Abstract
Objective
To determine whether midtrimester insulin resistance (IR) is associated with subsequent preeclampsia.
Study Design
This is a secondary analysis of 10,154 nulliparas administered vitamin C and E or placebo daily from 9-16 weeks' gestation until delivery. Of these, 1,187 women had fasting plasma glucose and insulin tested between 22 and 26 weeks' gestation. IR was calculated by the homeostasis model assessment (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI).
Results
Obese women were twice as likely to have a HOMA-IR ≥ 75th percentile. Hispanic and African-American women had a higher percentage ≥ 75th percentile for HOMA-IR than Caucasians (42.2, 27.2 and 16.9%, respectively, p<0.001). HOMA-IR ≥ 75th percentile was higher among the 85 nulliparas who subsequently developed preeclampsia compared with women who remained normotensive (40.5% vs. 24.8%; adjusted odds ratio 1.9, 95% confidence interval [1.1-3.2]). QUICKI results were similar to HOMA-IR.
Conclusion
Midtrimester maternal IR is associated with subsequent preeclampsia.
Keywords: Insulin resistance, low risk nulliparas, risk for preeclampsia
Introduction
Insulin resistance (IR) was first proposed in 19361 and has been established to play a major role in Type II diabetes mellitus and in the pathogenesis of hypertension, dyslipidemias, and coronary artery disease. Virtually all obese women with hypertension have elevated insulin2 and the highest levels occur in obese women with excessive abdominal adipose tissue.3 Insulin resistance is a hallmark of obesity, and in pregnant women, obesity is a consistent risk factor for preeclampsia.
Normal pregnancy is characterized by lower fasting, higher postprandial glucose values and hyperinsulinemia. After an oral glucose meal, gravid women demonstrate both prolonged hyperglycemia and hyperinsulinemia as well as greater suppression of glucagon.4 This cannot be explained by a decreased metabolism of insulin because its half-life during pregnancy is not changed.5 Instead, this response is consistent with a pregnancy-induced state of peripheral insulin resistance, the purpose of which is likely to ensure a sustained postprandial supply of glucose to the fetus. Indeed, insulin sensitivity in late normal pregnancy is 45 to 70 percent lower than that of nonpregnant women.6-7
Our objective was to determine whether increased maternal midtrimester insulin resistance is associated with subsequent preeclampsia. To test this hypothesis, we performed a secondary analysis of a subgroup of low risk nulliparas from a multicenter, randomized trial of daily vitamin C and E supplementation versus placebo for prevention of complications of pregnancy-associated hypertension.8
Materials and Methods
This was a secondary analysis of the randomized trial in 10,154 nulliparas administered vitamin C and E or placebo daily from 9-16 weeks' gestation until delivery conducted at the 16 clinical centers that were members of the Eunice Kennedy Shriver National Institute of Child Health and Human Developmental Maternal-Fetal Medicine Units Network between 2003 and 2008. Full details of the study design and technique of data collection have been previously described.8 Women included in the trial had blood samples collected at randomization, 24 and 32 weeks' gestation, and at admission for delivery. Information was collected as to whether the women fasted for 12 hours or more even though they were not specifically instructed to fast for any of these visits. Women were included in this secondary analysis if they had a blood sample collected between 22-26 weeks' gestation and had fasted for 12 hours or more prior to the blood collection. The study was approved by the Institutional Review Boards of each clinical site and the data coordinating center.
The diagnosis of hypertension was based on blood-pressure measurements obtained during or after the 20th week of pregnancy, excluding intraoperative blood pressures and intrapartum systolic pressures. Severe pregnancy-associated hypertension was defined as a systolic pressure of 160 mmHg or more or a diastolic pressure of 110 mmHg or more on two occasions 2 to 240 hours apart, or a single blood-pressure measurement that was severely elevated and that led to treatment with an antihypertensive medication. Mild pregnancy-associated hypertension was defined as a systolic pressure between 140 and 159 mmHg or a diastolic pressure between 90 mmHg and 109 mmHg on two occasions 2 to 240 hours apart. Mild preeclampsia was defined as mild pregnancy-associated hypertension with documentation of proteinuria within 72 hours before or after an elevated blood-pressure measurement. Proteinuria was defined as total protein excretion of 300 mg or more in a 24-hour urine sample or 2+ or higher on dipstick testing, or a protein-to-creatinine ratio of 0.35 or more if a 24-hour urine sample was not available. Severe preeclampsia was defined as preeclampsia with either severe pregnancy-associated hypertension or protein excretion of 5 g or more in a 24-hour urine sample or as mild pregnancy-associated hypertension with oliguria (<500 ml in a 24-hour urine sample), pulmonary edema (confirmed by radiography), or thrombocytopenia (platelet count of <100,000 per cubic millimeter). Preeclampsia included mild and severe preeclampsia, HELLP syndrome and eclampsia. To determine the diagnosis of preeclampsia, deidentified medical charts of all women with pregnancy-associated hypertension were reviewed centrally by at least three reviewers.
Determination of Insulin Resistance
Insulin resistance was calculated from fasting maternal plasma glucose and insulin concentrations obtained between 22 and 26 weeks' gestation. Insulin resistance was calculated using the surrogate indices of homeostasis model assessment (HOMA) and also the quantitative insulin sensitivity check index (QUICKI).9-10 Surrogate indirect indices describe glucose-insulin homeostasis by empiric non-linear equations. The intent of the empiric equations is to accommodate glucose ranges, assure reduced suppression of hepatic glucose production and to allow the use of total insulin assays. The equations for the indirect indices are:
The surrogate indices impute a dynamic ∃-cell function (insulin as stimulated by maternal glucose) from fasting steady state data.11
Insulin and glucose assays were performed at the Metabolism Core Laboratory at the University of Alabama at Birmingham. Glucose was measured on the Stanbio Sirrus, an automated spectrophotometric chemistry analyzer using a glucose-oxidase methodology. The intra-assay coefficient of variation (CV) was 1.21% and the inter-assay CV was 3.065%. Insulin was measured on the TOSOH AIA-600 II analyzer which uses a chemiluminescence technology. Insulin was measured using a two-site immunoenzymatic assay. The intra-assay CV was 4.42% and the inter-assay CV was 1.49%.
Statistical Analysis
Insulin resistance was evaluated across body mass index categories using the Cochran-Armitage test for trend. Other categorical variables were compared using the chi-square test. Percentiles for each week of gestation were determined for insulin, glucose, HOMA-IR and QUICKI using the data from the normotensive, non-proteinuric women from this cohort. For each marker (insulin, glucose, HOMA-IR, QUICKI), the Breslow-Day test for homogeneity was used to determine if there was a difference in the effect of body mass index among women who were Hispanic, African American, or Caucasian or other. Multivariable logistic regression analysis was used to calculate odds ratios and included race or ethnic group, body mass index and blood pressure at study entry (9-16 weeks gestational age), treatment group (vitamins, placebo) and gestational age at sampling. For all statistical tests, a nominal p value less than 0.05 was considered to indicate statistical significance; no adjustments were made for multiple comparisons. Analyses were performed using SAS software (Cary, NC).
Results
A total of 10,154 women were randomized in the parent trial, and outcome data were available for 9,969 women. While 68% of these women had a study sample available between 22-26 weeks' gestation, only 1,187 (12%) women had a 12 hour or more fasting sample available for analysis. Population characteristics of women with and without fasting samples are detailed in Table 1. Body mass index (BMI) was measured at study entry (9-16 weeks' gestation). Fasting samples were available in 14.2% of Caucasians, 10.3% of African Americans and 10.0% of Hispanics. While there were statistically significant differences in maternal age, education level and diastolic blood pressure, these differences were small and not clinically meaningful. Of the 1,187 women included in this secondary analysis, 22% were African-American, 26% Hispanic and 52% Caucasian or other. Fifty-two percent were under or normal weight and 48% were overweight or obese.
Table 1. Population Characteristics of Women with and Without Fasting Insulin and Glucose Measured Between 22-26 Weeks Gestation *.
| Characteristic | Women with fasting samples N=1,187 |
Women without fasting samples N=8,782 |
p-value |
|---|---|---|---|
|
| |||
| Maternal age (years) | 23.6 ± 4.9 | 23.5 ± 5.3 | 0.03 |
|
| |||
| Gestational age at study entry (weeks) | 12.3 ± 1.8 | 13.5 ± 2.1 | <0.01 |
|
| |||
| Race | <0.01 | ||
| Hispanic | 307 (25.9) | 2,776 (31.6) | |
| African American | 260 (21.9) | 2,258 (25.7) | |
| Caucasian / Other | 620 (52.2) | 3,748 (42.7) | |
|
| |||
| BMI at study entry # | 0.13 | ||
| <18.5 (underweight) | 39 (3.3) | 235 (2.7) | |
| 18.5-24.9 (normal) | 580 (48.9) | 4,325 (49.3) | |
| 25.0-29.9 (overweight) | 286 (24.1) | 2,335 (26.6) | |
| 30.0-39.9 (obese) | 228 (19.2) | 1,561 (17.8) | |
| ≥ 40.0 (morbidly obese) | 54 (4.5) | 323 (3.7) | |
|
| |||
| Treatment group | 0.40 | ||
| Vitamins | 581 (48.9) | 4,412 (50.2) | |
| Placebo | 606 (51.1) | 4,370 (49.8) | |
|
| |||
| Previous pregnancy < 20 weeks | 296 (24.9) | 1,991 (22.7) | 0.08 |
|
| |||
| Smoked during pregnancy | 202 (17.0) | 1,349 (15.4) | 0.14 |
|
| |||
| Education level (years) | 13.0 ±2.6 | 12.8 ± 2.7 | <0.01 |
|
| |||
| Blood pressure at study entry | |||
| Systolic | 109 ± 10 | 109 ± 10 | 0.35 |
| Diastolic | 66 ± 8 | 65 ± 8 | 0.04 |
Plus-minus values are means ± SD.
Study entry was 9-16 weeks' gestation
Insulin and glucose levels did not differ by whether women were in the vitamin or placebo treated group. The 75th percentile for insulin, glucose and HOMA-IR and the 25th percentile for QUICKI were chosen after other cutoffs were considered and the greatest significance was achieved with these percentiles. The frequency of glucose, insulin, and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile significantly increased with increasing body mass index (trend p<0.0001). At midtrimester, obese women were approximately two times more likely than normal weight women to have a fasting glucose, insulin, and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile (Table 2).
Table 2. Maternal body mass index (BMI) and fasting glucose and insulin levels, and insulin resistance (HOMA-IR and QUICKI) at midgestation *.
| BMI | N | Glucose ≥ 75th %ile (%) |
Insulin ≥ 75th %ile (%) |
HOMA-IR ≥ 75th %ile (%) |
QUICKI < 25th %ile (%) |
|---|---|---|---|---|---|
| < 18.5 | 39 | 15.4 | 17.9 | 15.4 | 15.4 |
| 18.5-24.9 | 580 | 20.9 | 18.0 | 18.0 | 18.2 |
| 25.0-29.9 | 286 | 29.7 | 28.7 | 28.7 | 28.4 |
| 30.0-39.9 | 228 | 36.4 | 37.7 | 37.3 | 37.3 |
| ≥ 40 | 54 | 53.7 | 51.9 | 50.0 | 51.9 |
Trend p<0.0001
Hispanic women had a higher percentage of glucose, insulin and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile compared with African-Americans and Caucasians (p<0.001, Table 3). Compared with Caucasian women, African-American women had a higher percentage of insulin and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile (p<0.001, Table 3) but not glucose at or above the 75th percentile (p=0.86, Table 3). There was a significant interaction between race and BMI (under/normal weight, overweight/obese) for glucose, insulin and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile. Among the 568 overweight or obese women, 48% of Hispanics, 34% of African-Americans and 28% of Caucasians had a HOMA-IR at or above the 75th percentile.
Table 3.
Maternal race/ethnicity and fasting glucose and insulin levels, and insulin resistance (HOMA-IR and QUICKI) at midgestation.
| Race | N | Glucose ≥ 75th %ile (%) |
Insulin ≥ 75th %ile (%) |
HOMA-IR ≥ 75th %ile (%) |
QUICKI < 25th %ile (%) |
|---|---|---|---|---|---|
| Hispanic | 307 | 39.4 | 43.1 | 42.2 | 41.8 |
| African American | 260 | 23.5 | 26.8 | 27.2 | 27.2 |
| Caucasian/Other | 620 | 22.9 | 17.0 | 16.9 | 17.2 |
As expected, the 44 women (3.7%) in the cohort with gestational diabetes mellitus were significantly more likely to have a glucose and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile than women without gestational diabetes (glucose: 59% vs. 26%, p<.0001; HOMA-IR: 43% vs. 25%, p=0.007; QUICKI 43% vs. 25%, p=0.007). While the gestational diabetic women were more likely to have an insulin level at or above the 75th percentile compared with non-diabetic women, this was not statistically significant (39% vs. 25%, p=0.05).
In the overall cohort, 85 women developed preeclampsia, 592 remained normotensive and non-proteinuric, and 510 had an elevated blood pressure or proteinuria but not preeclampsia. Only 8 of the 85 women who developed preeclampsia had gestational diabetes mellitus. Fasting maternal glucose, insulin, and HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile were significantly more likely among those who subsequently developed preeclampsia compared with women who remained normotensive and non-proteinuric (p<0.05, Table 4). A HOMA-IR at or above the 75th percentile had a sensitivity of 40% and specificity of 75% for subsequent preeclampsia with a positive predictive value of 19% and a negative predictive value of 90%. (Values for the QUICKI analyses were identical to the HOMA-IR.) Multivariable analyses confirmed midtrimester fasting insulin, HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile to be significantly associated with preeclampsia as compared to women with no hypertension or proteinuria (Table 4). The 510 women with an elevated blood pressure or proteinuria were similar to the normotensive, non-proteinuric women in regard to a HOMA-IR greater or equal 75th percentile or QUICKI less than the 25th percentile (24% vs 25%, p=0.8).
Table 4.
Midgestation fasting glucose, insulin, and insulin resistance (HOMA-IR and QUICKI) and subsequent preeclampsia.
| Measure | Preeclampsia (%) N=85 | Normal (%) N=592 | OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| Glucose ≥ 75th %ile | 37.6 | 26.5 | 1.7 [1.0-2.7] | 1.5 [0.9-2.5] |
| Insulin ≥ 75th %ile | 40.5 | 25.3 | 2.0 [1.3-3.2] | 1.8 [1.0-3.1] |
| HOMA-IR ≥ 75th %ile | 40.5 | 24.8 | 2.1 [1.3-3.3] | 1.9 [1.1-3.2] |
| QUICKI < 25th %ile | 40.5 | 25.0 | 2.0 [1.3-3.3] | 1.9 [1.1-3.2] |
Adjusted for race or ethnic group, body mass index and blood pressure at study entry, treatment group, and gestational age at sampling.
Comment
After controlling for body mass index, race, ethnicity, treatment group, enrollment blood pressure and gestational age at sampling, midtrimester fasting HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile remain a significant risk factor for subsequent preeclampsia. In low risk nulliparas increasing BMI or Hispanic/African-American ethnicity/race were significantly associated with HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile between 22 and 26 weeks' gestation.
Insulin resistance describes a decreased sensitivity to insulin in regard to glucose disposal and to inhibition of hepatic glucose production. The gold standard for direct testing of insulin resistance is by euglycemic glucose clamp testing.12 Direct testing is time consuming, labor intense, expensive, requires an experienced operator and it is not feasible for epidemiologic studies, large clinical trials or for routine clinical use. We used two indirect surrogates in this analysis, the homeostasis model assessment (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) methods. These indirect indices are dependent on a required fasting basal state (12 or more hours), glucose in the normal range, and the assumption that insulin levels are stable and that hepatic glucose production is constant. Glucose homeostasis is a feedback loop involving hepatic glucose production and insulin secretion from beta cells.9 HOMA-IR and QUICKI describe the glucose-insulin homeostasis loop by empiric non-linear equations. They accommodate glucose ranges, assure reduced suppression of hepatic glucose production, allow the use of total insulin assays, and impute a dynamic beta cell function (insulin stimulated by glucose) from fasting steady-state data.10-11 In nonpregnant women, HOMA-IR and QUICKI have a reasonable linear correlation with direct evaluation using the glucose clamp to assess insulin sensitivity/resistance.11,13-14 We are not aware of any reports comparing the glucose clamp to the HOMA-IR or QUICKI methodology in pregnant women.
The use of indirect indices of insulin resistance may not be generalizable from a single testing facility due to the lack of a standardized insulin assay.15-18 Thus, cutoff points for IR require development of the 75th percentile HOMA-IR and the 25th percentile for QUICKI at each testing facility. It is also important to note that population differences may have an effect on the utility of surrogate indices to reflect insulin resistance. Alvarez and colleagues found that surrogate indices may be more accurate in African-American versus white Americans and more accurate in overweight versus normal weight adults.19
Parretti and colleagues20 assessed insulin sensitivity in 829 gravidas at 16 to 20 and at 26 to 30 weeks' gestation. Their HOMA-IR and QUICKI insulin sensitivity analysis results were similar and at 16-20 weeks gestation had a sensitivity of 79-85% to predict subsequent preeclampsia with a specificity of 97% for both analyses. Our data confirms a significant relationship with a HOMA-IR at or above the 75th percentile and QUICKI less than the 25th percentile at 22 to 26 weeks' gestation with subsequent preeclampsia although with a lower sensitivity of 40% and specificity of 75%. The higher sensitivity and specificity of the Parretti report may relate to their more homogeneous population (Italians of white race), their exclusion of women with gestational diabetes mellitus, their selection of women with a body mass index of between 19 and 25 kg/m2 or to their method of calculation of the 75 to 100 percentile (HOMA-IR) or the 0 to 25 percentile (QUICKI) quartiles. In our report, race, ethnicity, and maternal weight significantly increased the percent of women whose HOMA-IR was at or above the 75th percentile and whose QUICKI was less than the 25th percentile. Sierra-Laguado and associates21 have also reported that midtrimester log-HOMA analysis was significantly associated with subsequent preeclampsia. Within their cohort of 572 normotensive pregnant women at a gestational age of less than 30 weeks the 18 women who developed preeclampsia had a higher log-HOMA than 72 controls matched by body mass index, gestational and maternal age at enrollment.
Roberts and Gammill22 have emphasized the importance of controlling for maternal weight and for insulin resistance testing prior to the clinical appearance of preeclampsia. Parretti et al.20 enrolled lean gravidas and Sierra-Laguado et al21 matched for maternal weight. Gravidas in both reports were assessed early in pregnancy prior to clinically evident preeclampsia. We also determined fasting glucose and insulin concentrations prior to clinically evident preeclampsia (22 to 26 weeks' gestation) and our analyses controlled for maternal weight and other potential risk factors for preeclampsia. Roberts and Gammill22 concluded that even if the midtrimester HOMA-IR is only 20% predictive of subsequent preeclampsia that it would be similar to the “gold standard” of uterine artery Doppler, also 20%, and which entails more complex and costly assessment of risk.23
In summary, maternal midtrimester insulin resistance increased significantly (HOMA-IR at or beyond the 75th percentile or QUICKI less than the 25th percentile) with increasing body mass index and among Hispanic and African-American women. Midtrimester maternal IR is associated with a significantly increased risk of subsequent preeclampsia.
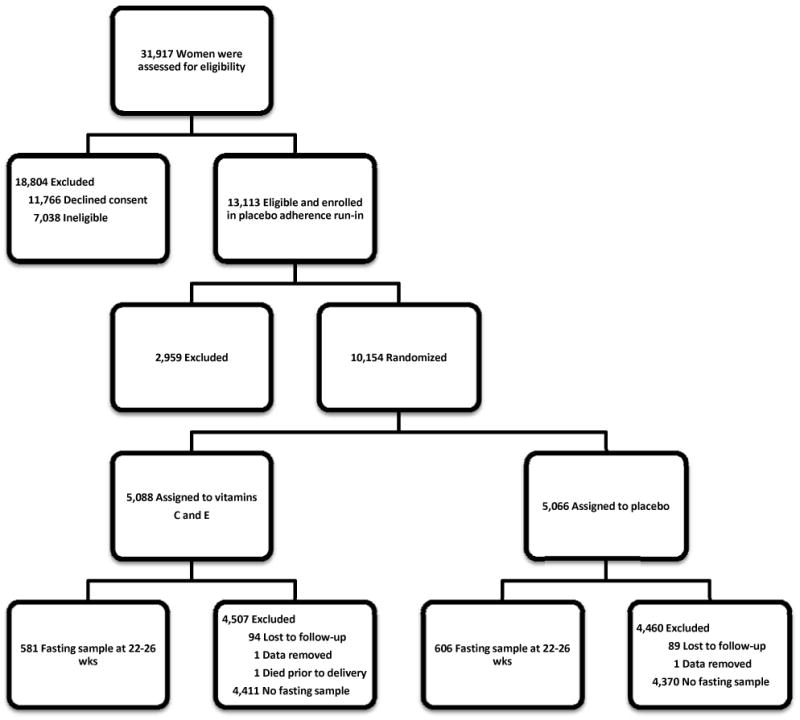
Figure 1. Enrollment, randomization, and follow-up of participants.
Acknowledgments
The wishes to thank the following Subcommittee members who participated in protocol development and coordination between clinical research centers (Sabine Bousleiman, R.N.C., M.S.N., M.P.H. and Margaret Cotroneo, R.N.), protocol/data management and statistical analysis (Elizabeth Thom, Ph.D.), and protocol development and oversight (Gail D. Pearson, M.D., Sc.D.)
The projected described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD34208, HD27869, HD40485, HD40560, HD40544, HD34116, HD40512, HD21410, HD40545, HD40500, HD27915, HD34136, HD27860, HD53118, HD53097, HD27917, and HD36801]; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources [M01 RR00080, UL1 RR024153, UL1 RR024989] and its contents do not necessarily represent the official view of NICHD, NHLBI, NCRR or NIH.
Footnotes
This paper will be presented as oral and poster presentations at the 31st Society for Maternal-Fetal Medicine 2011 Annual Meeting in San Francisco, CA, Thursday, February 10, 2011 and Saturday, February 12, 2011.
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of Alabama at Birmingham, Birmingham, AL – D.J. Rouse, A. Northen, P. Files, J. Grant, M. Wallace, K. Bailey
University of Pittsburgh, Pittsburgh, PA – S. Caritis, T. Kamon, M. Cotroneo, D. Fischer
University of Utah, Salt Lake City, UT – P. Reed, S. Quinn (LDS Hospital), V. Morby (McKay-Dee Hospital), F. Porter (LDS Hospital), R. Silver, J. Miller (Utah Valley Regional Medical Center), K. Hill
Columbia University, New York, NY – S. Bousleiman, R. Alcon, K. Saravia, F. Loffredo, A. Bayless (Christiana), C. Perez (St. Peter's University Hospital), M. Lake (St. Peter's University Hospital), M. Talucci
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Boggess, K. Dorman, J. Mitchell, K. Clark, S. Timlin
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – J. Bailit, C. Milluzzi, W. Dalton, C. Brezine, D. Bazzo
University of Texas Southwestern Medical Center, Dallas, TX – J. Sheffield, L. Moseley, M. Santillan, K. Buentipo, J. Price, L. Sherman, C. Melton, Y. Gloria-McCutchen, B. Espino
Northwestern University, Chicago, IL – M. Dinsmoor (Evanston NorthShore), T. Matson-Manning, G. Mallett
University of Texas Health Science Center at Houston, Houston, TX – S. Blackwell, K. Cannon, S. Lege-Humbert, Z. Spears
Brown University, Providence, RI – J. Tillinghast, M. Seebeck
The Ohio State University, Columbus, OH – J. Iams, F. Johnson, S. Fyffe, C. Latimer, S. Frantz, S. Wylie
Drexel University, Philadelphia, PA – M. Talucci, M. Hoffman (Christiana), J. Benson (Christiana), Z. Reid, C. Tocci
Wake Forest University Health Sciences, Winston-Salem, NC – M. Harper, P. Meis, M. Swain
Oregon Health & Science University, Portland, OR – W. Smith, L. Davis, E. Lairson, S. Butcher, S. Maxwell, D. Fisher
University of Texas Medical Branch, Galveston, TX – J. Moss, B. Stratton, G. Hankins, J. Brandon, C. Nelson-Becker, G. Olson, L. Pacheco
Wayne State University, Detroit, MI – G. Norman, S. Blackwell, P. Lockhart, D. Driscoll, M. Dombrowski
The George Washington University Biostatistics Center, Washington, DC – E. Thom, T. Boekhoudt, L. Leuchtenburg
National Heart, Lung, and Blood Institute, Bethesda, MD – G. Pearson, V. Pemberton, J. Cutler, W. Barouch
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – S. Tolivaisa
References
- 1.Himsworth HP. Diabetes mellitus: its differentiation into insulin-sensitive and insulin-insensitive types. Lancet. 1936;227:127–30. doi: 10.1093/ije/dyt203. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, editors. Obesity. Williams Obstetrics. 23rd. New York, NY: McGraw Hill; 2010. pp. 946–57. [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. Weight Control: Assessment and management. Clinical updates in women's health care. 2003;II(3) [Google Scholar]
- 4.Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids late in normal pregnancy. Am J Obstet Gynecol. 1981;140:730–6. [PubMed] [Google Scholar]
- 5.Lind T, Bell S, Gilmore E, et al. Insulin disappearance rate in pregnant and non-pregnant women, and in non-pregnant women given GHRIH. Eur J Clin Invest. 1977;7:47. doi: 10.1111/j.1365-2362.1977.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 6.Butte NF. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;7:1256S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 7.Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: Roles in fetal development and metabolic programming. Horm Res. 2006;65:41. doi: 10.1159/000091505. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent adverse outcomes with pregnancy associated hypertension. N Engl J Med. 2010;362:1282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 11.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 13.Radziuk J. Insulin sensitivity and its measurement: structural commonalities among the methods. J Clin Endocrinol Metab. 2000;85:4426–33. doi: 10.1210/jcem.85.12.7025. [DOI] [PubMed] [Google Scholar]
- 14.Wallace TM, Levy JC, Matthews Dr. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 15.Staten MA, Stern MP, Miller WG, Steffes MW, Campbell SE for the Insulin Standardization Workgroup. Insulin assay standardization: leading to measure of insulin sensitivity and secretion for practical clinical care. Diabetes Care. 2010;33:205. doi: 10.2337/dc09-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcovina S, Bowsher RR, Miller WG, et al. Standardization of insulin immunoassays: Report of the American Diabetes Association Workgroup. Clin Chem. 2007;53:711–6. doi: 10.1373/clinchem.2006.082214. [DOI] [PubMed] [Google Scholar]
- 17.Manley SE, Luzio SD, Stratton IM, Wallace TM, Clark PM. Preanalytical, analytical, and computational factors affect homeostasis model assessment estimates. Diabetes Care. 2008;31:1877–83. doi: 10.2337/dc08-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manley SE, Stratton EM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem. 2007;53:922–32. doi: 10.1373/clinchem.2006.077784. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez JA, Bush NC, Hunter GR, Brock DW, Gower BA. Ethnicity and weight status affect the accuracy of proxy indices of insulin sensitivity. Obesity. 2008;16:2739–44. doi: 10.1038/oby.2008.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parretti E, Lapolla A, Dalfrà M, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449–53. doi: 10.1161/01.HYP.0000205122.47333.7f. [DOI] [PubMed] [Google Scholar]
- 21.Sierra-Laguado J, Garcia RG, Celedón J, et al. Determination of insulin resistance using the homeostatic model assessment (HOMA) and its relation with the risk of developing pregnancy-induced hypertension. Am J Hypertens. 2007;20:437–42. doi: 10.1016/j.amjhyper.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JM, Gammill H. Insulin resistance in preeclampsia. Hypertension. 2006;47:341–2. doi: 10.1161/01.HYP.0000205123.40068.84. [DOI] [PubMed] [Google Scholar]
- 23.Papageorghious AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2004;18:383–96. doi: 10.1016/j.bpobgyn.2004.02.003. [DOI] [PubMed] [Google Scholar]


