analysis of Ly6a transcripts in bone marrow cell populations. (E) Expression of Sca-1 on B220+CD43+–gated CD24+ bone marrow cells. The data are representative of two independent experiments.
Promiscuous expression of NK cell-specific genes in Ehet and ERhet mice suggests that transcription factors required to activate their expression may be up-regulated in the haploinsuficient B cell progenitors. To address this question, we performed qRT-PCR to test whether transcripts of the Id2 and Nfil3 (E4BP4) genes, which have been linked with the generation of mature NK cells in vivo [22, 23], are increased in the mutant B cells. Significant changes in Id2 transcripts were not detected in any CD117+CD19+ cells from mice of the four genotypes (data not shown). However, Nfil3 transcripts were significantly increased in CD117+CD19+ cells of Ehet and ERhet mice (3.9-fold, p=0.041 and 5.8-fold, p=0.036, respectively; Fig. 2B). Significant differences in these transcripts were not observed between pre-B cells of the four genotypes. Thus, promiscuous expression of Nfil3/E4BP4 may contribute to the expression of NK cell specific genes in the mutant mice.
Simultaneous expression of CD19 and NK cell transcripts in B cells occurs in the presence of Pax5
The expression of NK cell markers on CD19+ B cells and co-expression of B and NK cell transcripts suggests that signals necessary for B lineage commitment are decreased in the haploinsufficent cells. Expression of the Cd19 and Cd79a (encoding Igα) genes is dependent critically on the B lineage commitment factor Pax5 [24-26]. Previously, we detected relatively normal amounts of Pax5 transcripts in populations of pro-B and pre-B cells of Ehet and ERhet mice [15]. This suggests that the presence of NK cell transcripts in early B cells is not due to the silencing of Pax5 genes, but it is dependent on sufficient dosage of EBF1.
To demonstrate the co-expression of B and NK cell-specific transcripts in single cells, we employed multiplex RT-PCR analysis on purified CD117+CD19+CD244+ bone marrow cells from ERhet mice. All cells expressed Hprt, which was amplified as a ubiquitously expressed control (data not shown). Forty-one percent of cells were positive for Cd244 transcripts. The percentage of cells expressing Cd244 was less than expected, which may have been due to low levels of transcripts in many of the sorted cells. Importantly, of the cells that expressed Cd244 transcripts, 95% (38/40) expressed transcripts of the B cell commitment factor Pax5 and 100% (40/40) expressed the BCR signaling component Cd79a (Fig. 2C). Additionally, Cd160 and Klrb1c transcripts were present in 20% (8/40) and 17.5% (7/40) of cells, respectively. These data clearly indicate the co-expression of B cell and NK cell programs in committed CD19+ pro-B cells when EBF1 expression is reduced. Furthermore, these data suggest that EBF1 functions similarly to, and perhaps in conjunction with Sfpi-1/PU.1, which represses expression of NK cell-specific genes including Cd244, Klra2 and Klrb1b in a dose-dependent manner [27].
Ly6a/Sca-1 expression is increased in pro-B/pre-B cells with reduced EBF1 expression
To evaluate the possibility that cells from ERhet mice transitioned incompletely to ‘committed’ CD19+ B cells, we assessed expression of Ly6a (encoding Sca-1), a marker of hematopoietic progenitors [28]. When compared to pro-B cells of WT mice, Ly6a transcripts were increased significantly in Rhet, Ehet and ERhet pro-B cells (2.7-, 4.2- and 7.7-fold, respectively; p=0.0002 for ERhet) (Fig. 2D, upper panel). Furthermore, Ly6a transcripts were increased 5.1-fold (p=0.029) compared to levels in WT late pro-/pre-B cells (Fig. 2D, lower panel). When evaluated by flow cytometry, expression of Sca-1 was increased dramatically on pro-B cells (Fr. B-C′) from the mutant mice (Fig. 2E). The Sca-1+CD24+ population of B220+CD43+-gated bone marrow cells increased 6.7-fold from WT to ERhet mice (8% to 54%, respectively). This population was increased to a lesser extent in the single mutants (22% for Rhet and 32% for Ehet). These data further confirm that the haploinsufficient cells are less differentiated than developing B cells from WT mice.
Increased expression of EBF1 reduces NK cell-specific transcripts
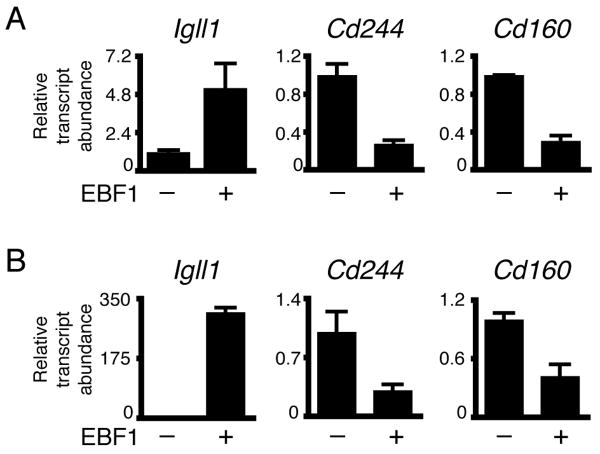
Our data suggested that the partial loss of B cell identity in Ehet and ERhet cells is largely due to Ebf1 haploinsufficiency. To address this hypothesis, ERhet pro-B cells were infected with retroviruses to express control GFP or EBF1(18-591) as separate proteins. Purified GFP+ cells were analyzed for expression of specific transcripts using qRT-PCR (Fig. 3A). Enforced expression of EBF1 increased B cell-specific Igll1 (λ5) transcripts 5-fold (p=0.065). In contrast, Cd244 and Cd160 transcripts were reduced significantly by EBF1 (70% and 80%, respectively; p=0.0014 and <0.0001, respectively). We conclude that expression of EBF1 is sufficient to repress NK cell-specific genes in ERhet pro-B cells. Previously, Ebf1−/− B220+IL-7R+ progenitors were shown to have NK, myeloid and T cell lineage potential; however, enforced expression of EBF1 repressed myeloid cell-specific genes and prevented alternate fates. The ability of EBF1 to repress non-B cell fate decisions was linked with repression of fate-promoting transcription factors including Sfpi1, Cebpa, Id2 and Id3 [10,11].
FIGURE 3.
Enforced expression of EBF1 increases B cell-specific and decreases NK cell-specific transcripts in pro-B cells of ERhet mice and Ebf1-/-Pax5-/- fetal liver cells. (A) qRT-PCR analysis of pro-B cells of ERhet mice expressing control GFP or EBF1(18-591)(co-expressed with GFP). (B) qRT-PCR analysis of Ebf1-/-Pax5-/- fetal liver cells expressing EBF1(18-429) and GFP. B220+ uninfected cells were analyzed as a control. All transcripts were normalized to Hprt transcripts (mean±SEM). Data are from three independent experiments.
To confirm that the reduced expression of NK lineage genes by EBF1 does not require Pax5, we reconstituted Ebf1-/-Pax5-/- fetal liver cells with EBF1 (Fig. 3B). Quantities of Igll1 transcripts in B220+ Ebf1-/-Pax5-/- fetal liver cells expressing EBF1(18-429) increased 309-fold (p<0.0001) compared to transcripts in control B220+ cells. Cd244 and Cd160 transcripts were reduced 69% and 58%, respectively (p=0.0282 and 0.02154, respectively) in the presence of EBF1. Together, these data demonstrate the ability of EBF1 to repress genes that are expressed promiscuously in B cells of ERhet mice. Notably, this activity is observed in the absence of Pax5.
Concluding Remarks
Here, we demonstrated that markers of NK and undifferentiated cells are expressed promiscuously on bone marrow-derived pro-B cells, but not on CD25+ pre-B, mIgM+ B cells or peripheral B cells of Ehet and ERhet mice. The data suggest a partial loss of B cell identity that is due largely to Ebf1 haploinsufficiency and is exacerbated by Runx1 haploinsufficiency. This is of particular interest because the expression of most genes does not change due to haploinsufficiency [29]. The synergy between Ebf1 and Runx1 genes in ERhet mice is highly significant because compound effects were not observed in Pax5+/−Runx1+/−, Ebf1+/−Ikzf1+/− or Ebf1+/−Gfi1+/− mice (K.L. and J.H., and H. Xu and H. Singh, unpublished data). Furthermore, enforced expression of EBF1 in ERhet pro-B cells increased the expression of B lineage-specific genes while repressing expression of NK-specific genes. Similar results were obtained in Ebf1−/−Pax5−/− fetal liver cells suggesting that EBF1 may repress genes to enforce B lineage commitment in the absence of Pax5. These observations, together with our detection of Pax5 transcripts in single ERhet cells that expressed NK cell transcripts, suggest that EBF1 contributes directly to B lineage commitment. This hypothesis is strengthened further by the loss of NK lineage potential in common lymphoid progenitors that express EBF1 [30]. The data do not detract from the seminal importance of Pax5 in the regulation of B lineage commitment [17,26], but they advocate strongly that the two factors regulate commitment together.
Materials and Methods
Mice
Ebf1+/− mice and Runx1+/− mice were described previously [15]. All experiments were approved by the Institutional Animal Care and Use Committee at National Jewish Health.
Antibodies and flow cytometry
Cell staining, analysis and sorting were performed as described previously [15] with the following antibody additions: CD24-biotin (BD Pharmingen, San Diego, CA, USA) with SA-APC-Cy7 (Biolegend, San Diego, CA, USA) for detection, CD244-Alexa Fluor 647 (eBioscience, San Diego, CA, USA) and Sca-1(Ly6a/e)-PE and CD244.2-PE (BD-Pharmingen, San Jose, CA, USA).
RNA isolation and quantitative RT-PCR (qRT-PCR)
RNA isolation, preparation of cDNA and qRT-PCR for detection of transcripts were performed as described [15]. Data are reported as mean±SEM. For single cell PCR, individual CD117+CD19+CD244+ bone marrow cells were sorted into wells of 96 well plates using a MoFlo XDP (Beckman Coulter, Brea, CA). Cells were quick frozen and processed prior to detection of indicated transcripts or control Hprt transcripts as described [30]. Primers used in these studies are listed in Supporting Information Table 2.
Preparation of recombinant retroviruses, transduction of cells and analysis
Retroviruses for expression of EBF were described previously (14). Bone marrow cells were isolated as described without depletion (15). ERhet pro-B cells were expanded in 10% complete IMDM containing 5 ng/mL IL-7 and Flt-3L (R&D Systems, Minneapolis, MN). On days 5 and 6, cells were transduced by spinfection at 2000 rpm at 25°C for 2 hours with retroviral supernatants containing 4 μg/mL polybrene (Sigma, St. Louis, MO) and 10 mM HEPES, pH7.4. Following spinfection, cells were returned to expansion media. Transduced cells were purified on day 4 using a MoFlo XDP (Beckman Coulter). Ebf1−/−Pax5−/− fetal liver cells (14) were transduced by a single spinfection with 10 μg/mL polybrene. At 96 hours, transduced, B220+ cells were purified as above and RNA was isolated as described previously [15].
Statistical analysis
Data shown were pooled from at least three experiments. Two-tailed, unpaired Student t-tests were calculated with Prism (Graphpad Software, Inc, La Jolla, CA). Values of p < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Laurent Gapin for helpful discussions. We are indebted to Yuan Zhuang, Rudolf Grosschedl, Dan Littman and Nancy A. Speck for providing Ebf1+/− and Runx1+/− mice and to Harinder Singh for sharing unpublished results. We thank José Garcia and Kathryn Tuttle for excellent technical assistance. This work and J.H. and K.L. were supported by National Institutes of Health Grants R01 AI54661, R01 AI81878 and P01 AI22295. S.F. was supported by NIH Postdoctoral Training Grant 5 T32 AI007405. L.G. was supported by NCI grant P20 CA103680-05. M.S. was supported by the Swedish Medical Research Council and the Univ. of Linköping. S.Z. and R.M. were supported by the Swedish Cancer Foundation.
Abbreviations
- COE-1
Collier-Olf-EBF
- EBF1
Early B cell factor 1
- Hprt
hypoxanthine/guanine phosphoribosyl transferase
- Klrb1c
killer cell lectin-like receptor, subfamily A, member 7
- Ly6a/Sca-1
Lymphocyte antigen 6, complex A/stem cell antigen 1
- mIgM
membrane IgM
- NK1.1
Natural Killer antigen 1.1
- O/E-1
Olf-EBF-1
- WT
wild type
- Ehet
Ebf1+/−
- Rhet
Runx1+/−
- ERhet
Ebf1+/−Runx1+/−
- Sfpi1-1/PU.1
Spleen focus forming virus (SFFV) proviral integration oncogene/Purine-rich box-1
- E2A
E12 and E47 factors encoded by Tcfe2a
- Nfil3/E4BP4
Nuclear factor, interleukin 3-regulated/E4 promoter-binding protein 4
- Id2
inhibitor of DNA binding 2
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Davidson EH. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution. Academic Press; San Diego: 2006. [Google Scholar]
- 2.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Northrup DL, Allman D. Transcriptional regulation of early B cell development. Immunol Res. 2008;42:106–117. doi: 10.1007/s12026-008-8043-z. [DOI] [PubMed] [Google Scholar]
- 4.Seet CS, Brumbaugh RL, Kee BL. Early B cell factor promotes B lymphopoiesis with reduced interleukin-7 responsiveness in the absence of E2A. J Exp Med. 2004;199:1689–1700. doi: 10.1084/jem.20032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina KL, Pongubala JMR, Reddy KL, Lancki DW, DeKoter RP, Kieslinger M, Grosschedl R, Singh H. Defining a regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Dias S, Silva H, Jr, Cumano A, Viera P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi K, Kasai H, Watanabe A, Lai AY, Kondo M. IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining Early B cell factor expression. J Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pongubala JM, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E, Thomas M, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nature Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 11.Thal M, Carvalho TL, He T, Kim HG, Gao H, Hagman J, Klug CA. Ebf1-mediated down-regulation of Id2 and Id3 is essential for specification of the B cell lineage. Proc Natl Acad Sci USA. 2009;106:552–557. doi: 10.1073/pnas.0802550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treiber T, Mandel EM, Pott S, Gyory I, Firner S, Liu ET, Grosschedl R. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity. 32:714–725. doi: 10.1016/j.immuni.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier H, Ostraat R, Gao H, Fields S, Shinton SA, Medina KL, Ikawa T, et al. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat Immun. 2004;5:1069–1077. doi: 10.1038/ni1119. [DOI] [PubMed] [Google Scholar]
- 15.Lukin K, Fields S, Lopez D, Cherrier M, Ternyak K, Ramirez J, Feeney AJ, Hagman J. Compound haploinsufficiencies of Ebf1 and Runx1 genes impede B cell lineage progression. Proc Natl Acad Sci U S A. 107:7869–7874. doi: 10.1073/pnas.1003525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolink A, ten Boekel E, Melchers F, Fearon DT, Krop I, Andersson J. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J Exp Med. 1996;183:187–194. doi: 10.1084/jem.183.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 18.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol. 2001;31:1900–1909. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 24.Kozmik Z, Wang S, Dorfler P, Adams B, Busslinger M. The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol Cell Biol. 1992;12:2662–2672. doi: 10.1128/mcb.12.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzsimmons D, Hodsdon W, Wheat W, Maira SM, Wasylyk B, Hagman J. Pax-5 (BSAP) recruits Ets proto-oncogene family proteins to form functional ternary complexes on a B cell-specific promoter. Genes Dev. 1996;10:2198–2211. doi: 10.1101/gad.10.17.2198. [DOI] [PubMed] [Google Scholar]
- 26.Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- 27.Kamath MB, Houston IB, Janovski AJ, Zhu X, Gowrisankar S, Jegga AG, DeKoter RP. Dose-dependent repression of T-cell and natural killer cell genes by PU.1 enforces myeloid and B-cell identity. Leukemia. 2008;22:1214–1225. doi: 10.1038/leu.2008.67. [DOI] [PubMed] [Google Scholar]
- 28.Bradfute SB, Graubert TA, Goodell MA. Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–843. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Qian W, Zhang J. Gene dosage and gene duplicability. Genetics. 2008;179:2319–2324. doi: 10.1534/genetics.108.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Månsson R, Zandi S, Welinder E, Tsapogas P, Sakaguchi N, Bryder D, Sigvardsson M. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.