Abstract
Bias in cytokine responses has been proposed as a contributing mechanism to pathogenesis in persistent HIV or hepatitis C virus (HCV) infections. We investigated whether coinfection with HCV modifies the profile of antigen-specific cytokine secretion in women persistently infected with HIV compared to women with single HIV or HCV infection. The T helper response to HIV, HCV and cytomegalovirus (CMV) as a positive viral control was dominated by type 1 cytokines (interleukin- [IL] 2, interferon- [IFN] γ and tumor necrosis factor- [TNF] α), with IFN-γ as the most abundantly secreted. IL-4, IL-5 and IL-10 were low in healthy controls and patients. Robust CMV-specific responses contrasted with curtailed HCV-specific responses in HCV-infected women. The overall anti-viral profile was dominated by Th1 cytokines even in coinfected women but both type 1 and type 2 responses were reduced in HIV-infected women and more extensively in women with HCV/HIV coinfection.
Keywords: T helper cells, cytokines, infectious diseases, hepatitis C virus, HIV
INTRODUCTION
Factors produced by T helper cells are important for the immune control of persistent viral infections such as HIV and HCV. Since the initial description of a dichotomy in the factors, e.g. cytokines produced by T helper cells (1), the role of polarized type 1 or type 2 responses in antiviral immunity, particularly during the chronic phase of infection has been debated. Potent HIV-specific T helper responses are detected during the acute phase of HIV (2–4) infection, or in subjects with long-term nonprogression (4, 5). For HCV, resolution of infection is associated with robust CD4+ responses to HCV (6–8). In contrast, memory T helper responses can rarely be detected during chronic infection with either HIV (3, 4) or HCV (7–9) infection.
A feature of HIV infection is the progressive loss of CD4+ T cells (10, 11). Consequently, polyclonal as well as antigen-specific proliferative responses are compromised, a functional alteration likely preceding any detectable decline in CD4+ T cell numbers (12–14). The dysfunction in the T cell compartment is also evident in substantially reduced cytokine expression, prominently IL-2 and to a lesser extent IL-4 and IL-10 (15–17).
The incidence of HCV coinfection is relatively high in HIV-infected patients (18, 19), and progression to HCV-mediated liver disease is faster in coinfected individuals (20–22). Recent studies indicate that T helper cell proliferation to HCV is more extensively compromised compared to HIV in coinfected patients (23). However, whether the cytokine response is involved in accelerated disease progression during coinfection or whether the cytokine profile is altered by HCV infection in HIV-infected patients has not been extensively investigated. We aimed to further the understanding of cell-mediated reactivity in HCV/HIV coinfection by investigating the Th1/Th2 cytokine profile in blood lymphocytes. This study compared the capacity for secretion of IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ; in response to HIV, HCV and CMV as positive control among women with single HCV or HIV infection and women dually infected with both viruses and in healthy subjects seropositive for CMV. Responses to HCV and HIV were detected in about one third of women infected with HCV or HIV respectively. Responses to CMV were more frequent and more potent than responses to HCV or HIV, with a clear type 1 profile and IFN-γ as the cytokine most abundantly secreted. The frequency of IFN-γ, TNF-α and IL-10 responders was significantly reduced among coinfected (HCV+ HIV+) women. Levels of TNF-α and IL-10 were also significantly reduced in coinfected women compared to women with single HIV infection. Potent cytokine responses to CMV contrasted sharply with curtailed responses to HCV in women singly infected with HCV. Overall, type 1 cytokines dominate the profile of antigen-specific T helper responses both in patients persistently infected with the highly pathogenic viruses HCV and HIV, and in healthy CMV-seropositive controls.
MATERIAL AND METHODS
Subjects
This is a cross-sectional study nested within the Women’s Interagency HIV Study (WIHS, 24). HIV+ and HIV- women with confirmed HCV infection status enrolled in the Southern California WIHS were evaluated as part of one of their scheduled visits. Inclusion criteria were confirmed HIV or HCV infection and female gender, and the patients were sequentially added to the study during Nov 2000 and Oct 2003. Immune responses to viral antigens were investigated in a group of 70 infected women, of which 33 were coinfected (HIV+/HCV+), 27 were HIV+ only and 10 were HCV+ only. Healthy donors (n = 43) recruited for a vaccine study and without any medical condition were used as a control group. The median age for the control group was 48.5 years, 38/43 were women (88.4%) and 38/43 were Caucasians (88.4%). Written consent was obtained from all subjects, and the institutional review boards of the University of Southern California and the City of Hope Medical Center approved the study. For analysis of responses to CMV, serostatus of all participants was verified by ELISA (Sigma Diagnostics).
HIV and HCV Viral Loads
Plasma HIV RNA levels were determined using NASBA (Nucleic Acid Sequence Based Amplification) assay (NucliSens, bioMerieux, Inc., lower limit of detection 25 copies/mL). HCV RNA was detected by PCR assay using Amplicor HCV Monitor 2.0 Roche Diagnostics®, with a dynamic range of 600–850,000 IU/mL.
PBMC Stimulation
Fresh PBMC were cultured in serum-free medium (X-vivo 20, BioWhittaker) at a density of 1 × 105 cells/well in 96-well round-bottomed plates. Antigens used in cell proliferation were recombinant HIV-1 p25/p24 gag (5μg/mL, Viral Therapeutics Inc), recombinant HCV NS3 (1 μg/mL, Fitzgerald Industries), CMV whole virus lysate (10 μg/mL, Microbix Biosystems Inc) or medium alone for 3–5 days. Both recombinant p24/p25 and NS3 were produced in yeast. Phytohaemagglutinin (1 μg/mL, PHA, Murex Biotech Limited) was used as polyclonal control.
Cytometric Bead Array (CBA) Assay
Cell culture supernatants for cytokine profile were recovered after 3 and 5 days of stimulation and kept frozen until batch analysis. The CBA assay was performed in undiluted tissue culture supernatants, except for samples after PHA stimulation that were tested at 1:2 dilutions, as described by the supplier (BD Biosciences). For this assay, soluble cytokines are captured on microparticles and then measured using a fluorescence-based detection system and flow cytometry analysis as described previously (25, 26). A series of 10 dilutions from cytokine standards were run in each assay for generation of standard curves. Samples were analyzed in a FACSCalibur flow cytometer using the BD CBA Analysis Software. For statistical analysis, results are presented as cytokine indexes by calculating the ratio between stimulated and nonstimulated cultures. For the differentiation between responders and low/nonresponders, responders were classified as such if a cytokine result was ≥ mean ± 3SD of background release in PBMC from healthy controls (n = 43). For each study subject, the highest value for each cytokine (either day 3 or day 5) was used for statistical analysis.
Depletion of CD4+ T Cells
PBMC were depleted of CD4+ T cells by immunomagnetic separation. Briefly, cell suspensions were incubated for 30 min at 4ºC with anti CD4-coated magnetic beads (Dynabeads, Dynal) at a 10:1 ratio. Bound cells were removed by application of a magnetic field. The resulting preparation after one-round of separation contained 1.5–2% CD4+ T cells. PBMC depleted of CD4+ T cells were recounted and stimulated with antigens or PHA control, and supernatants were tested for secreted cytokines as described above.
Study Design and Statistical Analyses
Student’s t-test was used for the comparison of continuous variables between two groups. Analysis of variance (ANOVA) was used for the comparison of continuous variables between three or more groups. Further comparisons between any two groups within these multiple groups were conducted using Scheffe’s method. Proportions between categorical groups were compared using Chi-squared test or Fisher’s exact test. Pearsons correlation coefficient was used to study the relationship between Th1 and Th2 cytokines. General linear regression model was used to explore the association between Th1 responses and other parameters. In these analyses, when log10 transformation was used, the value of 0 was assigned for those with undetectable measures. Two-tailed p value <0.05 was considered statistically significant.
RESULTS
Viral Load and CD4+ Counts in Women with Single HIV or HCV Infection and Coinfected Women
A group of 70 women singly or dually infected with HIV and/or HCV were evaluated for virus-specific T helper cell function. Of these, 33 were coinfected (HIV+/HCV+), 27 were infected only with HIV (HIV+) and 10 were infected only with HCV (HCV+). Demographic and clinical characteristics of the study groups are described in Table I. Age and ethnicity were similar among patient groups. IV drug use was higher among HCV+ women (p < 0.0001), either coinfected with HIV or not. PBMC from 43 healthy individuals were used as control group, of which 22 were seropositive for CMV. Among study patients, 97% of HCV+/HIV+, 93% of HIV+ and 90% of HCV+ were seropositive for CMV.
Table I.
Demographic and Clinical Characteristics of the Study Population
| Characteristics | Group | HCV+/HIV+ | HIV+ | HCV+ | p |
|---|---|---|---|---|---|
| N | 33 | 27 | 10 | ||
| IV drug use | Ever | 26 (79) | 1 (4) | 9 (90) | <0.0001 |
| CMV sero-reactivity | 32 (97) | 25 (93) | 9 (90) | ||
| HAART-last 6 months | Yes | 21 (66) | 21 (78) | ns | |
| Length of HAARTa (years) | 2.81 | 3.61 | ns | ||
| CD4 (cells/μl)a | 459 | 499 | 1043b | <0.0001 | |
| Nadir CD4a | 268 | 250 | 863b | <0.0001 | |
| ALT (U/L)a | 69c | 39 | 76 | <0.05 | |
| HIV (Log 10 RNA copies/mL)a | 2.7 | 2.52 | ns | ||
| HCV (Log 10 IU/mL)a | 5.33 | 5.19 | ns |
Note. Data are no. (%) of women, unless otherwise indicated. Ns: not significant.
Group mean.
Significantly higher than the other 2 groups
Significantly higher compared to the HIV+ group.
CD4+ T cell counts were similar in HIV+/HCV+ and HIV+ women (p = 0.56), but lower than in HCV+ women (p < 0.0001). Also, mean CD4 nadirs were similar between HIV+/HCV+ and HIV+ women but lower than in HCV+ women (p < 0.0001). Mean ALT value among HIV+/HCV+ women was significantly higher than in HIV+ women (p < 0.05), but similar to HCV+ women (p = 0.81).
Mean log10 HIV-1 viral load was similar in HIV+/HCV+ and HIV+ women (2.70 and 2.52 respectively, p = 0.59). Also, mean log10 HCV RNA levels were similar in HIV+/HCV+ women and HCV+ women (5.33 and 5.19 respectively, p = 0.80).
CD4 Responses Specific for HCV and HIV
The cytokine profile of T helper cells in HIV and/or HCV infected women in response to HIV p24 and HCV NS3 proteins was investigated. For each cytokine, responders were classified as such if a cytokine result was ≥ mean ± 3SD of background release in PBMC from healthy controls (n = 43). Thirty nine % (13 of 33) of HIV+/HCV+ women and 30% (3 of 10) in the HCV+ group responded to NS3 stimulation with secretion of 1 or more cytokines (Table II). Among responders to NS3, TNF-α was the most commonly secreted cytokine in HIV+/HCV+ women (33%) and IFN-γ along with IL-2 in HCV+ women (20% for both cytokines). Despite detectable responses in some patients, the group means were not statistically different from healthy controls.
Table II.
Frequency of Cytokine Responses Among HIV and/or HCV Infected Women and Healthy Controls
| Antigen | Group | Number of cases | Cytokine responders (%)
|
|||||
|---|---|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-5 | IL-10 | IFN-γ | TNF-α | |||
| NS3 (HCV) | HCV/HIV | 33 | 0 | 0 | 0 | 4 (12.1) | 3 (9.1) | 11 (33.3) |
| HCV | 10 | 2 (20.0) | 0 | 0 | 1 (10.0) | 2 (20.0) | 1 (10.0) | |
| p24 (HIV) | HCV/HIV | 33 | 1 (3.0) | 1 (3.0) | 1 (3.0) | 1 (3.0) | 4 (12.1) | 2 (6.1) |
| HIV | 27 | 1 (3.7) | 0 | 2 (7.4) | 1 (3.7) | 8 (29.6) | 3 (11.1) | |
| CMVa | HCV/HIV | 32 | 6 (18.8) | 1 (3.1) | 7 (21.9) | 9 (28.1) | 17 (53.1) | 13 (40.6) |
| HIV | 25 | 4 (16.0) | 0 | 9 (36.0) | 17 (68.0)b | 20 (80.0)b | 22 (88.0)b | |
| HCV | 9 | 9 (100)b | 0 | 5 (55.6) | 6 (66.7) | 9 (100)b | 8 (88.9)b | |
| Control | 22 | 10 (45.5)b | 1 (4.6) | 10 (45.5) | 18 (81.8)b | 19 (86.4)b | 18 (81.8)b | |
Note. For each cytokine, responders were classified as such if the result was ≥ mean ± 3SD of the spontaneous cytokine release by unstimulated PBMC from healthy controls.
Data from subjects seropositive for CMV.
Significantly higher frequency (p < 0.05) for each group compared to HCV+/HIV+.
For HIV-specific memory, stimulation with p24 elicited responses in 24% (8 of 33) in the HIV+/HCV+ group and 37% (10 of 27) in the HIV+ group. Among responders to p24, the most frequently secreted cytokine in the HIV+/HCV and HIV+ groups was IFN-γ (Table II). However, group means were not statistically different from healthy controls. Taken together, these results indicate a relatively low frequency as well as low levels of responses to HCV and HIV in women with single HCV or HIV infection, or coinfection. PBMC from healthy controls did not respond to stimulation with NS3 or p24 (data not shown).
CD4 Responses to CMV
Responses to CMV were comparatively more frequent than responses to p24 or NS3 in all infected groups, with 75%, 92% and 100% of overall responses among CMV-seropositive HIV+/HCV+ (n = 32), HIV+ (n = 25) and HCV+ (n = 9) women respectively (Table II). The frequency of responders for IFN-γ, TNF-α and IL-10 was significantly reduced in HCV+/HIV+ women compared to all other groups, whereas the frequency of IL-2 responders was low for both HCV+/HIV+ and HIV+ women. IFN-γ was the most abundant cytokine among HIV+/HCV+, HCV+ and healthy CMV-seropositive controls, whereas TNF-α was higher in the HIV+ group (Fig. 1A and Table III). Levels of IFN-γ were similar among HCV+ women and healthy controls but significantly higher compared to the HIV+/HCV+ and HIV+ groups. Levels of TNF-α were higher in HIV+ women compared to HIV+/HCV+ women, but similar to HCV+ women and healthy controls. Levels of IL-10 were significantly lower among HIV+/HCV+ women compared to all other groups (Fig. 1A). Levels of IL-2 were similar in HIV+ and HCV+/HIV+ women but significantly reduced compared to HCV+ women and controls. Overall, secretion of the type 2 cytokines IL-4 and IL-5 was low and no significant differences were observed among groups (data not shown). The cells responding to antigens were confirmed to be of the T helper type by depletion experiments: removal of CD4+ T cells resulted in >99% reduction in cytokine responses (Fig. 1B).
Fig 1.
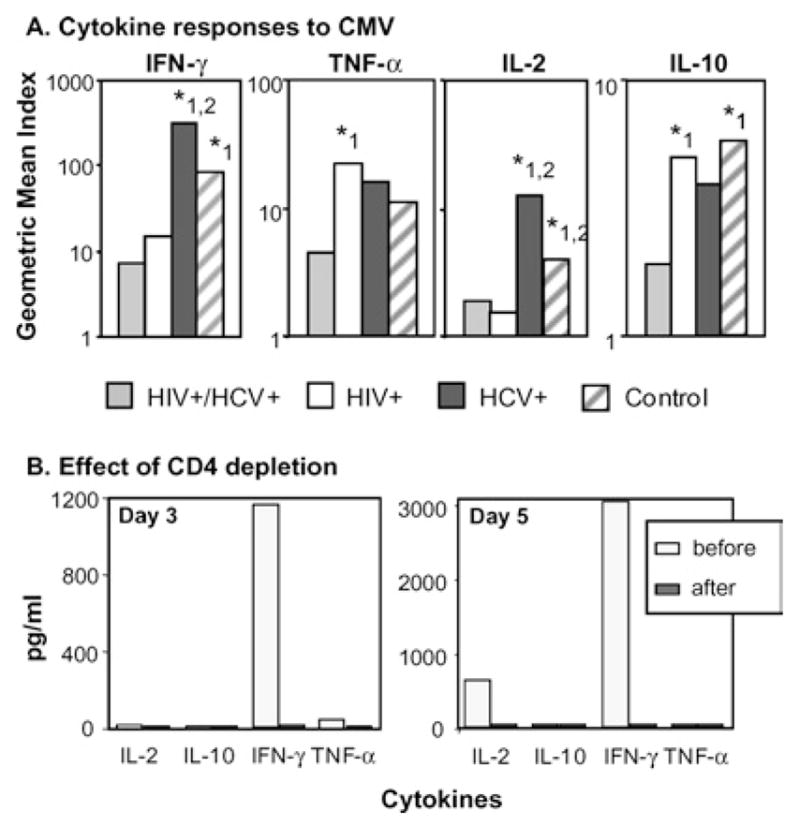
Analysis of memory cytokine responses to CMV by infection group. (A) Cell culture supernatants from PBMC stimulated with CMV were analyzed for secreted IFN-γ, TNF-α, IL-2 and IL-10 and as described in the section on “Materials and Methods.” Levels of secreted IL-4 and IL-5 were low or undetectable, and not significantly different between the groups (data not shown). Bars represent group means of individual cytokine indexes. Note the different scales used to allow for visualization of responses in all groups. Levels of secreted IFN-γ, TNF-α, IL-2 and IL-10 were not statistically different between HCV+ women (black bars) and healthy controls (striped bars). Above the indicated bars: *1, p < 0.05 compared to HIV+/HCV+ women (gray bars); *2, p < 0.05 compared to HIV+ women (white bars). (B) Depletion of CD4+ T cells abolishes the cytokine responses to CMV (IL-4 and IL-5 were below the detection limit of the assay both before and after depletion). Data (from a HCV+ patient) representative of 8 control experiments with similar results performed in singly infected women and healthy controls.
Table III.
CMV-Specific T Helper Cytokines Indexes
| Test group | Geometric mean | 95% confidence interval |
p value
|
||
|---|---|---|---|---|---|
| HCV | HCV | Control | |||
| IFN-γ: HCV/HIV | 7.49 | 3.16–17.76 | ns | <0.05 | <0.05 |
| HIV | 15.96 | 6.47–39.35 | <0.05 | ns | |
| HCV | 315.07 | 94.17–1054.16 | ns | ||
| Control | 83.97 | 38.46–183.31 | |||
| TNF-α: HCV/HIV | 4.44 | 2.71–7.26 | <0.05 | ns | ns |
| HIV | 21.86 | 11.24–42.50 | ns | ns | |
| HCV | 15.92 | 7.58–33.47 | ns | ||
| Control | 11.30 | 6.91–18.48 | |||
| IL-10: HCV/HIV | 2.01 | 1.43–2.84 | <0.05 | ns | <0.05 |
| HIV | 4.95 | 3.34–7.33 | ns | ns | |
| HCV | 3.94 | 2.59–6.01 | ns | ||
| Control | 5.81 | 4.34–7.77 | |||
Note. Cytokine indexes were calculated for each patient as the ratio of CMV-specific secretion and spontaneous release of the same cytokine. Values expressed as geometric mean of each patient group. Ns: not significant.
Type 1 Responses Dominate the Cytokine Profile of CMV-Specific T Helper Cells
The substantial level of responses to CMV allowed for a more detailed analysis of the cytokine profile specific for this antigen. For this, the indexes for type 1 (IL-2, IFN-γ and TNF-α) and type 2 cytokines (IL-4, IL-5 and IL-10) were calculated for each patient (Fig. 2). Interestingly, we found both in infected women and healthy controls that only those women with relatively high secretion of type 1 cytokines had also substantial secretion of type 2 cytokines (p < 0.0001 for the overall Th1/Th2 correlation). Among HIV-infected and HIV+/HCV+ coinfected women, levels of secreted cytokines were low for both type 1 and type 2 but still type 1 was detected to higher extent.
Fig 2.
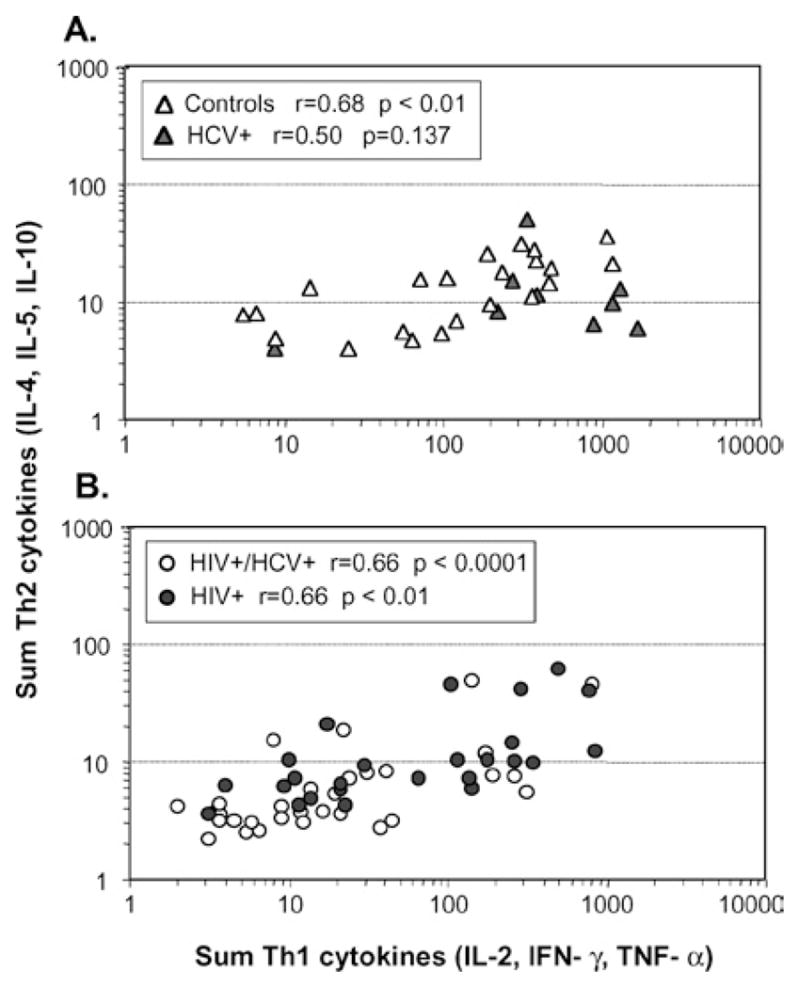
Substantial secretion of type 2 cytokines is only observed in subjects with high type 1 secretion. Cytokine indexes in response to CMV stimulation were grouped in type 1 (IL-2, IFN-γ and TNF-α) and type 2 (IL-4, IL-5 and IL-10), and plotted for each subject. The correlation between the sum of type 1 and type 2 cytokines was statistically significant (p ≤ 0.01) for healthy controls but not for HCV+ women (p = 0.137, panel A) as well as for HIV+ (p ≤ 0.0001) and HIV+/HCV+ women (p ≤ 0.01, panel B).
The role of covariates on the levels of Th1 cytokines among HIV-infected women was investigated in multivariate analysis. For CMV, Th1 responses in HIV+/HCV+ women were significantly associated with HIV viral load (p = 0.038) even after adjusting for HCV viral load (data not shown). However, no significant association was observed for CD4 counts. Similarly, no significant association was observed for responses to NS3 and p24 in multivariate analysis. Together, these results implicate that HIV viremia in coinfected women has a larger impact than HCV viremia on the levels of cytokine responses to CMV.
DISCUSSION
This is, to our knowledge, the first study investigating extensively the Th1/Th2 cytokine profile in patients with multiple viral infections and demonstrates a significant dominance of type 1 responses specific for three persistent viral infections. Our data also highlight an important quantitative difference in levels of cytokine responses in single HIV or HCV infections compared to coinfection, and appear to reflect the combined impact of the dual pathogenic infection on the immune system. For HIV, HCV and CMV, either in single or multiple infections, IFN-γ was the most commonly and abundantly secreted cytokine, although TNF-α was also readily detectable among women with single HIV infection. The comparatively high levels of IFN-γ and TNF-α secreted in response to viral proteins indicate that, even in patients with reduced CD4 counts because of HIV infection, T helper cells contribute significantly to viral control by direct secretion of anti-viral cytokines.
The quantitative difference in cytokine levels was most evident in responses to CMV, which were more readily detected than responses to HIV or HCV. CMV is a ubiquitous member of the herpes virus family, eliciting potent memory responses in asymptomatic but chronically infected individuals (27–29). Although CMV-specific T helper responses are reduced in HIV infection compared to healthy subjects, the responses are relatively stronger than HIV-specific responses (30–32). Thus, responses to CMV are useful to monitor anti-viral responses in patients with T cell dysfunction.
As observed before, responses specific for NS3 in chronic HCV infection were low compared to responses observed during acute HCV infection (7–9, 33). Contrasting the scarcity of HCV-specific responses in HCV+ women, cytokine secretion in response to CMV was robust. This dichotomy may be due to loss of HCV-specific CD4+ responses, which has been described during persistent HCV viremia (7). Because CD4 counts were normal in HCV+ women, it is likely that populations of CMV-specific CD4 memory cells were intact. Activity of regulatory T cells specific for HCV may also limit immune responses, although this remains to be investigated. Other factors may also have an impact on immune function in single HCV infection. It is conceivable that activation of monocytes/macrophages by HCV may favor CMV replication in those cells, leading to higher CMV antigenic load in these patients. In addition, several studies indicate that HCV proteins have the capacity to modulate lymphocyte survival by pro- and anti-apoptotic mechanisms (34, 35), which may also have an impact on the level of CMV-specific memory cytokine response. Although HCV can infect monocytes and dendritic as well as T and B cells besides hepatocytes (36–38), the effect of HCV infection on the function of those lymphocytes remains to be investigated.
Immune responses, particularly cytotoxicity by CD8+ T cells, have been implicated in the pathogenesis of HCV infection. Studies in subjects with chronic HCV infection indicate that secretion of IFN-γ might be linked to the degree of liver damage (39, 40). In our study, HCV-specific responses were detectable in 30–40% of coinfected or HCV-infected women and IFN-γ responses only in 9% of coinfected women. Overall our results were in agreement with low proliferation to NS3 but better preserved responses to other antigens reported earlier (41). However, our multivariate analysis did not detect any significant association between HCV viral load and responses to NS3. Noteworthy, serum levels of ALT were similar among coinfected and HCV-infected women, which suggest less extensive liver damage in coinfected women and contrasted with reported higher ALT values in a predominantly male population (41). Further investigations are needed to clarify whether the low frequency of cytokine responses, including IFN-γ, in coinfected women is indeed reflecting less extensive liver damage in our patients.
Scarcity of memory T cell responses has also been observed in chronic HIV infection (3, 4), which in the presence of HIV replication may be due to exhaustion of antigen-specific T cells, as shown in other infection models (42, 43). Other mechanisms affecting T cell function, such as altered tryptophan catabolism by the enzyme indoleamine 2,3-deoxygenase (44) have not been ruled out. However, Th1 cells appear to be more susceptible to tryptophan starvation than Th2 cells (45) which does not correlate with our results of decline in both Th1 and Th2 functions. Despite similar CD4 counts in HIV+/HCV+ and HIV+ women, cytokine secretion specific for CMV was more extensively compromised in coinfected women, suggesting that monitoring of T cell function should complement the routine control of CD4 counts in these patients. Further long-term studies are required to clarify whether the reduced cytokine responses in coinfected women may be associated with increased risk for disease progression.
In summary, this study demonstrates a profile dominated by Th1 cytokines in anti-viral responses to HIV, HCV and CMV during persistent infection, a pattern consistently observed even in multiple-infected subjects. It also indicates that compared to singly infected women, the T helper response of women coinfected with HIV and HCV is markedly reduced. Finally, this study highlights the importance of monitoring simultaneous responses to unrelated viruses such as CMV in subjects infected with HCV or HIV, where responses specific for HIV and HCV are clearly curtailed. Additional studies are needed to evaluate mechanism(s) behind reduced HCV-specific T helper responses, as well as the clinical implications of reduced cytokine responses in coinfected women.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, Herminia Palacio); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Alvaro Munoz, Stephen J. Gange). The WIHS is funded ∼ by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute of Dental and Craniofacial Research (grants U01-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (grant UO1-CH-32632) and the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). Financial support for the present study: NIAID (AI-52065 and U01-HD32632) to AK and NIH, Division of AIDS (RO1-AI43267 and R21-AI44313) and NCI (RO1-CA77544 and PO1-CA30206), and U. of CA AIDS Research Program (ID02-BRI-054) to DJD. NIH (MO1-RR00043-38) supports the City of Hope GCRC (satellite of USC GCRC). The Laboratory of Vaccine Research is supported by a charitable gift from Bea and Edwin Wolfe. We gracefully thank Drs. Eva Operskalski and Marek Nowicki for critical reading the manuscript.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Altfeld M, Addo MM, Kreuzer KA, Rockstroh JK, Dumoulin FL, Schliefer K, et al. TH1 to TH2 shift of cytokines in peripheral blood of HIV-infected patients is detectable by reverse transcriptase polymerase chain reaction but not by enzyme-linked immunosorbent assay under nonstimulated conditions. J Acquir Immune Defic Syndr. 2000;23:287–294. doi: 10.1097/00126334-200004010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Oxenius A, Price DA, Easterbrook PJ, O’Callaghan CA, Kelleher AD, Whelan JA, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 5.Kalams SA, Buchbinder SP, Rosenberg ES, Billingsley JM, Colbert DS, Jones NG, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 8.Botarelli P, Brunetto MR, Minutello MA, Calvo P, Unutmaz D, Weiner AJ, et al. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology. 1993;104:580–587. doi: 10.1016/0016-5085(93)90430-k. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann RM, Diepolder HM, Zachoval R, Zwiebel FM, Jung MC, Scholz S, et al. Mapping of immunodominant CD4+ T lymphocyte epitopes of hepatitis C virus antigens and their relevance during the course of chronic infection. Hepatology. 1995;21:632–638. [PubMed] [Google Scholar]
- 10.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 11.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 12.Giorgi JV, Fahey JL, Smith DC, Hultin LE, Cheng HL, Mitsuyasu RT, et al. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987;138:3725–3730. [PubMed] [Google Scholar]
- 13.Miedema F, Petit AJ, Terpstra FG, Schattenkerk JK, de Wolf F, Al BJ, et al. Immunological abnormalities in human immunodeficiency virus (HIV)- infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerici M, Hakim FT, Venzon DJ, Blatt S, Hendrix CW, Wynn TA, Shearer GM. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J, Bass HZ, Fahey JL. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993;151:5031–5040. [PubMed] [Google Scholar]
- 17.Graziosi C, Gantt KR, Vaccarezza M, Demarest JF, Daucher M, Saag MS, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. and the Swiss HIV Cohort Study. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 19.Daar ES, Lynn H, Donfield S, Gomperts E, O’Brien SJ, Hilgartner MW, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183:589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 20.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 21.Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 22.Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, Hernandez-Quero J, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 23.Lauer GM, Nguyen TN, Day CL, Robbins GK, Flynn T, McGowan K, et al. Human immunodeficiency virus type 1-hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J Virol. 2002;76:2817–2826. doi: 10.1128/JVI.76.6.2817-2826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The women’s interagency HIV study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 25.Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, et al. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non- allergics. J Immunol Methods. 2001;254:109–118. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 26.Oliver KG, Kettman JR, Fulton RJ. Multiplexed analysis of human cytokines by use of the FlowMetrix system. Clin Chem. 1998;44:2057–2060. [PubMed] [Google Scholar]
- 27.Sester M, Sester U, Gartner B, Kubuschok B, Girndt M, Meyer-hans A, et al. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J Virol. 2002;76:3748–3755. doi: 10.1128/JVI.76.8.3748-3755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitmansour AD, Waldrop SL, Pitcher CJ, Khatamzas E, Kern F, Maino VC, et al. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J Immunol. 2001;167:1151–1163. doi: 10.4049/jimmunol.167.3.1151. [DOI] [PubMed] [Google Scholar]
- 29.Alp NJ, Allport TD, van Zanten J, Rodgers B, Sissons JG, Borysiewicz LK. Fine specificity of cellular immune responses in humans to human cytomegalovirus immediate-early 1 protein. J Virol. 1991;65:4812–4820. doi: 10.1128/jvi.65.9.4812-4820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villacres MC, Lacey SF, La Rosa C, Krishnan R, Auge C, Longmate J, et al. Human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy maintain activated CD8+ T cell subsets as a strong adaptive immune response to cytomegalovirus. J Infect Dis. 2001;184:256–267. doi: 10.1086/322028. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg A, Wohl DA, Barrett RJ, van der Horst C. Inconsistent reconstitution of cytomegalovirus-specific cell-mediated immunity in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2001;184:707–712. doi: 10.1086/322859. [DOI] [PubMed] [Google Scholar]
- 32.Komanduri KV, Viswanathan MN, Wieder ED, Schmidt DK, Bredt BM, Jacobson MA, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 33.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 34.Zhu N, Ware CF, Lai MM. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001;283:178–187. doi: 10.1006/viro.2001.0896. [DOI] [PubMed] [Google Scholar]
- 35.Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, et al. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laskus T, Radkowski M, Piasek A, Nowicki M, Horban A, Cianciara J, et al. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: Evidence of active replication in monocytes/macrophages and lymphocytes. J Infect Dis. 2000;181:442–448. doi: 10.1086/315283. [DOI] [PubMed] [Google Scholar]
- 37.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. The presence of active hepatitis C virus replication in lymphoid tissue in patients coinfected with human immunodeficiency virus type 1. J Infect Dis. 1998;178:1189–1192. doi: 10.1086/515682. [DOI] [PubMed] [Google Scholar]
- 38.Sansonno D, Iacobelli AR, Cornacchiulo V, Iodice G, Dammacco F. Detection of hepatitis C virus (HCV) proteins by immunofluorescence and HCV RNA genomic sequences by non-isotopic in situ hybridization in bone marrow and peripheral blood mononuclear cells of chronically HCV- infected patients. Clin Exp Immunol. 1996;103:414–421. doi: 10.1111/j.1365-2249.1996.tb08296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson MW, Jaksic A, Price P, Cheng W, McInerney M, French MA, et al. Interferon-gamma Response by Peripheral Blood Mononuclear Cells to Hepatitis C Virus Core Antigen Is Reduced in Patients with Liver Fibrosis. J Infect Dis. 2003;188:1533–1536. doi: 10.1086/379252. [DOI] [PubMed] [Google Scholar]
- 40.Anthony DD, Post AB, Valdez H, Peterson DL, Murphy M, Heeger PS. ELISPOT analysis of hepatitis C virus protein-specific IFN-gamma-producing peripheral blood lymphocytes in infected humans with and without cirrhosis. Clin Immunol. 2001;99:232–240. doi: 10.1006/clim.2001.5018. [DOI] [PubMed] [Google Scholar]
- 41.Valdez H, Anthony D, Farukhi F, Patki A, Salkowitz J, Heeger P, et al. Immune responses to hepatitis C and non-heoatitis C antigens in hepatitis C virus infected and HIV-1 coinfected patients. AIDS. 2000;14:2239–2246. doi: 10.1097/00002030-200010200-00004. [DOI] [PubMed] [Google Scholar]
- 42.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus- specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs D, Forsman A, Hagberg L, Larsson M, Norkrans G, Reibnegger G, et al. Immune activation and decreased tryptophan in patients with HIV-1 infection. J Interferon Res. 1990;10(6):599–560. doi: 10.1089/jir.1990.10.599. [DOI] [PubMed] [Google Scholar]
- 45.Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, et al. T cell apoptosis by kynurenines. Avd Exp Med Biol. 2003;527:183–190. doi: 10.1007/978-1-4615-0135-0_21. [DOI] [PubMed] [Google Scholar]


