Abstract
Background
Adenoid cystic carcinoma is a locally aggressive salivary gland neoplasm which has a poor long term prognosis. A chromosomal translocation involving the genes encoding the transcription factors MYB and NFIB has been recently discovered in these tumors.
Methods
MYB translocation and protein expression was studied in 37 adenoid cystic carcinomas, 112 other salivary gland neoplasms, and 409 non salivary gland neoplasms by FISH and immunohistochemistry. MYB translocation and expression status in adenoid cystic carcinoma was correlated with clinicopathologic features including outcome, with a median follow up of 77.1 months (range: 23.2–217.5) for living patients.
Results
A balanced translocation between MYB and NFIB is present in 49% of adenoid cystic carcinomas but is not identified in other salivary gland tumors or non-salivary gland neoplasms. There is no apparent translocation of MYB in 35% of the cases. Strong Myb immunostaining is very specific for adenoid cystic carcinomas but is only present in 65% of all cases. Interestingly, Myb immunostaining is confined to the basal cell component though the translocation is present in all the cells. Neoplasms with MYB translocation demonstrate a trend towards higher local relapse rates, but the results are not statistically significant with current case numbers.
Conclusions
MYB translocation and expression are useful diagnostic markers for a subset of adenoid cystic carcinomas. The presence of the translocation may be indicative of local aggressive behavior but a larger cohort may be required to demonstrate statistical significance.
Keywords: Adenoid cystic carcinoma, salivary gland tumors, MYB, NFIB, translocation, immunohistochemistry
Background
Adenoid cystic carcinoma is a salivary gland tumor with locally aggressive behavior and a poor prognosis. A cytogenetic abnormality has been identified in several cases involving a t(6;9) (q22–23; p23–24) translocation5. Recently it has been found that the gene encoding the transcription factor MYB is fused with the NFIB transcription factor in 6/11 cases of adenoid cystic carcinoma from the salivary gland and breast6.
The discovery of this potentially pathognomic translocation suggests that this could be useful for diagnostic, prognostic and potentially therapeutic purposes. To ascertain the diagnostic and prognostic utility of the MYB translocation and of elevated levels of MYB expression, we examined by FISH and immunohistochemistry the status of MYB in 37 adenoid cystic carcinomas, 112 other salivary gland tumors and 409 non-salivary gland neoplasms.
Materials and Method
Tissue microarray construction
The 52 cases of adenoid cystic carcinoma used to construct the tissue microarray (TMA) represented samples from patients treated in the Stanford Department of Radiation Oncology who had specimens stored in the Surgical Pathology archives at Stanford Hospital and Clinics.
The 112 cases for the non-adenoid cystic salivary gland TMA were identified by searching the Surgical Pathology archives from January 1, 1995 to January 10, 2010 for salivary gland, palate, sublingual and buccal specimens with a diagnosis of mucoepidermoid carcinoma (23 cases), acinic cell carcinoma (7), polymorphous low grade carcinoma (12), myoepithelioma (5), myoepithelial or epithelial-myoepithelial carcinoma (4), pleomorphic adenoma (benign mixed tumor)/cellular pleomorphic adenoma/carcinoma ex pleomorphic adenoma (35), oncocytic hyperplasia/oncocytoma/oncocytic carcinoma (12), basal cell adenoma/adenocarcinoma (9), salivary duct carcinoma (3), adenosquamous carcinoma (5).
The TMAs of non-salivary gland neoplasms were previously constructed and described elsewhere3. All salivary gland and non salivary gland cases were reviewed by a pathologist for diagnostic accuracy.
The TMAs were constructed by using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD) following techniques described in ref. 17. 600-μM cores were taken from salivary gland tumor samples archived at the Stanford University Medical Center Department of Pathology between 1995 and 2009.
Clinical features of patients
The TMA consisted of tumor tissues from 52 patients with primary adenoid cystic carcinoma arising from either major or minor salivary glands located in head and neck sites. All patients were treated at Stanford for their cancer between 1973–2006 and this retrospective study was approved by the Stanford Institiutional Review Board (IRB). Of these, fifteen cases were excluded due to negative oligo-dT staining (see below) indicating poor tissue quality in nine cases, and uninterpretable FISH reading in six cases. Table 1 shows patient distributions for both analyzable and non-analyzable samples. As shown, most analyzable patients were treated with surgery followed by radiation therapy (73%). A small percentage of analyzable patients were treated with surgery alone for small tumors (11%) and the remaining patients were treated with either neutron beam radiotherapy or a combination of chemoradiotherapy with photon beam for unresectable tumors. The distribution of the analyzable and non-analyzable samples were similar except for a statistically higher percentage of adenoid cystic carcinoma with solid component in the non-analyzable group and a non-statistically higher percentage of nodal involvement in the analyzable group.
Table 1.
Patient, tumor and treatment characteristics of analyzable and non-analyzable patients who were included on the tissue micro array
| Characteristics | Analyzable patients (N = 37) | Non-analyzable patients (N = 15) | P value |
|---|---|---|---|
| Mean Age | 51 | 52 | 0.80 |
|
| |||
| Gender | > 0.99 | ||
| Female | 13 (35%) | 5 (33%) | |
| Male | 24 (65%) | 10 (67%) | |
|
| |||
| Salivary gland type | 0.18 | ||
| Major | 13 (35%) | 2 (13) | |
| Minor | 24 (65%) | 13 (87%) | |
|
| |||
| T-stage | 0.72 | ||
| 1 | 5 (14%) | 1 (7%) | |
| 2 | 11 (30%) | 3 (20% | |
| 3 | 5 (14%) | 3 (20%) | |
| 4 | 16 (42%) | 8 (53%) | |
|
| |||
| Nodal Status | 0.41 | ||
| Negative | 30 (81%) | 14 (93%) | |
| Positive | 7 (19%) | 1 (7%) | |
|
| |||
| Metastasis | >0.99 | ||
| Negative | 35 (94%) | 14 (93%) | |
| Positive | 2 (6%) | 1 (7%) | |
|
| |||
| Solid Compent | 0.02 | ||
| No | 26 (70%) | 5 (33%) | |
| Yes | 11 (30%) | 10 (67%) | |
|
| |||
| Perineural invasion | > 0.99 | ||
| No | 11 (30%) | 4 (27%) | |
| Yes | 26 (70%) | 11 (63%) | |
|
| |||
| Treatment | 0.37 | ||
| Surgery alone | 4 (11%) | 1 (7%) | |
| Surgery + PORT | 27 (73%) | 13 (86%) | |
| Neutron beam alone | 4 (11%) | 0 | |
| Chemoradiation (photon beam) | 2 (5%) | 1 (7%) | |
|
| |||
| Relapse | 0.55 | ||
| Yes | 18 (49%) | 9 (60%) | |
| No | 19 (51%) | 6 (40%) | |
|
| |||
| Survival status | 0.54 | ||
| Alive | 22 (59%) | 7 (47%) | |
| Dead | 15 (41%) | 8 (53%) | |
|
| |||
| Follow Up | 0.87 | ||
| mean | 96.5 months | 99.4 months | |
FISH probe construction FISH method and interpretation
The FISH come-together and break-apart assay was performed using three BAC probes: two probes flanking the MYB gene (RP11-104D9-centromeric, RP11-1105M6-telomeric) and one probe located at the 3′ end of the NFIB gene (RP11-413D24-telomeric) from the Human BAC Library RPCI-11 (BACPAC Resources Centre, Children’s Hospital Oakland Research Institute, Oakland, CA). The FISH assay was performed as previously described elsewhere9. In brief, 6 micron thick sections of the TMA slides were pre-treated with citric acid buffer pH 6.0. Metaphases and metaphase slides were produced using standard methods. BACs were directly labeled with either Cy3 or Cy5 (GE Healthcare Biosciences, NJ). The chromosomal locations of all BACs were validated using normal metaphases (results not shown). Probe labeling and FISH was performed using Vysis reagents according to manufacturer’s protocols (Vysis, IL). Slides were counterstained with 4,6-diamidino 2-phenylindole (DAPI) for microscopy. Signals were interpreted manually, and images were captured by using the Ariol software (Applied Imaging, CA). A cutoff of ≥30 breaks of the two MYB probes per 100 nuclei was selected for a positive score.
IHC antibody, staining, titering use of Oligo dT, and interpretation
The primary antibodies used were Myb (clone 5E11, 1:200, citrate retrieval, Vector VectaStain Elite ABC kit), p63 (clone 4A4, 1:200, DAKO, Carpinteria, CA, USA), and Kit (rabbit polyclonal, 1:50; DAKO, Carpinteria, CA). The Myb staining was titered on a TMA with 150 cases of neoplastic and non-neoplastic tissues. A titer that demonstrated strong staining in the two adenoid cystic carcinoma cores, weak staining in normal thymus as a positive control, and no non specific staining in other tissues was chosen. We first assessed the quality of the tissue preservation by performing RNA in situ hybridization for oligo-dT. The oligo-dT probe hybridizes to the polyA tail of mRNA and should strongly stain all well preserved cells. A number of steps in the processing of clinical specimens can lead to degradation of macromolecules with RNA being among the most sensitive. Thus, RNA preservation (and oligo-dT hybridization) is a good indicator of tissue quality. Eleven adenoid cystic carcinoma and three other salivary gland tumors had poor oligo-dT staining and were excluded from further study.
Myb and Kit immunohistochemistry was independently evaluated by two pathologists and interpretation discrepancies were discussed to achieve consensus. For Myb, only nuclear staining was considered positive. Lesional tissues with strong staining in greater than 50% of the neoplastic cells were scored as strong positive. Weak or strong staining in less than 50% of the cells was scored as weak positive. Less than 5% staining was scored as negative. Kit staining was scored as positive if greater than 10% of the cells demonstrated membrane or cytoplasmic reactivity.
Statistical analysis
Statistical analysis was performed using the Statview statistical software (Computing Resource Center, San Monica, CA). The Fisher Exact and the Student T-tests were used to compare the patient, tumor and treatment characteristics between analyzable and non-analyzable patient groups. The Kaplan-Meir method was used to calculate time to local progression (TTLP). Log-rank statistics were used to compare survival curves between different FISH groups. Multivariate analysis was not performed due to the small sample size.
Results
To determine clinical utility of MYB translocation and the resulting Myb fusion protein expression, we studied adenoid cystic carcinoma and other salivary gland tumors in the adenoid cystic carcinoma diagnostic differential by both FISH and immunohistchemistry on TMAs. Of 52 patients with adenoid cystic carcinoma with available tissues, nine had negative oligo dT staning, indicating poor tissue quality, leaving 43 patients for further analysis. Of these, the FISH assay was interpretable in 37 adenoid cystic carcinomas: 18 (49%) cases have a FISH pattern of a translocation involving MYB and NFIB (Figure 1A); 6 (16%) cases have an abnormal FISH pattern suggestive of a translocation of MYB but not necessarily involving NFIB (Figure 1B–D), and 13 (35%) cases showed no evidence of MYB translocation based on the probes used. Three atypical patterns were noted (Figure 1, B–D): MYB 3′, MYB 5′, and NFIB all come together, extra 5′ MYB without association with NFIB, and MYB split without association with NFIB. There was no evidence of MYB and NFIB translocation in the 112 other, non- adenoid cystic carcinoma, salivary gland tumors. In one case (donor ID 20387), a patient with a history of treated adenoid cystic carcinoma developed a presumed recurrent tumor at the original site that had histology more characteristic of a cellular mixed tumor than adenoid cystic carcinoma. Immunohistochemistry at the time of diagnosis could not provide support for adenoid cystic carcinoma. The tricolor FISH assay demonstrated a MYB translocation in this case supporting the diagnosis of recurrent adenoid cystic carcinoma. Another case of epithelial myoepithelial carcinoma (donor ID 18351) also demonstrated an abnormal FISH pattern.
Figure 1.
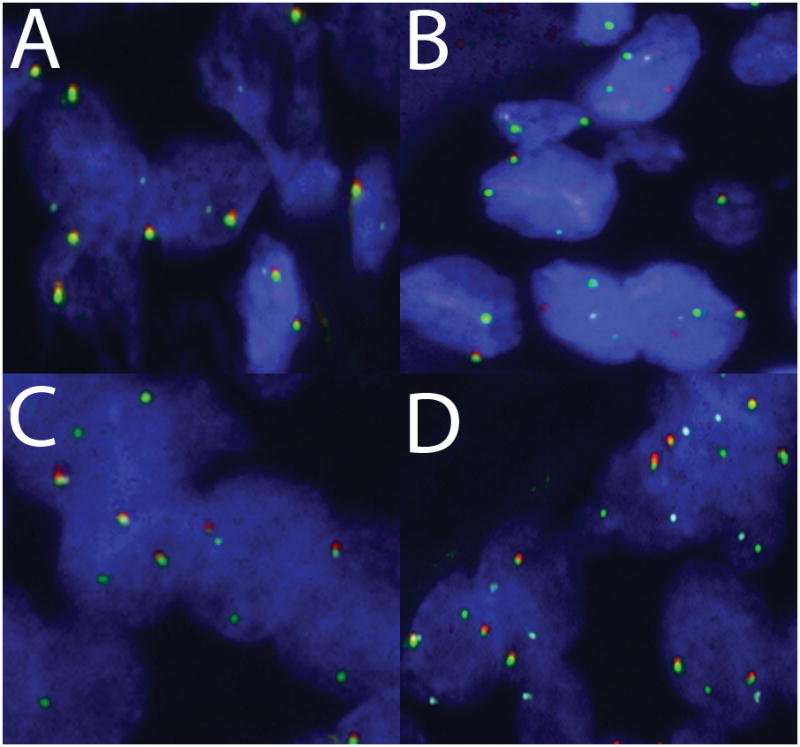
FISH break-apart and come-together tricolor patterns in adenoid cystic carcinoma (Red = 3′ MYB, Green = 5′ MYB, and Blue = NFIB). A) Classic FISH translocation pattern for adenoid cystic carcinoma: 5′ MYB splits from 3′ MYB to associate with NFIB. B–D) Three atypical patterns B) MYB 3′, MYB 5′, and NFIB all come together, C) extra 5′ MYB without association with NFIB, D) MYB split without association with NFIB.
The MYB translocation with NFIB results in a fusion protein that contains the amino-terminal ~90% of the Myb protein. We therefore used a monoclonal mouse antibody directed at the N-terminus of Myb to stain a TMA containing the adenoid cystic carcinoma cases, 112 other salivary gland tumors, and 409 non salivary gland tumors.
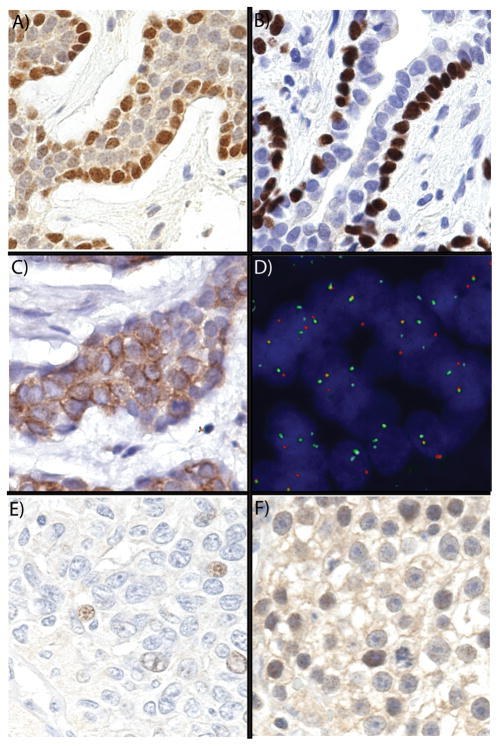
Strong Myb expression as measured by immunohistochemistry was present in 24 of 37 (65%) of well preserved adenoid cystic carcinoma cases (see oligo-dT in Materials and Methods). The Myb expression was predominately present in the nucleus. A comparison with the p63 and Kit staining patterns indicated that Myb expression was confined to neoplastic cells with a basal phenotype (Figure 2A–C). The protein levels decreaseas the cells mature to luminal cells despite the continued presence of the MYB-NFIB translocation in these cells (Figure 2D).
Figure 2.
Myb expression in adenoid cystic carcinoma and other salivary gland tumors and carcinomas. A) Basal pattern of Myb expression in adenoid cystic carcinoma; B) Basal pattern of p63 in adenoid cystic carcinoma; C) Luminal pattern of Kit expression; D) FISH demonstrating translocation in both basal and luminal cells; E) Very low expression in low grade polymorphous adenocarcinoma; F) Myb expression seminoma.
Table 2 shows the distribution of Myb and Kit staining for adenoid cystic carcinoma and other salivary gland tumors. Myb was strongly expressed in 14 of 18 (78%) adenoid cystic carcinoma cases with the balanced pattern of MYB-NFIB translocation (Table 1). Myb was likewise expressed in 4 of 6 (67%) of adenoid cystic carcinoma cases with an abnormal FISH pattern involving MYB. 6 of 13 (46%) adenoid cystic carcinoma cases with a normal FISH pattern were positive for Myb. Myb was expressed focally and at very low levels in rare non-adenoid cystic carcinoma salivary gland tumors (Table 2 and Figure 2E). Diffuse and strong staining was highly specific for adenoid cystic carcinoma among salivary gland tumors. In contrast, Kit expression was observed in a variety of different salivary gland tumors included in this cohort (Table 2).
Table 2.
Distribution of Myb positivity by immunohistochemistry in 37 adenoid cystic carcinomas (ACC) and other salivary gland tumors.
| # cases | Myb strong positive | Myb weak positive | % Myb positive | KIT positive | % KIT positive | |
|---|---|---|---|---|---|---|
| ACC - MYB/NFIB translocation | 18 | 14 | 0 | 78% | 18 | 100% |
| ACC - Abnormal MYB FISH | 6 | 4 | 0 | 67% | 6 | 100% |
| ACC - Normal MYB FISH | 13 | 6 | 0 | 46% | 13 | 100% |
| myoepithelial carcinoma | 4 | 0 | 2 | 50% | 0 | 0% |
| polymorphous adenocarcinoma | 12 | 0 | 4 | 33% | 8 | 67% |
| myoepithelioma | 5 | 0 | 1 | 20% | 2 | 40% |
| pleomorphic adenoma | 16 | 0 | 1 | 6% | 7 | 44% |
| cellular pleomorphic adenoma | 19 | 0 | 1 | 5% | 14 | 74% |
| mucoepidermoid carcinoma | 23 | 0 | 1 | 4% | 10 | 43% |
| basal cell adenoma | 9 | 0 | 0 | 0% | 8 | 89% |
| oncocytoma | 9 | 0 | 0 | 0% | 8 | 89% |
| adenosquamous carcinoma | 5 | 0 | 0 | 0% | 1 | 20% |
| acinic cell carcinoma | 7 | 0 | 0 | 0% | 6 | 86% |
| salivary duct carcinoma | 3 | 0 | 0 | 0% | 2 | 67% |
| oncocytic hyperplasia | 3 | 0 | 0 | 0% | 3 | 100% |
Table 3 shows Myb expression in non-salivary gland carcinomas. As shown, Myb is expressed in a number of other solid tumors. This expression was generally low and focal, but a subset of breast carcinomas (5 of 17, 29%), seminomas (7 of 21, 33%) (Figure 2F), colorectal carcinomas (3 of 11, 27%) and 3 of 3 thymomas demonstrated strong staining.
Table 3.
Myb immunohistochemical expression in other, non-salivary, carcinomas.
| # Cases | Myb reactivity | % positive | |
|---|---|---|---|
| Thymoma | 4 | 3 | 75% |
| Lymphoma | 7 | 5 | 71% |
| Breast carcinoma | 14 | 5 | 36% |
| Colonic adenocarcinoma | 11 | 3 | 27% |
| Testicular carcinoma | 32 | 8 | 25% |
| Head and neck squamous cell carcinoma | 11 | 2 | 18% |
| Esophageal carcinoma | 10 | 1 | 10% |
| Urothelial carcinoma | 11 | 1 | 9% |
| Ovarian carcinoma | 11 | 1 | 9% |
| Melanoma | 23 | 2 | 9% |
| Renal carcinoma | 13 | 1 | 8% |
| Brain neoplasia | 18 | 0 | 0% |
| Non small cell lung cancer | 18 | 0 | 0% |
| Liver neoplasia | 14 | 0 | 0% |
| Pancreastic neoplasia | 12 | 0 | 0% |
| Prostatic carcinoma | 12 | 0 | 0% |
| Uterine carcinoma | 11 | 0 | 0% |
| Cervical carcinoma | 10 | 0 | 0% |
| Thyroid neoplasia | 9 | 0 | 0% |
| Gastric carcinoma | 8 | 0 | 0% |
| Adrenal neoplasia | 7 | 0 | 0% |
| Mesothelioma | 5 | 0 | 0% |
Myb expression and MYB translocation status was correlated with clinicopathologic features of these 37 well-measured cases which had a median clinical follow up of 74.7 months (range: 21.0–246.5) for all patients and 77.1 (23.2–217.5) for living patients. 14/22 (64%) of living patients had follow up greater than 5 years. In addition, of the 22 patients who were alive at the last follow up, 10/22 (45%) already had relapsed and 4/12 (33%) of non-relapsing patients were followed for more than 10 years. Numerically, the local relapse rate was highest for adenoid cystic carcinoma cases with a balanced MYB-NFIB translocation (Figure 3). The cases with an abnormal MYB FISH pattern have an intermediate rate of local relapse. However, these differences are not statistically significant. Table 4 shows the correlation between MYB translocation (including those with abnormal MYB FISH pattern) or Myb expression with other clinical-pathologic parameters. There was a trend to more perineural invasion (PNI) noted for adenoid cystic carcinoma with balanced MYB FISH translocation as compared to those without any translocation. 15 of 18 (83%) of adenoid cystic carcinomas with balanced translocation had PNI as compared 7/13 (54%) of patients without any translocation (chi square p = 0.07, fisher exact test 0.11). When the patients with abnormal MYB FISH pattern were also included, the trend was still there but the difference became less statistically significant (Table 4). Interestingly, two patients, who presented with metastasis at diagnosis, did not have MYB translocation but did have strong Myb protein expression. There were no other statistically significant correlations between either MYB translocation or Myb expression with any other patient or tumor characteristics in this small patient cohort.
Figure 3.
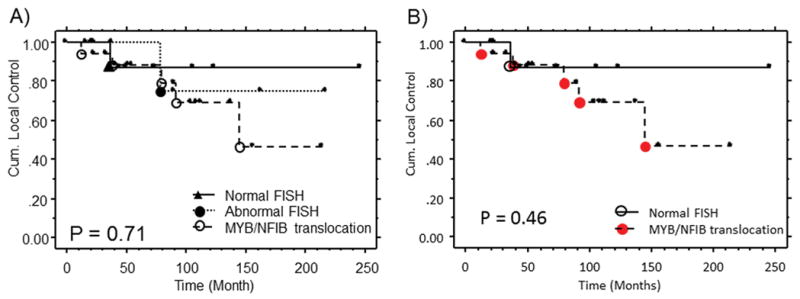
MYB FISH pattern in adenoid cystic carcinoma with local relapse outcome curves with (A) and without (B) the abnormal FISH patterns group.
Table 4.
Correlation between MYB translocation by FISH and Myb protein expression by immunohistochemistry with other clinical pathologic parameter
| Parameter | FISH Translocation | Myb IHC | ||||
|---|---|---|---|---|---|---|
| Present | Absent | p-value | Negative | Positive | p-value | |
| Average Age | 54 | 49 | 0.31 | 54 | 49 | 0.28 |
| Female: Male | 10:14 | 3:10 | 0.31 | 5:8 | 8:16 | >0.00 |
| Salivary gland type Major: Minor |
8:16 | 5:8 | >0.99 | 6:7 | 7:17 | 0.47 |
| Nodal involvement No: yes |
20:4 | 10:3 | 0.68 | 10:3 | 20:4 | 0.68 |
| Metastasis at diagnosis No: yes |
24:0 | 11:2 | 0.11 | 13:0 | 22:2 | 0.53 |
| Perineural invasion No:Yes |
5:19 | 6:7 | 0.14 | 5:8 | 6:18 | 0.46 |
| Solid component No: yes |
17:7 | 9:4 | >0.99 | 11:2 | 15:9 | 0.26 |
Discussion
A number of salivary gland neoplasms have been associated with recurrent chromosomal abnormalities and resulting gene fusions. This includes pleomorphic adenoma (PLAG1 or NFIB and -HMGA2), mucoepidermoid carcinoma (MAML2-TORC1), and Warthin’s tumor (MAML2-TORC1)8. Recently, a recurrent t(6;9) translocation was found in adenoid cystic carcinoma6. Subsequently, it was determined that the translocation leads to the fusion of MYB and NFIB. This fusion leads to the deregulation of the expression of Myb and is likely to be a critical step in oncogenesis for adenoid cystic carcinoma.
The finding of a recurrent gene fusion and subsequent aberrant expression involving MYB provides an opportunity for developing a clinically useful biomarker for diagnosis and prognosis. To address this question we determined the MYB gene and Myb protein status for a series of analyzable 37 adenoid cystic carcinoma cases, 112 other salivary gland neoplasms, and 409 non-salivary gland tumors. We found that the balanced translocation involving MYB and NFIB was present in approximately in one-half of adenoid cystic carcinoma cases (18 of 37, 49%). An additional subset of cases (6 of 37, 16%) had an abnormal MYB FISH pattern suggestive of an unusual translocation of MYB but not involving NFIB. It is possible that this might be in part due to local duplication of MYB, as has been reported in human T-ALL cells4. As seen in other salivary gland tumor translocations, a significant subset of cases did not have apparent MYB involvement, suggesting an alternative mechanism for oncogenesis. Overall, 65% of adenoid cystic carcinoma cases have a FISH pattern suggestive of MYB translocation. This is consistent with previously published chromosomal analyses suggesting that only a subset of adenoid cystic carcinomas harbor the recurrent chromosomal rearrangements.
Myb protein expression is seen in a similar fraction of adenoid cystic carcinomas (27 of 37, 65%). It is highly correlated with MYB FISH translocation (18 of 24, 75%) but also positive in a subset of cases without the MYB translocation (6 of 13, 46%). Among salivary gland tumors, strong diffuse Myb expression is restricted to adenoid cystic carcinomas. Focal weak nuclear staining of Myb was seen in a small subset of salivary gland tumors and this could pose a diagnostic problem if used in the clinical setting on small samples (e.g. fine needle aspirates). Nevertheless, Myb protein expression or MYB translocation as demonstrated by FISH may be helpful for the diagnosis of difficult salivary gland lesions. In a survey of 409 non salivary gland neoplasms, Myb protein expression was seen in a significant subset of breast cancers, colorectal carcinomas, thymomas, and germ cell tumors. This suggests that some caution should be exercised if Myb protein expression is to be used in the diagnosis of metastatic adenoid cystic carcinoma.
Outcome studies provide some evidence to suggest there is clinical prognostic significance to the presence of the MYB translocation. In our study of 37 well measured cases, there is a trend to an increase in local relapse with cases that have a balanced MYB-NFIB translocation. There is also the suggestion that these cases are more likely to demonstrate perineural invasion. These results are similar to the findings in low grade mucoepidermoid carcinoma which show an outcome difference between tumors that bear a translocation versus those that do not. Previous studies have demonstrated that the MECT1-MAML2 translocation is present in a subset of low grade mucoepidermoid carcinomas (and Warthin’s tumors). Patients with low grade mucoepidermoid carcinoma and the MECT1-MAML2 translocation have a statistically significantly lower risk of local recurrence, distance recurrence, and death1.
Extensive work has been done on the biology of MYB in relation to cell development and differentiation7. However, comparatively little is known about MYB in tumorigenesis in humans. We found that Myb expression is restricted to the basal cell phenotype within adenoid cystic carcinoma. Myb expression is lost in the luminal cells. This is surprising in that both the basal cells and luminal cells have the MYB-NFIB translocation. These results suggest that there are intact regulatory mechanisms in these neoplastic cells that can shut off expression of the MYB-NFIB fusion transcript and/or regulate levels of the fusion protein. It is possible that the oncogenically active cells (including the tumor-initiating cells) are present mainly in the basal cell population. Kit expression is seen in the luminal cells. Because of high Kit expression in these tumors, investigators have tried to target Kit expressing adenoid cystic carcinomas with imatinib (a Kit inhibitor) without success2. The finding that MYB is only expressed in basal cells and not in the cells that express Kit suggest that strategies to target Kit may have been directed at the wrong cell phenotype or that Myb might lie upstream of Kit and be a more important target in adenoid cystic carcinoma.
In summary, we have found that over 50% of adenoid cystic carcinoma tumors harbor MYB translocations and increased Myb expression, a feature which is unique compared to other non- adenoid cystic carcinomas salivary gland cancers and provides a useful diagnostic tool. There was a trend for increased perineural invasion and higher rate with local relapse in patients with balanced MYB-NFIB translocation. Drawbacks of this study included its small sample size, the large percentage of non-analyzable patients due to poor tissue quality, heterogeneity of treatment approaches and the long period over which the patient data and tissues were collected. However, the follow-up is quite mature for adenoid cystic carcinoma with over half of living patients having more than five years follow-up. The results of this study will need to be validated against a cohort of larger and more homogeneously treated adenoid cystic carcinoma patients. If validated, MYB will serve as a new target for the management of this difficult condition.
Acknowledgments
Financial support: Stanford Cancer Center Translational Seed Funding
Footnotes
Conflict of interest statement: The authors and the study sponsors have no conflict of interest in this work.
References
- 1.Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–81. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 2.Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–90. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan C, Higgins JP, West RB, et al. Microtubule-associated protein-2 is a sensitive marker of primary and metastatic neuroblastoma. Am J Surg Pathol. 2009;33:1695–704. doi: 10.1097/PAS.0b013e3181b0ebdc. [DOI] [PubMed] [Google Scholar]
- 4.Lahortiga I, De Keersmaecker K, Van Vlierberghe P, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet. 2007;39:593–5. doi: 10.1038/ng2025. [DOI] [PubMed] [Google Scholar]
- 5.Nordkvist A, Mark J, Gustafsson H, et al. Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes Chromosomes Cancer. 1994;10:115–21. doi: 10.1002/gcc.2870100206. [DOI] [PubMed] [Google Scholar]
- 6.Persson M, Andrén Y, Mark J, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–4. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–34. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 8.Stenman G. Fusion oncogenes and tumor type specificity--insights from salivary gland tumors. Semin Cancer Biol. 2005;15:224–35. doi: 10.1016/j.semcancer.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci U S A. 2006;103:690–5. doi: 10.1073/pnas.0507321103. [DOI] [PMC free article] [PubMed] [Google Scholar]